Abstract
The Atlantic Forests outside of the Amazon region in Brazil are low-frequency malaria hotspots. The disease behaves as a zoonosis maintained by nonhuman primates (NHPs), especially howler monkeys. Between 2016 and 2018, Brazil witnessed the largest yellow fever outbreak since 1980, resulting in massive declines in these NHP populations. However, reports of malaria cases continued in transmission areas. This scenario motivated this survey to determine the frequency of infection of the anophelines by Plasmodium species. Mosquitoes were captured using Shannon traps and CDC light traps and identified as to species based on morphological characters. The screening for malaria parasites targeted only Anopheles species belonging to the subgenus Kerteszia, the proven primary malaria vector. A TaqMan qPCR assay using ribosomal primers (18S rRNA gene) was performed in a Step One Plus Real-time PCR to detect Plasmodium species. Seven hundred sixty field-caught anophelines divided into 76 pools were examined. Out of 76 tested pools, seven (9.21%) were positive. Three pools were Plasmodium malariae-positive, and four were Plasmodium vivax-positive. The anopheline infection was expressed as the maximum infection rate (MIR), disclosing a value of 0.92%, indicative of a steady state. Such stability after the yellow fever outbreak suggests that other species of NHPs could support transmission.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Malaria is a public health challenge in Brazil, especially in the Amazon region, where more than 99% of cases currently occur (Buery et al. 2021). The remaining 1% of the occurrences outside the Brazilian Amazon region may be imported or autochthonous. Autochthonous cases of the disease in the extra-Amazon region are found specifically in areas surrounded by the Atlantic Forest, located in the southeastern and southern parts of the country, zones previously known to be free of malaria since the 1960s. The popular name for the disease in such areas is Bromeliad malaria. It was named after bromeliads (Bromeliaceae), a native plant of the Atlantic Forest that serves as the development local of the vector. Immature forms of the transmitting mosquito Anopheles (Kerteszia) cruzii develop in the water that accumulates in the leaf axials of these plants (Consoli and Lourenço-de-Oliveira 1994).
The sporadic cases of Bromeliad malaria are found more frequently in rural communities, where there are dense fragments of preserved forest. The infected individuals usually report recent excursions into the woods for recreational (e.g., ecotourism) or professional activities (e.g., agriculture), and it is precisely the interior of the forest that Anopheles (K.) cruzii, the incriminated vector, inhabits (Ferreira et al. 2021). The clinical presentation is mainly one of few or no symptoms, with low parasitemia, representing an unexpected clinical pattern for the disease (Cerutti et al. 2007). Plasmodium vivax is the main parasite responsible for human infections, but Plasmodium malariae and Plasmodium falciparum also occur in a lower proportion (Laporta et al. 2015; Buery et al. 2017).
Bromeliad-malaria ecology also appears to involve the participation of nonhuman primates (NHPs), especially those belonging to the genus Alouatta (Duarte et al. 2008; Abreu et al. 2019; Monteiro et al. 2020). Epidemiological studies conducted in Atlantic Forest areas have found natural Plasmodium infection in NHPs, with Plasmodium brasilianum and Plasmodium simium being the species implicated as etiologic agents. Interestingly, Plasmodium brasilianum and Plasmodium simium are morphologically and genetically indistinguishable from the human parasites Plasmodium malariae and Plasmodium vivax, respectively. These last two can infect humans when they enter forested areas (Goldman et al. 1993; Tazi and Ayala 2011). Furthermore, entomological studies in Bromeliad-malaria areas have shown that P. vivax/P. simium and P. malariae/P. brasilianum frequently infect Anopheles (K.) cruzii (Buery et al. 2018; Demari-Silva et al. 2020. 2020). This evidence suggests that once infected, Anopheles (K.) cruzii can transmit Plasmodium species from humans to NHPs or vice versa. This hypothesized transmission dynamic is possible due to acrodendrophilic behavior and the vertical dispersion of such an incriminated vector (Deane et al. 1984; Ferreira et al. 2021). In addition, the vertical distribution associated with the close contact between NHPs and humans is possibly sufficient to enable repeated spillover and spillback events (Fig. 1) (Deane et al. 1966).
Studies carried out over the last few years in Atlantic Forest areas indicated that the predominant simian host for P. brasilianum and P. simium is Alouatta guariba clamitans (howler monkey), which is indigenous to South America (Deane, 1992; Duarte et al. 2008; Abreu et al. 2019; Monteiro et al. 2020).
In addition to their evident susceptibility to malaria infection, howler monkeys are also considered highly susceptible to yellow fever virus (YFV), the etiologic agent of yellow fever (YF). Yellow fever is an acute viral disease transmitted by sylvatic mosquitoes from the Hemagogus and Sabethes genera. Unlike malaria, YFV infections cause severe and often fatal illnesses for NHPs belonging to the Alouatta genus (Leal et al. 2016).
Between 2016 and 2018, Brazil witnessed the largest YFV outbreak since 1980, with at least 2251 human YFV cases and 15,000 epizootic events (Silva et al. 2020). This outbreak caused the deaths of many howler monkeys, resulting in massive and rapid declines in these NHP populations in the Brazilian Atlantic Forest. In this context, assuming the hypothesis of howler monkeys participating in the Bromeliad-malaria transmission cycle, their population reduction would exhaust the source of infection — especially for anophelines of the subgenus Kerteszia — and consequently impact the malaria transmission cycle. Therefore, anophelines have become relevant biological indicators of malaria in the Atlantic Forest. Hence, analyzing anopheline infectivity while the population of the potential NHP reservoir is reduced will help to clarify the role and influence of alternative hosts/reservoirs in the disease cycle. Additionally, it will shed some light on epidemiological uncertainties regarding the transmission dynamics of bromeliad malaria.
Materials and methods
Study area
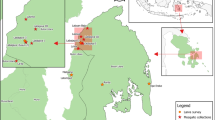
The capture of adult Anopheles mosquitoes occurred in Valsugana Velha (Fig. 2), a rural area of Santa Teresa municipality, located in the central mountainous region of Espírito Santo, southeastern Brazil (19°58′05.2″S, 40°34′40.8″W). The choice of this area relates to the previous detection of local autochthonous human malaria cases. Moreover, there were records of natural Plasmodium infection in NHPs and anopheline specimens on the same site (Cerutti et al. 2007; Rezende et al. 2009; Buery et al. 2018). Valsugana Velha is a receptive area for malaria, as it has an ecosystem that allows the cohabitation of vector mosquitoes and reservoir hosts.
Santa Teresa has a total area of 683.032 km2, of which approximately 219.253 km2 corresponds to the Atlantic Forest biome. Atlantic Forest is a significant rainforest in Brazil, composed of a great diversity of biotopes, which provides adequate conditions for the development of mosquitoes (Secretaria de Meio Ambiente e Recursos Hídricos 2018). According to the Köppen classification, Santa Teresa has a warm temperate climate, with an average temperature of 22 °C during summer and 18 °C during winter. The average annual rainfall for this area is 1491.6 mm, with two climatic seasons: the drier period from June to October and the rainy period from November to May (Instituto Capixaba de Pesquisa, Assistência Técnica e Extensão Rural 2021).
Sampling, identification, and storage of Anopheles females
Anopheles female mosquitoes were sampled monthly (once a month) from May 2019 to April 2020 using two types of light traps: (1) Shannon light trap, with the help of an oral suction tube: its setting inside the forest allows the capture of anophelines for 4 h (06:00–10:00 p.m.). (2) CDC automatic (Center for Disease Control) trap with CO2 baits (200 g of dry ice): the team installed two CDC traps inside the forest, the first at a height of 1.5 m above the ground and the second at a height of 10 m in the canopy. The CDC traps remained turned on for 12 h from the night (06:00 p.m.) until the morning (06:00 a.m.).
The procedures involving the collected mosquitoes included euthanasia by flash freezing, storage in polyethylene cages, and transportation to the Entomology and Malacology Centre of Espírito Santo (Núcleo de Entomologia e Malacologia do Espírito Santo — NEMES/ES). In NEMES/ES, the investigators identified the specimens at the species level based on their morphological characters using the identification keys of Consoli and Lourenço-de-Oliveira (1994). After the identification process, the mosquitoes were pooled into groups of up to 10 specimens based on their collection date and trap type. Each pool was placed in an Eppendorf tube and stored at − 20 °C until DNA extraction for the detection of malaria parasites.
Molecular procedures
Due to the large number of mosquitoes collected during the study, screening for malaria parasites targeted only Anopheles species belonging to the subgenus Kerteszia, considering their proven implication as the main malaria vectors in the Atlantic Forest areas.
DNA extraction and detection of natural Plasmodium infection
DNA extraction from each pool of mosquitoes (maximum of 10 specimens/pool) was performed using the DNeasy® Blood & Tissue kit (Qiagen, Hilden, Germany), according to the manufacturer’s instructions. To detect P. falciparum, P. malariae/P. brasilianum, and P. vivax/P. simium, a TaqMan qPCR assay using ribosomal primers (18S rRNA gene) was performed in a Step One Plus Real-time PCR System (Applied Biosystems), as previously described by Bickersmith et al. (2015). The same protocol (Bickersmith et al. 2015) was applied to detect P. malariae/P.brasilianum, but the primers and probes used followed the description of Rougemont et al. (2004).
Each PCR used a total of 20 μl, containing 2 μl of genomic DNA, 7.5 μl of TaqMan Universal Master Mix II with UNG, 0.15 µl of the probe (10 µM), 0.45 µl of each primer, forward and reverse (10 µM), and 4.45 μl of ultrapure water. The positive control was DNA from P. falciparum, P. malariae/P. brasilianum, and P. vivax/P. simium. An aliquot of ultrapure water was the negative control. The thermocycler conditions were 50 °C for 2 min, followed by 95 °C for 10 min, 50 cycles at 95 °C for 15 s, and 60 °C for 1 min.
Minimum infection rate
To evaluate the infection rate of the collected Anopheles (K.) cruzii, the minimum infection rate (MIR) was calculated using the following formula: MIR = (x/n) × 100, where x is the number of positive pools and n is the total number of mosquitoes tested (Paiva et al. 2006). The minimum infection rate was estimated, assuming that each positive pool contained at least one infected specimen.
Ethics statement
No specific permissions were needed. The study did not involve endangered or protected species.
Results
Composition and distribution of Anopheles (Kerteszia) mosquitoes
The study collected 1259 Anopheles (K.) cruzii specimens from May 2019 to April 2020. The CDC light trap located in the canopy (approximately 10 m) collected a total of 1236 (98.17%) Anopheles (K.) cruzii. In turn, the Shannon light trap and CDC light trap located on the ground level collected 13 (1.03%) and 10 (0.79%) specimens of Anopheles (K.) cruzii, respectively.
Plasmodium species detection by qPCR
The screening involved testing mosquitoes for P. falciparum, P. malariae/P. brasilianum, and P. vivax/P. simium because in Santa Teresa, detection of these species occurred in different hosts, including humans, howler monkeys, and anophelines. A total of 760 field-caught Anopheles (Kerteszia) cruzii divided into 76 pools were examined. Out of 76 tested pools, seven (9.21%) were positive. Three pools of Anopheles (K.) cruzii were P. malaria-positive, and four pools were P. vivax-positive. Plasmodium falciparum was not detected in this study. The rate of Plasmodium species infection of Anopheles (K.) cruzii was expressed as MIR (Table 1). All seven Plasmodium-positive pools came from the CDC light trap located in the canopy. Figure 3 shows the seasonal distribution of Plasmodium-positive sample pools.
Minimum infection rate of Plasmodium species in Anopheles (K.) cruzii
The minimum infection rate for Anopheles (K.) cruzii was 0.92. In analyzing the infections individually by Plasmodium malariae and Plasmodium vivax, the MIR was 0.39 and 0.53, respectively. It was not possible to calculate the MIR of the Anopheles (K.) cruzii for Plasmodium falciparum, since no positive pools for this parasite species were found.
Discussion
Several reports have shown that Anopheles (K.) cruzii, incriminated as the principal vector for human and simian malaria in the Brazilian Atlantic Forest, has an acrodendrophilic habit (Deane et al. 1971; Rezende et al. 2009; Buery et al. 2018). Consistent with the literature, the present study confirmed this species’ preference for tree canopies. Furthermore, the natural infection of Anopheles (K.) cruzii by P. vivax/P. simium and P. malariae/P.brasilianum found in this study corroborate the primary importance of this vector in the transmission of malaria in Atlantic Forest areas. Altogether, these data support the hypothesis that the specific breeding conditions of Anopheles (K.) cruzii favor their predominance in the canopy, where they find NHPs to be their principal blood source. The distribution of this vector in the sylvatic environment fits the hypothesized malaria transmission cycle in the Atlantic Forest, which seems to occur mainly among NHPs in the tree canopy, with humans being accidental hosts.
The nonspecificity of molecular techniques in identifying parasites of nonhuman origin is a limitation of the present study. However, several studies have suggested that P. vivax and P. malariae are the same species as P. simium and P. brasilianum, respectively, based on their genetic and morphological similarities (Goldman et al. 1993; Escalante et al. 1998; Leclerc et al. 2004; Lim et al. 2005; Tazi and Ayala 2011). Such evidence raises the possibility of the routine transfer of the parasites from one host species to another, making source identification less relevant. Moreover, the observed anopheline preference for the canopy reinforces the conclusion of a simian origin of the identified parasites.
In addition, another limitation of this study was that Anopheles Kerteszia identification was made only by morphological criteria. To minimize the possibility of misidentification, we improved the accuracy through evaluation by experienced entomologists. However, molecular methods in conjunction with morphological identification are the gold standard and should be recommended.
Valsugana Velha is a study area where human autochthonous malaria cases occur sporadically. Investigations at the site allowed the capture of a small number of mosquitoes of the species Anopheles (K.) cruzii at ground level. Rezende et al. (2009) and Buery et al. (2018) observed natural infection by P. vivax/P simium in the same area in one pool of the species Anopheles (K.) cruzii, captured by a CDC light trap placed on the ground. Deane et al. (1971) highlighted the versatility of the species Anopheles (K.) cruzii in moving between the canopy and the ground level as a risk factor for transmission of parasites from the genus Plasmodium between canopy-dwelling simian hosts and ground-dwelling humans.
Considering the putative transmission cycle of malaria in the Atlantic Forest, several lines of evidence suggest that NHPs may act as a source of new Plasmodium infections for humans and vice versa. Such a transference would depend on a competent mosquito species acting as a bridge between these different host species occupying different strata in the forest. Surveys such as those conducted by Deane et al. (1966) and Brasil et al. (2017) reported transfers of Plasmodium from NHPs to humans or from humans to NHPs. However, the possible species of NHPs involved in this shared transmission cycle are the object of speculation. Recent evidence suggests that NHPs of the genus Alouatta sp. should be the principal potential reservoir of malaria in the Atlantic Forest. The surveillance of simian malaria performed over the last few years in the Brazilian Atlantic Forest disclosed in Alouatta sp. the highest prevalence of infection by P. brasilianum and P. simium, species indistinguishable from the human Plasmodium species P. malariae and P. vivax, respectively (Duarte et al. 2006, 2008; Yamasaki et al. 2011; Abreu et al. 2019; Monteiro et al. 2020; Assis et al. 2021).
The epidemiological relevance of the Alouatta sp. genus for malaria in Atlantic Forest areas became the object of scrutiny after their drastic population reduction promoted by the sylvatic yellow fever outbreak, which spread in the Atlantic Forest areas of southern and southeastern Brazil in 2016. As postulated by Abreu et al. (2019), the expectation was that the population decrease of the supposed principal reservoir of malaria in the Atlantic Forest would affect its transmission dynamics, resulting in a decreased infection rate of the predominant vector Anopheles (K.) cruzii. However, such an infection rate reduction did not occur, mainly in Espírito Santo state. The stability of the vector infection rate is evident in the present study, as four pools of Anopheles (K.) cruzii had infection by Plasmodium vivax, giving an infection rate of 0.53. Such a rate is similar to that of Rezende et al. (2009) in a study conducted in Valsugana Velha (MIR = 0.5), the same area of this study. The overall infection rate for malaria parasites found in the examined mosquito pools was 0.92. Buery et al. (2018) found a similar minimum infection rate in Anopheles (K.) cruzii in the same study area (MIR = 0.96). However, the MIR in the Anopheles (K.) cruzii from Atlantic Forest areas of São Paulo is distinctly different. Duarte et al. (2013) and Neves et al. (2013) found an MIR for the Anopheles (K.) cruzii of 0.09 and 0.24, respectively.
The stability of Plasmodium infection rates in Anopheles (K.) cruzii after the yellow fever outbreak suggests that other species of NHPs could support the transmission cycle in areas of the Atlantic Forest. This would be particularly relevant during the reduction in the population of Alouatta specimens. In Rio de Janeiro state, an area of malaria autochthonous cases, the studies disclosed natural infection in NHPs of the Sapajus genus by P. vivax/P. simium and P. malariae/P. brasilianum (de Alvarenga et al. 2015). More recently, in the municipality of Santa Teresa — the setting of this study — researchers identified the presence of P. falciparum and P. malariae/P. brasilianum in the feces of Sapajus nigritus (manuscript in preparation). These results further support the hypothesis that a variety of NHPs can act as reservoirs for malaria in Atlantic Forest areas, thus contributing to the persistence of the parasite in the environment.
Despite these results, questions remain. Therefore, investigative studies exploring potential reservoirs in the Atlantic Forest areas are necessary and should be encouraged. However, such studies should not focus only on the diagnosis of infection. They should mainly seek to understand the infection course and profile in the putative wild hosts.
Data availability
Not applicable.
References
Abreu FVSD, Santos ED, Mello ARL et al (2019) Howler monkeys are the reservoir of malarial parasites causing zoonotic infections in the Atlantic forest of Rio de Janeiro. PLOS Negl Trop Dis 13:e0007906. https://doi.org/10.1371/journal.pntd.0007906
Assis GMPD, Alvarenga DAMD, Costa Pereira MDO et al (2021) Profiling humoral immune response against pre-erythrocytic and erythrocytic antigens of malaria parasites among neotropical primates in the Brazilian Atlantic Forest. Front Cell Infect Microbiol 11:395. https://doi.org/10.3389/fcimb.2021.678996
Bickersmith SA, Lainhart W, Moreno M, Chu VM, Vinetz JM, Conn JE (2015) A sensitive, specific and reproducible real-time polymerase chain reaction method for detection of Plasmodium vivax and Plasmodium falciparum infection in field-collected anophelines. Mem Inst Oswaldo Cruz 110:573–576. https://doi.org/10.1590/0074-02760150031
Brasil P, Zalis MG, Pina-Costa A et al (2017) Outbreak of human malaria caused by Plasmodium simium in the Atlantic Forest in Rio de Janeiro: a molecular epidemiological investigation. Lancet Glob Health 5:1038–1046. https://doi.org/10.1016/S2214-109X(17)30333-9
Buery JC, Rodrigues PT, Natal L, Salla LC, Loss AC, Vicente CR, Rezende HR, Duarte AMRC, Fux B, Malafronte RS, Falqueto A, Cerutti Junior C (2017) Mitochondrial genome of Plasmodium vivax/simium detected in an endemic region for malaria in the Atlantic Forest of Espírito Santo state, Brazil: do mosquitoes, simians and humans harbour the same parasite? Malar J 16:437. https://doi.org/10.1186/s12936-017-2080-9
Buery JC, Rezende HR, Natal L, Silva LS, Menezes RMT, Fux B, Malafronte RS, Falqueto A, Cerutti Junior C (2018) Ecological characterization and infection of anophelines (Diptera: Culicidae) of the Atlantic Forest in the southeast of Brazil over a 10 year period: has the behaviour of the autochthonous malaria vector changed? Mem Inst Oswaldo Cruz 113:111–118. https://doi.org/10.1590/0074-02760170225
Buery JC, de Alencar FEC, Duarte AMRC, Loss AC, Vicente CR, Ferreira LM, Fux B, Medeiros MM, Cravo P, Arez AP, Cerutti Junior C (2021) Atlantic Forest malaria: a review of more than 20 years of epidemiological investigation. Microorganisms 9:132. https://doi.org/10.3390/microorganisms9010132
Cerutti C, Boulos M, Coutinho AF, Hatab MCL, Falqueto A, Rezende HR, Duarte AMRC, Collins W, Malafronte RS (2007) Epidemiologic aspects of the malaria transmission cycle in an area of very low incidence in Brazil. Malar J 6:33. https://doi.org/10.1186/1475-2875-6-33
Consoli RAGB, Lourenco-de-Oliveira R (1994) Principais mosquitos de importancia sanitaria no Brasil. Fiocruz, Rio de Janeiro
de Alvarenga DAM, de Pina-Costa A, de Sousa TN, Pissinatti A, Zalis MG, Suaréz-Mutis MC, Lourenço-de-Oliveira R, Brasil P, Daniel-Ribeiro CT, Brito CFA (2015) Simian malaria in the Brazilian Atlantic Forest: first description of natural infection of capuchin monkeys (Cebinae subfamily) by Plasmodium simium. Malar J 14:1–9. https://doi.org/10.1186/s12936-015-0606-6
Deane LM (1992) Simian malaria in Brazil. Mem Inst Oswaldo Cruz 87:1–20. https://doi.org/10.1590/S0074-02761992000700001
Deane LM, Deane MP, Neto JF (1966) Studies on transmission of simian malaria and on a natural infection of man with Plasmodium simium in Brazil. Bull World Health Organ 35:805–808
Deane LM, Deane MP, Ferreira Neto JA, Almeida FB (1971) On the transmission of simian malaria in Brazil. Rev Inst Med Trop Sao Paulo 13:311–319
Deane LM, Ferreira Neto JA, Lima MM (1984) The vertical dispersion of Anopheles (Kerteszia) cruzi in a forest in southern Brazil suggests that human cases of malaria of simian origin might be expected. Mem Inst Oswaldo Cruz 79:461–463. https://doi.org/10.1590/S0074-02761984000400011
Demari-Silva B, Laporta GZ, Oliveira TMP, Sallum MAM (2020) Plasmodium infection in Kerteszia cruzii (Diptera: Culicidae) in the Atlantic tropical rain forest, southeastern Brazil. Infect Genet Evol 78:104061. https://doi.org/10.1016/j.meegid.2019.104061
Duarte AMR, Porto MA, Curado I, Malafronte RS, Hoffmann EHE, Oliveira SG, Silva AMJ, Kloetzel JD, Gomes AC (2006) Widespread occurrence of antibodies against circumsporozoite protein and against blood forms of Plasmodium vivax, P. falciparum and P.malariae in Brazilian wild monkeys. J Med Primatol 35:87–96. https://doi.org/10.1111/j.1600-0684.2006.00148.x
Duarte AMR, Malafronte RS, Cerutti C, Curado I, Paiva BR, Maeda AY, Yamasaki T, Summa MEL, Neves DVDA, Oliveira SG, Gomes AC (2008) Natural Plasmodium infections in Brazilian wild monkeys. Reservoirs for human infections? Acta Trop 107:79–85. https://doi.org/10.1016/j.actatropica.2008.05.020
Duarte AMR, Pereira DM, de Paula MB, Fernandes A, Urbinatti PR, Ribeiro AF, Mello MHSH, Matos MO, Mucci LF, Fernandes LN, Natal D, Malafronte RS (2013) Natural infection in anopheline species and its implications for autochthonous malaria in the Atlantic Forest in Brazil. Parasit Vectors 6:1–6. https://doi.org/10.1186/1756-3305-6-58
Escalante AA, Freeland DE, Collins WE, Altaf AL (1998) The evolution of primate malaria parasites based on the gene encoding cytochrome b from the linear mitochondrial genome. Proc Natl Acad Sci U S A 95:8124–8129. https://doi.org/10.1073/pnas.95.14.8124
Ferreira LM, Rezende HR, Buery JC, Silva LS, Figueiredo TCC, Fux B, Duarte AMR, Cerutti Junior C (2021) Residual malaria of Atlantic Forest systems and the influence of anopheline fauna. Parasitol Res 120:2759–2767. https://doi.org/10.1007/s00436-021-07238-0
Goldman IF, Qari SH, Millet PG, Collins WE, Altaf AL (1993) Circumsporozoite protein gene of Plasmodium simium, a Plasmodium vivax like monkey malaria parasite. Mol Biochem Parasitol 57:177–180. https://doi.org/10.1016/0166-6851(93)90257-x
Instituto Capixaba de Pesquisa, Assistência Técnica e Extensão Rural. (2021). Programa de Assistência Técnica e Extensão Rural – PROATER 2020–2023. https://incaper.es.gov.br/media/incaper/proater/municipios/Santa_Teresa.pdf [acessed on 25 Nov 2021].
Laporta GZ, Burattini MN, Levy D, Fukuya LA, Oliveira TMP, Maselli LMF, Conn JE, Massad E, Bydlowski SP, Sallum MAM (2015) Plasmodium falciparum in the southeastern Atlantic forest: a challenge to the bromeliad-malaria paradigma? Malar J 14:181. https://doi.org/10.1186/s12936-015-0680-9
Leal SG, Romano APM, Monteiro RV, Melo CB, Vasconcelos PFC, Castro MB (2016) Frequency of histopathological changes in Howler monkeys (Alouatta sp.) naturally infected with yellow fever virus in Brazil. Rev Soc Bras Med Trop 49:29–33. https://doi.org/10.1590/0037-8682-0363-2015
Leclerc MC, Durand P, Gauthier C, Patot S, Billote N, Menegon M, Severini C, Ayala FJ, Renaud F (2004) Meager genetic variability of the humn malária agent Plasmodium vivax. Proc Natl Acad Sci U S A 101:14455–14460. https://doi.org/10.1073/pnas.0405186101
Lim CS, Tazi L, Ayala FJ (2005) Plasmodium vivax: Recente world expansion and genetic identify to Plasmodium simium. Proc Natl Acad Sci U S A 102:15523–15528. https://doi.org/10.1073/pnas.0507413102
Monteiro EF, Fernandez-Becerra C, Araujo MDS et al (2020) Naturally acquired humoral immunity against malaria parasites in non-human primates from the Brazilian Amazon Cerrado and Atlantic Forest. Pathogens 9:525. https://doi.org/10.3390/pathogens9070525
Neves A, Urbinatti PR, dos Santos MR, Fernandes A, Paganini WS, Natal D (2013) Malaria outside the Amazon region: natural Plasmodium infection in anophelines collected near an indigenous village in the Vale do Rio Branco, Itanhaém, SP, Brazil. Acta Trop 125:102–106. https://doi.org/10.1016/j.actatropica.2012.08.014
Paiva BRD, Secundino NFC, Nascimento JCD, Pimenta PFP, Galati EAB, Andrade Junior HF, Malafronte RS (2006) Detection and identification of Leishmania species in field-captured phlebotomine sandflies based on mini-exon gene PCR. Acta Trop 99:252–259. https://doi.org/10.1016/j.actatropica.2006.08.009
Rezende HR, Soares RM, Cerutti Junior C, Alves IC, Natal D, Urbinatti PR, Yamasaki T, Falqueto A, Malafronte RS (2009) Entomological characterization and natural infection of anophelines in an area of the Atlantic Forest with autochthonous malaria cases in mountainous region of Espírito Santo State, Brazil. Neotrop Entomol 38:272–280. https://doi.org/10.1590/S1519-566X2009000200017
Rougemont M, Van Saanen M, Sahli R, Hinrikison HP, Bille J, Jaton K (2004) Detection of four Plasmodium species in blood from humans by 18S rRNA gene subunit-based and species-specific real-time PCR assays. J Clin Microbiol 42:5636–5643. https://doi.org/10.1128/JCM.42.12.5636-5643.2004
Secretaria de Meio Ambiente e Recursos Hídricos (2018) Atlas da Mata Atlântica do estado do Espírito Santo. https://seama.es.gov.br/atlas-da-mata-atlantica-es [accessed on 25 Nov 2021].
Silva NIO, Sacchetto L, de Rezende IM, Trindade GS, LaBeaud AD, Thoisy B, Drumond BP (2020) Recent sylvatic yellow fever virus transmission in Brazil: the news from an old disease. Virol J 17:1–12. https://doi.org/10.1186/s12985-019-1277-7
Tazi L, Ayala FJ (2011) Unresolved direction of host transfer of Plasmodium vivax v. P. simium and P. malariae v P Brasilianum. Infect Genet Evol 11:209–221. https://doi.org/10.1016/j.meegid.2010.08.007
Yamasaki T, Duarte AM, Curado I, Summa MEL, Neves DVDA, Wunderlich G, Malafronte RS (2011) Detection of etiological agents of malaria in howler monkeys from Atlantic Forests, rescued in regions of São Paulo city, Brazil. J Med Primatol 40:392–400. https://doi.org/10.1111/j.1600-0684.2011.00498.x
Funding
This research was supported by the Research and Innovation Support Foundation of Espírito Santo (FAPES-Brazil; grant number 344/2018). L. M. F. received a Coordination for the Improvement of Higher Education Personnel master’s scholarship (CAPES-Brazil; grant number 88882.385044/2019–01). A. C. L. was supported by CNPq (grant number 300964/2022–6).
Author information
Authors and Affiliations
Contributions
Conceptualization: Lucas Mendes Ferreira and Crispim Cerutti Júnior; performed the research: Lucas Mendes Ferreira and Helder Ricas Rezende; performed the data analysis: Lucas Mendes Ferreira and Ana Maria Ribeiro de Castro Duarte; writing — original draft preparation: Lucas Mendes Ferreira and Crispim Cerutti Júnior; writing — review and editing: Julyana Cerqueira Buery, Crispim Cerutti Júnior, Filomena Euridice Carvalho de Alencar, Ana Maria Ribeiro de Castro Duarte, Blima Fux and Ana Carolina Loss.; figure production: Lucas Mendes Ferreira.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
All the authors have read and agreed to the published version of the manuscript.
Conflict of interest
The authors declare no competing interests.
Additional information
Handling Editor: Una Ryan
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ferreira, L.M., Rezende, H.R., Fux, B. et al. Anopheles (Kerteszia) cruzii infected by Plasmodium in the Atlantic Forest indicates that the malaria transmission cycle is maintained even after howler monkeys’ population decline. Parasitol Res 121, 3627–3634 (2022). https://doi.org/10.1007/s00436-022-07689-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-022-07689-z