Abstract
Neosporosis is the major cause of abortion and reproductive failures in cattle, leading to significant economic losses. In this study, we evaluated the impact of Neospora caninum infection on oxidative stress (OS) markers and local cytokine mRNA expression at the placenta, as well as its effect on the progesterone (P4) serum levels and systemic cytokine profile in a pregnant mouse model. Infected pregnant mice (NC-1 group) showed increased percentages of fetal losses and IFN-γ serum levels, decreased serum progesterone, increased placental mRNA expression levels of both Th1-type (IFN-γ and TNF-α) and Th2-type (IL-4) cytokines, and inhibited expression of TGF-β1 (Treg) compare to control dams (CONTROL group). In addition, lipid peroxidation and ROS were increased, whereas the antioxidant enzymes, superoxide dismutase (SOD), and catalase (CAT) activities were modified in the placentae of infected mice compared to control mice. These findings demonstrate that multiple factors, including placental OS, are involved in fetal losses associated with N. caninum infection in mice, thus OS contribution to the placental physiopathology of neosporosis in other hosts must not be ruled out.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neosporosis is a worldwide distributed infectious disease caused by Neospora caninum, an apicomplexan parasite recognized as the major etiological agent of abortion in cattle (Innes 2007). Despite cattle can be horizontally infected with N. caninum, the vertical transmission through the placenta is largely the major route of infection (López-Pérez et al. 2010). Prime-infection or recrudesce of bovine neosporosis during pregnancy may result in fetal death, the birth of a weak calf, or the birth of a clinically normal but persistently infected calf (Rosbottom et al. 2011). The mechanisms underlying fetal death are unclear, but it has been suggested that it could be a result of (i) the direct injury of placental or fetal tissues by the proliferating parasite; (ii) the indirect effect of the damage or inflammation of the placenta, leading to insufficient delivery of oxygen and nutrients to the fetus or its the immune expulsion, respectively (Quinn et al. 2002), and/or (iii) the result of the luteolytic effects caused by the increased release of maternal prostaglandins and inflammatory cytokines, suggesting an important immune component in bovine neosporosis (Innes 2007). In this regard, high levels of seruminterferon-γ (IFN-γ) in N. caninum infection during pregnancy may be considered as a double-edged sword; since, despite IFN-γ is essential for the development of host protective immunity against the parasite (Entrican 2002), a successful gestation has been thought to be accompanied by a bias away from T helper 1 (Th1)-type cytokines and toward T helper 2 (Th2)-type cytokines (Quinn et al. 2002), which also implies that high levels of peripheral IFN-γ would contribute to the disruption of pregnancy.
Beyond the uncertainties, it is clear that the placenta plays a key role in the pathogenesis of N. caninum infection in cattle (Entrican 2002).The placenta is a vital organ that forms a connection between the mother and the fetus, mediating nutrient, oxygen, and hormone exchanges between them, also acting as an immune protective barrier (Arck et al. 2007). The high metabolic demand that rules the normal physiology of the placenta defines it as an organ of active oxygenic metabolism, where not only reactive oxygen species (ROS) but also antioxidants are formed (Wu et al. 2016). In neosporosis, the role of ROS has been scarcely studied and remains controversial. Despite it has been suggested that ROS and its signaling pathway are crucial in the resistance against N. caninum infection both in vivo and in vitro (da Silva et al. 2017; Mota et al. 2020), a recent report by Tao et al. (2022) affirmed that increased ROS levels triggered by the infection were associated with mitochondrial damage in caprine endometrial epithelial cells in vitro, potentially contributing to the pathophysiology of abortion. Taking into account that the role of oxidative stress (OS) in the placenta of N. caninum-infected animals remains unexplored, the aim of the present report was to investigate OS markers, endocrine, and immune changes in the placenta of pregnant mice experimentally infected with N. caninum.
Material and methods
Animals, experimental design, and analyzed parameters
It has been proposed that pregnant models of N. caninum infection at mid-gestation are those that better mimic the dynamics and outcome of natural infections (Horcajo et al. 2016). In fact, it has been demonstrated that N. caninum infection at mid-gestation had the most severe consequences for the offspring when compared to infection in early or late gestation, and leads to the highest rate of vertical transmission and fetal mortality in mice (López-Pérez et al. 2008). Taking these data into account, the mouse model of congenital infection at mid-gestation described by López-Pérez et al. (2010) and reproduced in our laboratory (Bengoa-Luoni et al. 2019) was employed in this research. The sample size calculation was based on the percentage of NC-1 + placentae (58%) obtained by López-Pérez et al. (2010). In order to ensure independence among the samples of each group, we considered only one placenta per litter. The power of the experiment was set to P = 90% and alpha level to P = 0.05, resulting in a minimum sample size of n = 8 dams per group (García-García et al. 2013). Briefly, 40 BALB/c mice aged 8 to 10 weeks were randomly allocated in two groups (NC-1 and CONTROL) and mated in harem with male BALB/c mice (2 females/1 male) (Bioterium of the Faculty of Exact and Natural Sciences of the University of Buenos Aires, Argentina). The day of appearance of vaginal plug was considered as gestational day 0.5 (0.5. G.D). The pregnant mice obtained in NC-1 group (n = 8) were experimentally infected with 2 × 106 freshly purified NC-1 tachyzoites by s.c. injection in 200 μl of sterile PBS per mouse on 7.5 G.D. (NC-1 group), whereas the dams from the CONTROL group (n = 14) received only vehicle. On 17.5 G.D. (10days post-infection), dams were euthanized on CO2 chamber, and blood was collected by cardiac puncture.
Sera were separated and stored at − 70 °C to quantify systemic cytokines: interleukine 4 (IL4), interleukine 5 (IL-5), interleukine 10 (IL-10), and IFN γ, as well as progesterone (P4) levels. After performing cesarean section, the litter size was determined by counting feto-placental units, and viable ones were identified as described by López-Pérez et al. (2010). Placentae were carefully removed by dissection and immediately processed for the determination of total ROS by the membrane-permeant probe 5-(and-6)-chloromethyl-2′,7′-dichlorofluorescein diacetate (DCFH-DA), or frozen in liquid N2 and stored at − 70 °C to determine lipid peroxidation, catalase (CAT), and total superoxide dismutase (SOD) activities, expression of placental cytokines by RT-qPCR, and parasite load by qPCR.
The parameter “pregnancy loss” corresponds to the number of pregnant females with at least one dead fetus or reabsorbed decidua/total number of pregnant females with feto-placental units (percentage), whereas the “individual pregnancy loss rate” is defined as the ratio of dead fetuses + reabsorbed deciduas/total feto-placental units in each pregnant mouse (percentage).
All procedures involving animals were conducted in accordance with the Animal Care and Use Committee of Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), 1996, and the studies were approved by the Independent Ethics Committee for the Care and Use of Experimental Animals of National University of General San Martin (C.I.C.U.A.E., IIB-UNSAM, protocol number 006/21).
Progesterone and cytokine serum levels in dams
Serum P4 concentrations were determined by chemiluminescence immunoassay performed using ADVIA Centaur Immunoassay System (BAYER, Argentina). Serum cytokine levels (IFN- γ, IL-4, IL-5, and IL-10) were determined by capture ELISA commercial kits (Pharmingen; BD Biosciences, Sandiego, CA) following manufacturer’s instructions. Each sample was run in duplicate and two independent ELISAs were performed.
Cytokine expression and quantification of parasite load in placentae
Total RNA was isolated from frozen placentae by Trizol reagent following the manufacturer’s instructions (Invitrogen Life Technologies, Carlsbad, CA, USA). Reverse transcription was accomplished by using M-MLV reverse transcriptase (Promega, Wisconsin, USA), oligo dT20 (Invitrogen), and 4 μg of total RNA according to the manufacturer’s instructions. The steady-state mRNA levels of placental cytokines IL-4, IFN-γ, TNF-α, and transforming growth factor-β1 (TGF-β1) were analyzed by RT-qPCR. The β-actin gene (Actb) was used as reference gene based on previous studies in the mouse placenta (López-Pérez et al. 2010; Solano et al. 2016; Souza et al. 2023). Genomic DNA was extracted using Accuprep genomic DNA (gDNA) extraction kit (Bioneer, Korea) from 1 × 107 tachyzoites from N. caninum NC-1 strain or 50 mg of placental tissue following the manufacturer’s instructions. The parasite load in placental tissue and host DNA were quantified by qPCR as previously described by Bengoa-Luoni et al. (2019) in two different placentae from each mice of CONTROL (n = 28 placentae) and NC-1 (n = 16 placentae) groups. Primer sequences for all the experiments are listed in Supplementary Table 1.
Oxidative stress parameters
The ROS produced within placental cells were detected with the membrane-permeant probe 5-(and-6)-chloromethyl-2′,7′-dichlorofluorescein diacetate (DCFH-DA) as previously described (Lautraite et al. 2003). Briefly, placental cells (1 × 106 cells) obtained as described by (Clark and Chaouat 1986) were incubated in the dark with 10 uM DCFH-DA (Sigma Chemical Co.) for 25 min at 37 °C. Fluorescence was measured immediately after the addition of the probe (0 m) and after the incubation period (25 min) in total incubates (cells + exposure medium) directly in each well at an excitation wavelength of 488 nm and an emission wavelength of 530 nm. (Hitachi F2000 Fluorescence Spectrophotometer, Tokyo, Japan). The method used to assess thiobarbituric acid reactants (TBARS) quantifies the amount of malondialdehyde (MDA) present in cell homogenates that reacts with trichloroacetic acid (TCA)- thiobarbituric acid (TBA)-HCl (15% w/v; 0.375% w/v; and 0.25 M respectively) to give red species absorbing at 535 nm (TBARS) and was previously described by Sander et al. (2008). To evaluate total SOD and CAT activities, placentae were homogenized in 50 mM phosphate buffer (pH = 7.2), centrifuged at 3000 rpm for 10 min at 4 °C and supernatants were collected. Total SOD activity was assayed by measuring the inhibition of xanthine oxidase-dependent reduction of nitroblue tetrazolium (Moriconi et al. 2012). Supernatants were used in a reaction mixture containing 0.1 mM nitroblue tetrazolium, 0.1 mM EDTA, 50 M xanthine, and xanthine oxidase in 50 mM potassium phosphate buffer (pH 7.8). One unit of SOD is considered to be the amount of enzyme that inhibits the control rate by 50% (0.025 units min−1, absorbance at 560 nm). Catalase activity was measured as previously informed by Sander et al. (2008).
Statistical analysis
Statistical analyses were carried out using GraphPad Prism 7 Software (GraphPad, CA, USA). Results are informed as mean + SEM. Differences between groups were assessed by unpaired t tests followed by Welch’s correction or one-way analysis of variance (ANOVA) test (where appropriate).
Results and discussion
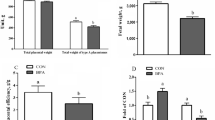
In the present study, we used a pregnant mouse model of N. caninum infection at mid-gestation because it has been demonstrated that these models better mimic the dynamics and outcome of natural neosporosis (Horcajo et al. 2016), making them especially interesting. This model is characterized by vertical transmission of neosporosis and increased neonatal death of offspring (Bengoa-Luoni et al. 2019), and in the current research, it was employed to evaluate oxidative, immunological and endocrine parameters involved in placental function. The litter size was similar between NC-1 and CONTROL groups (4.18 ± 0.49 vs. 4.71 ± 0.99, respectively; P > 0.05). However, the macroscopic evaluation of most of the uteri and placentae from infected mice showed abnormalities and presented, at least, one dead fetus or reabsorbed decidua when examined, which resulted in a pregnancy loss rate (number of pregnant females with at least one dead fetus or reabsorbed decidua/total number of pregnant females with feto-placental units) of 75% in the NC-1 group whereas there were no pregnancy losses in CONTROL group. In addition, the mean individual pregnancy loss rate (the percentage of dead fetuses and reabsorbed placentae per dam) was 21.30 ± 16.07% in NC-1 group vs. 0% in the CONTROL group. Since establishment and maintenance of pregnancy depend on P4, and this hormone is secreted mainly by the placenta during the middle and late gestational period in rodents (Arck et al. 2007), its serum levels were evaluated. Infected dams showed lower serum P4 levels than control ones (27.14 ± 2.90 pg/mL vs. 41.00 ± 0.58 pg/mL, respectively, P < 0.01; Supplementary Fig. 1), which demonstrated placental dysfunction. In addition, the present study reveals that N. caninum infected pregnant dams depicted a systemic pro-inflammatory cytokine profile, characterized by high serum levels of IFN-γ (256.1 ± 34.43 pg/mL in NC-1 dams vs. non-detectable levels in CONTROL dams; Supplementary Fig. 2a) and a limited increased in IL-5 (16.71 ± 0.62 in NC-1 dams vs. 12.07 ± 1.24 in CONTROL dams, P < 0.05; Supplementary Fig. 2b) when compared with control dams, whereas the levels of IL-4 or IL-10 were under the detection limit of the assay (both in NC-1 and CONTROL groups). Although IFN-γ is crucial for N. caninum restriction, its abundance at the placenta is detrimental to fetal development (Innes 2007). In the present report, high peripheral IFN-γ levels were unsuccessful in preventing neither the pregnancy losses nor the multiplication of N. caninum in the placenta, since N. caninum gDNA was detected in 93.75% of the NC-1 evaluated placentae (15 + /16 total), corresponding to 100% of the infected dams, with a mean parasite load of 13.15 ± 5.2 (pg parasite gDNA/100 ng total brain gDNA). Placental tissues are known to be a particularly endocrine-immune microenvironment which can be severely affected by infections, including N. caninum (Tang et al. 2023). In rodents, P4 present at the placenta is a potent inducer of Th2-type cytokines, which in turn, can inhibit the development and function of Th1 cells (Arck et al. 2007). Our results show that on the contrary to that observed systemically, N. caninum infection during middle pregnancy increased not only Th1-type cytokines (IFN−γ and TNF−α), but also IL-4 mRNA expression levels in NC-1 dams compared to those from controls (Fig. 1a, b, c, P < 0.05), whereas completely inhibited the expression of the regulatory cytokine, TGF-β1 (Fig. 1d). The dysregulation of TGF-β1is implicated in pregnancy-related diseases and in fact, the downregulation of TGF-β1 expression in N. caninum infected-trophoblast cell lines has been reported by Jiménez-Pelayo et al. (2019). Also, a recent study by Jia et al. (2020) showed that IFN−γ and IL-4 secreted by placental cells obtained from N. caninum infected mice were significantly higher compare to those from control mice. A recent study by Jiménez-Pelayo et al. (2019), suggest that pro-inflammatory cytokines could have a minor impact in placental damage by N. caninum than postulated previously, but other mechanisms should be implicated in the injury of the placenta in vivo. Here, the placentae obtained from NC-1 pregnant dams showed a significant increase in ROS levels (Fig. 2a, P < 0.05) when compared with those from CONTROL mice. Regarding neosporosis, despite similar results have been obtained in vivo and in vitro from different hosts and cell types (da Silva et al. 2017, 2020; Glombowsky et al. 2017; Mota et al. 2020; Tao et al. 2022), this is the first time that ROS and OS parameters are determined in the placenta of N. caninum-infected animals. In this sense, an important biomarker of OS is malondialdehyde (MDA) (Tonin et al. 2014). Our results show that N. caninum infection induced a significant increase in placental MDA content (Fig. 2b, P < 0.001), in accordance with data by Tonin et al. (2014) in brains from experimentally infected gerbils and by Glombowsky et al. (2017) in serum samples from natural infected cows. The counterparts of ROS are antioxidants, including the enzymes SOD and CAT (Wu et al. 2016). Our data showed that SOD activity was decreased (Fig. 2c, P < 0.05) while CAT activity was increased (Fig. 2d, P < 0.05) in placental tissues of N. caninum-infected dams. This modulation might be the biological response of the placental tissues to scavenge hydrogen peroxide (Biri et al. 2006), a potent toxic metabolite for living cells. However, the increased levels of ROS and MDA demonstrate that the antioxidant defense system was inefficient to protect cells from OS and its consequences. Recently, Tang et al. (2023) have demonstrated that N. caninum infection at the placenta of pregnant mice was associated with increased apoptosis. Taking into account that it is well established that excess OS kills cells either by necrosis or by apoptosis (Sinha et al. 2013), the OS contribution to the induction of apoptosis, and thus to the pathogenesis of congenital neosporosis must be considered. In summary, N. caninum infection in pregnant mice was associated with fetal losses, a systemic Th1-type biased cytokine profile, and placental dysfunction (denoted by decreased serum progesterone level). The invasion of the placenta by the parasites increased OS in the placenta and concomitantly induced a counterbalanced placental Th1/Th2-type cytokine response; both factors resulted inefficient to control parasite proliferation. These events would, in turn, affect the nutrient/oxygenic status of the growing fetus contributing, among other factors, to pregnancy loss, or in less severe cases, the vertical transmission of the parasite. Despite the important differences between the murine and the bovine placentae, these findings suggest that it would be of interest to investigate also the role of OS mediators in the physiopathology of the placenta from infected N. caninum cattle in order to evaluate them as potential targets for innovative treatments against neosporosis.
Relative gene expression of placental cytokines in N. caninum infected and control pregnant mice. a IFN-γ. b TNF-α. c IL-4. d TGF-β1. Placentae were obtained from N. caninum-infected (NC-1 group, n = 8 from different dams) and non-infected (CONTROL group, n = 14 from different dams) pregnant mice at 17.5 G.D (10 days post-infection). Each determination was performed in duplicate and results analyzed by the comparative cycle threshold method. Results are expressed as the mean of cytokine expression ± S.E.M. Significant differences were assessed by unpaired t test, followed by Welch’s correction.*P < 0.05, n.d., non-detectable expression levels
Parameters of placental oxidative stress in N. caninum-infected and control pregnant mice. a ROS (DCFH/DA). b Lipid peroxidation. c Superoxide dismutase (SOD) activity. d Catalase (CAT) activity. Placentae were obtained from N. caninum-infected (NC-1 group) and non-infected (CONTROL group) pregnant mice at 17.5 G.D (10 days post-infection). In a, b, c, and d, n = 5 and 6, respectively for NC-1 and CONTROL groups (obtained from different pregnant dams). Bars represents the mean ± S.E.M. Statistical differences were assessed by unpaired t test, followed by Welch’s correction.*P < 0.05, ***P < 0.001
Data availability
All data used are represented in the manuscript and are available to any researcher who wishes to use them for non-commercial purposes.
References
Arck P, Hansen PJ, Mulac Jericevic B, Piccinni MP, Szekeres-Bartho J (2007) Progesterone during pregnancy: endocrine-immune cross talk in mammalian species and the role of stress. Am J Reprod Immunol 58(3):268–279. https://doi.org/10.1111/j.1600-0897.2007.00512.x
Bengoa-Luoni SA, Corigliano MG, Sánchez-López E, Albarracín RM, Legarralde A, Ganuza A, Clemente M, Sander VA (2019) The potential of a DIVA-like recombinant vaccine composed by rNcSAG1 and rAtHsp81.2 against vertical transmission in a mouse model of congenital neosporosis. Acta Trop 198:105094. https://doi.org/10.1016/j.actatropica.2019.105094
Biri A, Kavutcu M, Bozkurt N, Devrim E, Nurlu N, Durak I (2006) Investigation of free radical scavenging enzyme activities and lipid peroxidation in human placental tissues with miscarriage. J Soc Gynecol Investig 13(5):384–388. https://doi.org/10.1016/j.jsgi.2006.04.003
Clark DA, Chaouat G (1986) Characterization of the cellular basis for the inhibition of cytolytic effector cells by murine placenta. Cell Immunol 102(1):43–51. https://doi.org/10.1016/0008-8749(86)90324-2
da Silva MV, Ferreira França FB, Mota CM, de Macedo JAG, Ramos EL, Santiago FM, Mineo JR, Mineo TW (2017) Dectin-1 compromises innate responses and host resistance against Neospora caninum infection. Front Immunol 8:245. https://doi.org/10.3389/fimmu.2017.00245
da Silva AS, Gebert RR, Reis JH, Baldissera MD, Souza CF, Barros LD, Garcia JL, Gris A, Mendes RE (2020) Experimental infection by Neospora caninum in gerbil reduces activity of enzymes involved in energy metabolism. Exp Parasitol 208:107790. https://doi.org/10.1016/j.exppara.2019.107790
Entrican G (2002) Immune regulation during pregnancy and host-pathogen interactions in infectious abortion. J Comp Pathol 126(79–94):6. https://doi.org/10.1053/jcpa.2001.0539
García-García JA, Reding-Bernal A, López-Alvarenga JC (2013) Cálculo del tamaño de la muestra en investigación en educación médica. Investig Educ Méd 2(8):217–224. https://doi.org/10.1016/S2007-5057(13)72715-7
Glombowsky P, Bottari NB, Klauck V, Fávero JF, Soldá NM, Baldissera MD, Perin G, Morsch VM, Schetinger MRC, Stefani LM, Da Silva AS (2017) Oxidative stress in dairy cows seropositives for Neospora caninum. Comp Immunol Microbiol Infect Dis 54:34–37. https://doi.org/10.1016/j.cimid.2017.07.007
Horcajo P, Regidor-Cerrillo J, Aguado-Martınez A, Hemphill A, Ortega-Mora LM (2016) Vaccines for bovine neosporosis: current status and key aspects for development. Parasite Immunol 38:709e723. https://doi.org/10.1111/pim.12342
Innes EA (2007) The host-parasite relationship in pregnant cattle infected with Neospora caninum. Parasitology 134:1903–1910. https://doi.org/10.1017/S0031182007000194
Jia L, Xie S, Li J, Li H, Wang H, Zhao S, Zhang S (2020) Establishment of a model of Neospora caninum infection in pregnant mice. Parasitol Res 119(11):3829–3837. https://doi.org/10.1007/s00436-020-06903-0
Jiménez-Pelayo L, García-Sánchez M, Regidor-Cerrillo J, Horcajo P, Collantes-Fernández E, Gómez-Bautista M, Hambruch N, Pfarrer C, Ortega-Mora LM (2019) Immune response profile of caruncular and trophoblast cell lines infected by high- (Nc-Spain7) and low-virulence (Nc-Spain1H) isolates of Neospora caninum. Parasit Vectors 12(1):218. https://doi.org/10.1186/s13071-019-3466-z
Lautraite S, Bigot-Lasserre D, Bars R, Carmichael N (2003) Optimisation of cell-based assays for medium throughput screening of oxidative stress. Toxicol InVitro 17(2):207–220. https://doi.org/10.1016/s0887-2333(03)00005-5
López-Pérez IC, Collantes-Fernández E, Aguado-Martínez A, Rodríguez-Bertos A, Ortega-Mora LM (2008) Influence of Neospora caninum infection in BALB/c mice during pregnancy in post-natal development. Vet Parasitol 155(3–4):175–183. https://doi.org/10.1016/j.vetpar.2008.05.018
López-Pérez IC, Collantes-Fernández E, Rojo-Montejo S, Navarro-Lozano V, Risco-Castillo V, Pérez-Pérez V, Pereira-Bueno J, Ortega-Mora LM (2010) Effects of Neospora caninum infection at mid-gestation on placenta in a pregnant mouse model. J Parasitol 96(5):1017–1020. https://doi.org/10.1645/GE-2347.1
Moriconi JI, Buet A, Simontacchi M, Santa-María GE (2012) Near-isogenic wheat lines carrying altered function alleles of the Rht-1 genes exhibit differential responses to potassium deprivation. Plant Sci 185–186:199–207. https://doi.org/10.1016/j.plantsci.2011.10.011
Mota CM, Lima-Junior DS, Ferreira França FB, Aguillón Torres JD, Barros PDSC, Santiago FM, Silva JS, Mineo JR, Zamboni DS, Mineo TWP (2020) Interplay between reactive oxygen species and the inflammasome are crucial for restriction of Neospora caninum replication. Front Cell Infect Microbiol 10:243. https://doi.org/10.3389/fcimb.2020.00243
Quinn HE, Ellis JT, Smith NC (2002) Neospora caninum: a cause of immunemediated failure of pregnancy? Trends Parasitol 18:391–394. https://doi.org/10.1016/s1471-4922(02)02324-3
Rosbottom A, Gibney H, Kaiser P, Hartley C, Smith RF, Robinson R, Kipar A, Williams DJ (2011) Upregulation of the maternal immune response in the placenta of cattle naturally infected with Neospora caninum. PLoS One 6(1):e15799. https://doi.org/10.1371/journal.pone.0015799
Sander VA, Piehl L, Facorro GB, Rubín de Celis E, Motta AB (2008) Regulation of functional and regressing stages of corpus luteum development in mice. Role of reactive oxygen species. Reprod Fertil Dev 20(7):760–9. https://doi.org/10.1071/rd08051
Sinha K, Das J, Pal PB, Sil PC (2013) Oxidative stress: the mitochondria-dependent and mitochondria-independent pathways of apoptosis. Arch Toxicol 87(7):1157–1180. https://doi.org/10.1007/s00204-013-1034-4
Solano ME, Thiele K, Kowal MK, Arck PC (2016) Identification of suitable reference genes in the mouse placenta. Placenta 39:7–15. https://doi.org/10.1016/j.placenta.2015.12.017
Souza JS, Farani PSG, Ferreira BIS, Barbosa HS, Menna-Barreto RFS, Moreira OC, Mariante RM (2023) Establishment of a murine model of congenital toxoplasmosis and validation of a qPCR assay to assess the parasite load in maternal and fetal tissues. Front Microbiol 14:1124378. https://doi.org/10.3389/fmicb.2023.1124378
Tang Z, Li H, Xie S, Zhao S, Zhang S, Wang H, Li N, Zhang X, Zhao F, Jia L (2023) A preliminary study on placental damage associated to experimental neosporosis in BALB/c mice. Parasitol Res 122(3):781–788. https://doi.org/10.1007/s00436-022-07771-6
Tao DL, Zhao SS, Chen JM, Chen X, Yang X, Song JK, Liu Q, Zhao GH (2022) Neospora caninum infection induced mitochondrial dysfunction in caprine endometrial epithelial cells via downregulating SIRT1. Parasit Vectors 15(1):274. https://doi.org/10.1186/s13071-022-05406-4
Tonin AA, da Silva AS, Thomé GR, Bochi GV, Schetinger MR, Moresco RN, Camillo G, Toscan G, Vogel FF, Lopes ST (2014) Oxidative stress in brain tissue of gerbils experimentally infected with Neospora caninum. J Parasitol 100(1):154–156. https://doi.org/10.1645/13-310.1
Wu F, Tian FJ, Lin Y, Xu WM (2016) Oxidative stress: placenta function and dysfunction. Am J Reprod Immunol 76(4):258–271. https://doi.org/10.1111/aji.12454
Funding
This work was supported by grants to Dr. Valeria A. Sander (PICT 2016–0310) and Dr. Marina Clemente (PICT 2016–0621) from Agencia Nacional de Promoción Científica y Tecnológica (Argentina). The study also received institutional support from the Universidad Nacional General de San Martín (UNSAM, Argentina).
Author information
Authors and Affiliations
Contributions
LFMM and VL contributed to the conception and design of the study, performed the experiments, and analyzed the data. MGC, ESL, and VAR performed part of the experiments. AL and AG maintain the mice and the parasite cultures, respectively. MC contributed with writing—review, editing, and funding acquisition, and VS participated in conceptualization, visualization, supervision, project administration, funding acquisition, and writing—review of the MS.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All procedures requiring animals were performed in agreement with institutional guidelines and were approved by the Independent Ethics Committee for the Care and Use of Experimental Animals of National University of General San Martin (C.I.C.U.A.E., IIB-UNSAM).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Section Editor: Daniel Howe
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Morales, L.F.M., Lagorio, V., Corigliano, M.G. et al. Dysfunction, oxidative stress markers, and cytokine expression in the placentae of mice experimentally infected with Neospora caninum. Parasitol Res 122, 3257–3263 (2023). https://doi.org/10.1007/s00436-023-07995-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-023-07995-0