Abstract
Leishmaniasis is a vector-borne parasitic disease caused by protozoa of the genus Leishmania. Twenty different species are known to cause disease in humans with varying degrees of pathology. These diseases are transmitted throughout the geographic range of phlebotomine sandflies, found between the latitudes 50°N and 40°S. This study explores antibody dependent enhancement (ADE) as the cause of disease exacerbation in heterologous exposure of L. major primed mice to L. infantum challenge. BALB/c mice received serum from L. major infected or naive mice. All mice were challenged with L. infantum and tissue parasite burdens were recorded. Animals that received anti-L. major serum exhibited significantly higher parasite burdens. Surprisingly, these parasite burdens were higher than those of mice infected with L. major and challenged with L. infantum. In vitro phagocytosis assays were carried out to measure parasite uptake in the presence of naive vs. anti-L. major serum. J774A.1 murine monocytes were cultured with either L. major or L. infantum in the presence of anti-L. major serum, naive serum, or no serum. Significantly higher rates of L. major uptake by J774A.1 cells occurred in the presence of anti-L. major serum, but no measurable increase of L. infantum phagocytosis was seen. Our results suggest that increased disease severity observed in vivo in mice previously exposed to L. major and challenged with L infantum is not a result of extrinsic ADE. We speculate that intrinsic ADE, due to biased memory T cell responses caused by Fcγ signaling, could account for disease exacerbation seen in the animal model.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The leishmaniases are a group of vector-borne diseases caused by protozoan parasites of the genus Leishmania. This genus is comprised of 53 species, 20 of which are known to infect humans (Akhoundi et al. 2016). It is estimated that there are up to 3 million new cases of leishmaniases each year leading to as many as 50,000 fatalities annually (Lozano et al. 2012; Mathers et al. 2007). The parasite is transmitted by biting phlebotomine sandflies which are found throughout the tropics and sub-tropics as well as in some temperate regions (Pigott et al. 2014). There are two dominant presentations of Leishmania infection, cutaneous leishmaniasis (CL) and visceral leishmaniasis (VL). CL is the most common form of infection and is characterized by open, weeping sores. Generally, CL will self-heal in weeks or months (Nadim et al. 1997; Markle and Makhoul 2004). VL causes a more diffuse infection that attacks the internal organs, particularly the spleen and liver. VL is characterized by fever, abdominal swelling, and a dark ashen coloration of the skin. This clinical form of leishmaniasis can be fatal when left untreated (Alvar et al. 2012).
Multiple studies have explored the potential for cross-protection between Leishmania species. One study showed that mice immunized with L. amazonensis have increased susceptibility to L. braziliensis (Silva et al. 2011). In this study, Silva and colleagues noted that disease exacerbation was associated with high titers of L. amazonensis antibody. These same researchers replicated and further elucidated these results in a subsequent publication (Silva et al. 2015). A number of studies have examined whether previous exposure to L. donovani or its constituent antigens can lend protective effects against L. major (Mitchell and Handman 1987; Rachamim and Jaffe 1993; Gicheru et al. 1997). These authors all noted heterologous protection against L. major. Other studies have endeavored to find potential cross-protection conferred by L. donovani against L. mexicana, L. amazonensis, L. tropica, and L. chagasi (Dey et al. 2014; Nico et al. 2014; Manson-Bahr 1961; Aguilar-Be et al. 2005). While cross-reactive immune responses are notable, it could be argued they are not reflective of real-world conditions. Parasites known to cause CL are more geographically widespread than their VL-inducing relatives, and CL is considerably more prevalent on a global scale (Pigott et al. 2014; Alvar et al. 2012). This suggests that individuals in endemic areas are more likely to have been exposed to/recovered from CL than VL. Furthermore, because CL is clinically mild whereas VL is virulent, cross protection generated by CL with heterologous leishmanial exposures could be advantageous from a public health perspective
Several inquiries have examined exposure to L. major as a foundation to study immunological cross-reactivity toward VL. However, the results are ambiguous. One publication examining the effects of heterologous immunization used L. major as an inoculum to confer future protection against L. chagasi (infantum) in susceptible BALB/c mice. The authors found no effect and observed similar parasite burden between naïve animals and those that had been primed with L. major (Streit et al. 2001). Another study also used an initial inoculation of L. major followed by challenge with L. infantum in the BALB/c mice. However, in this instance, the authors reported an increase in tissue parasite burden as well as a non-healing cytokine profile among the L. major-primed group when compared to the naive control group, indicating disease exacerbation (Nation et al. 2012). In a similar study but using Leishmania-resistant C57BL/6 mice, the authors noted lower L. infantum parasite burden in L. major recovered animals compared to the naïve control group, suggesting a protective effect (Romano et al. 2015). These contradictory findings speak to the complexity of the mechanisms at play and call for further investigation.
The findings of Nation et al. (2012) and those of Silva et al. (2015) suggest the possibility of antibody dependent enhancement (ADE), a potentiality in pathogens that utilize macrophages as a primary host cell. ADE is a phenomenon in which the binding of a pathogen to pre-existing antibodies enhances its entry into host cells, followed by its replication. ADE has been implicated in the pathogenesis of other diseases known to attack phagocytes, such as Dengue virus, yellow fever, Zika virus, and Chikungunya virus (Katzelnick et al. 2017; Gould and Buckley 1989; Bardina et al. 2017; Lum et al. 2018). Given Leishmania’s proclivity for macrophages, it is quite possible that the presence of cross-reactive antibodies from previous exposure could augment rates of infection.
Our present study investigates whether ADE plays a role in disease exacerbation of L. infantum infection after previous exposure to L. major as we reported earlier (Nation et al. 2012). Herein, we observed increased parasite burden in BALB/c mice infected with L. infantum after recovery from L. major infection or passive L. major-serum transfer. We further examined the role of L. major-recovered serum as it pertains to parasite uptake by macrophages in vitro. While increased parasite uptake was seen with L. major promastigotes, no corresponding increase was seen with L. infantum promastigotes. These results lead us to conclude that disease exacerbation is not caused by increased parasite uptake or opsonization alone. Taken as a whole, our current results exclude the possibility that extrinsic ADE leads to increased parasite entry of host cells. However, intrinsic ADE mediated by Fcγ signaling and associated IL-10 production may still be the cause of increased disease severity in L. major–L. infantum heterologous infections observed by our group and others.
Materials and methods
Animals
BALB/c mice were originally procured from Simonsen Laboratories (Gilroy, CA) and bred in the Central Washington University vivarium. They were housed under controlled light and temperature conditions with food and water provided ad libitum. All animal procedures were carried out in compliance with Central Washington University Institutional Animal Care and Use Committee (CWU-IACUC). The use of mice in our study was approved by CWU-IACUC under the protocol numbers A011704 and A101203.
Cells
J774A.1 murine macrophage line (ATCC TIB-67™) were used in phagocytosis assays and are known to be active in antibody dependent phagocytosis (Macura et al. 2007). Cells were cultured in high glucose DMEM with sodium pyruvate (GE Healthcare Bio-Sciences, USA) fortified with 6 mM L-glutamine and 10% heat-inactivated fetal calf serum or FCS (GeminiBio, USA). Cells were incubated at 37°C in a humidified atmosphere with 5% CO2.
Parasites
Leishmania parasites were cultured in Schneider’s complete insect medium (Sigma-Aldrich, USA) with 15% heat-inactivated fetal calf serum (FCS) and incubated at 24°C. Prior to culture, parasites were kept in liquid nitrogen and virulence was maintained by passage through BALB/c mice. Both L. major, strain MHOM/IL/79/LRC-L251 and L. infantum, MHOM/ES/92/LLM-320 were originally provided by Dr. Diane McMahon-Pratt (Yale School of Medicine, New Haven, CT).
Analysis of antibody responses by ELISA
Soluble Leishmania antigens (SLA) were generated from L. major or L. infantum promastigotes grown at 24°C, then collected by centrifugation at 912.5×g for 10 min at 4°C and washed 3× in phosphate-buffered saline (PBS). The suspension was then frozen overnight at −18°C to lyse cells. The solution was thawed and sonicated with a Microson Ultrasonic Cell disruptor XL (Misonix, Newtown, CT) at 11 W on ice for 3–15-s intervals. Protein concentration was determined using the Pierce BCA Protein Assay Kit (Thermo Fisher Scientific, Waltham, MA) with bovine serum albumin as the standard. SLA was adjusted to a concentration of 1 mg/ml and 100 μl per well was added to a 96-well plate and incubated overnight at 4°C. Plates were washed five times with PBS+0.05% Tween20 (PBS-T). Blocking buffer (PBS-T+1% dried non-fat milk) was added to each well (200 ul) and set for two hours at room temperature. The plates were again washed and 100 ul of serum samples were added to each well and allowed to set overnight at 4°C. Serum from three control animals and three infected animals were diluted 1:50 and 1:100 in blocking buffer and each dilution was assayed in duplicate. The plates were then washed and 100 μl secondary antibody solution was added. Goat anti-mouse IgG HRP (Bio-rad, Hercules, CA) diluted to 1:3000 in blocking buffer was added and incubated for two hours at room temperature. Plates were washed again and 100 μl HRP substrate solution (Thermo-Fisher, Rockfield, IL) was added. Absorbance at 405 nm was then measured using a BioTek plate reader (Winooski, VT). A two-tailed Student’s T test assuming unequal variances was used to compare serum affinity for L. major and L. infantum SLA.
In vivo experiments
Serum used for passive immunization was obtained from infected BALB/c mice that were given a sub-clinical, self-curing dose of 104 L. major metacyclic promastigotes in the left rear footpad. Blood serum was collected at 12 weeks post infection through cardiac puncture under isoflurane anesthesia. Control serum was obtained from age-matched animals. Serum was pooled within the control and L. major infected groups (Table 1).
There were three test groups consisting of 6 mice each. The first group, passively immunized with control serum, were treated with 200 μl fresh serum from uninfected age-matched mice via intraperitoneal (IP) injection. The second group, passively immunized with anti-L. major serum, were administered 200 μl fresh serum by IP injection. Serum donors and recipients were of the same cohort. The third group consisted of L. major recovered animals previously infected with 104 parasites 12 weeks prior to challenge. No serum was administered to the L. major recovered group. Following a 48-h incubation after passive immunization, all three groups were challenged with 107 L. infantum metacyclic promastigotes in 10 μl of PBS via intradermal injection to the right ear as previously described (Ahmed et al. 2003; Dondji et al. 2005). L. infantum-infected mice were euthanized four-weeks post infection. Draining lymph nodes (DLNs) and spleens were harvested. Our timeline of in vivo experiments is found on Table 1.
The parasite burden in infected mice was determined using a limiting dilution method as previously described (Titus et al. 1985; Soong et al. 1995; Ahmed et al. 2003; Dondji et al. 2008). Spleens and DLNs were homogenized under aseptic conditions, washed in PBS, and re-suspended in Schneider’s complete medium. The neat solution was diluted at 1:125 using Schneider’s complete. For the spleens, this first 1:125 dilution was then serially diluted three more times, yielding a final dilution of 1:15625. The DLNs were diluted four more times yielding a final dilution of 1:78125. Each dilution was plated 48 times on a 96-well plate. The plates were incubated at 24°C to allow promastigotes to emerge. Plates were examined under the microscope and the ratio of promastigote-positive and negative wells was recorded. Using these data, a mathematical curve was generated from which tissue parasite burden could be extrapolated as previously described (Dondji et al. 2005, 2008). The results are expressed with an averaged value ± standard errors of the parasite burdens for each group. An ANOVA test coupled with Tukey’s comparison was used to determine statistical significance.
Phagocytosis assays
Prior toin vitro experimentation, serum used to treat cells was produced and harvested as described below. Parasites were cultured until determined by visual inspection under the microscope that metacyclic promastigotes predominated the medium. Parasites were removed from the medium via centrifugation at 1000 rpm, 4°C for ten minutes. Metacyclic promastigotes were then isolated using a Percoll (Sigma-Aldrich, St. Louis, MO, USA) density gradient as previously described (Ahmed et al. 2003). Promastigotes were then suspended in sterile PBS to a concentration of 106 cells/ml. A 10 μl dose, delivering 104 promastigotes of L. major was injected into the left rear paw of BALB/c mice. The infection was allowed to progress for up to twelve months prior to serum collection. Control serum was collected from age/sex matched animals.
The J774A.1 cell line henceforth called J774 was used as a macrophage source. J774 cells and Leishmania were cultured, harvested and enumerated using a hemocytometer. Serum treatments were administered to Leishmania using 1μl serum to 1ml promastigote culture. Serum was added directly to culture medium and allowed to incubate for 30 min. Concentration of J774 macrophages was adjusted to 1.25 × 105/ml and reacted with Leishmania at a 1:10 ratio in culture. Reacting cells were incubated at 37°C in a humidified atmosphere with 5% CO2. Samples were collected in triplicate at 0, 12, 18, and 24 h. Once collected, samples were incrementally fixed to minimize clumping, first with 35% ethanol and then in 70% ethanol.
Prior to analysis, samples were re-suspended in PBS and adjusted to a concentration of 5 × 105 cells per milliliter. Cells were stained using propidium iodide (PI), 1 μg/ml. Flow cytometry was carried out using an S3 cell sorter (Bio-Rad, Hercules, CA). A multiparametric protocol was devised to quantify unbound parasites via forward scatter, side scatter, and PI fluorescence. Data on 3 × 104 events were collected from each sample. Data were then analyzed using FlowJo software (FlowJo, Ashland, OR). Statistical significance was determined using ANOVA test coupled with Tukey’s comparison.
Fluorescent microscopy
To demonstrate progressive infection of cells, fluorescent staining was used for photomicroscopy. Cells were harvested, enumerated, reacted, fixed, and stained as described for flow cytometry. In addition to PI being used to stain nuclei and kinetoplasts, membranes were counter stained using fluorescein isothiocyanate (FITC)-conjugated wheat germ agglutinin (WGA), 1 μg/ml (Vector Labs, Burlingame, CA, USA). Samples were incubated for 30 min in darkness and at room temperature. Photos were taken using a Leica DMRB microscope (Wetzlar, Germany) and Leica Application Suite software.
Results
Cross reactivity of L. major antibodies against L. infantum antigens was measured by ELISA. Wells were coated with either L. major SLA or L. infantum SLA. Serum from L. major exposed mice recognized both L. major and L. infantum antigen significantly above controls at both 50:1 and 100:1 serum dilution (Fig. 1). Control-unexposed mouse serum had no measurable binding, similar to no-serum controls. Serum from L. major-infected mice reacted strongly with L. infantum SLA despite never having been exposed to L. infantum. Results indicate substantial cross reactivity between the two parasites.
ELISA results measuring affinity of L. major antiserum for L. major and L. infantum soluble Leishmania antigen. Each bar represents the average of measurements in each experimental group. Serum dilutions of 1:50 and 1:100 were used. NS, no serum control; CS, control serum; Lm, L. major; Li, L. infantum. T test results for CS vs. Lm serum at any concentration and any Leishmania SLA was p < .0001. T test result for Lm SLA vs. Li SLA at 1:50 p < .01. T test result Lm SLA vs. Li SLA at 1:100 p < .001.
At four weeks post-challenge infection with L. infantum, mice were sacrificed (Table 1) and both the spleen and cervical LNs harvested for the evaluation of the total number of parasites in these tissues. The parasite burdens in the draining lymph nodes (Fig. 2) of L. major recovered mice had an average mean parasite burden of 1.08 ± 0.09 × 104, a significant difference over the naïve serum passive transfer group with the average mean at 0.21 ± 0.06 × 104 (p < 0.05). The group that underwent passive transfer of anti-L. major serum showed the highest parasite burden with a mean parasitemia of 1.83 ± 0.06 × 104. The parasite burden was significantly higher in the anti-L. major serum group relative to the L. major primed group in the draining lymph nodes (p < 0.05).
Parasite burden in the draining lymph node four weeks post L. infantum infection. Tissue parasite burden of mice (n = 6/treatment) challenged with 107 L. infantum after recovery from L. major infection (Lm recovered); passive transfer of naïve serum 48 h pre-infection (Naive serum); Passive transfer of L. major recovered serum 48 h pre-infection (Lm serum) ANOVA < .05 Tukey’s comparison: Lm recovered vs. Naive serum: p < .05, Lm recovered vs. Lm serum: p < .05, Naive serum vs. Lm serum: p < .001
The parasite burdens in the spleens four weeks post-challenge infection with L. infantum were evaluated (Fig. 3). L. major recovered mice had a higher average mean parasitemia at 1.13 ± 0.27 × 104 relative to the naïve serum passive transfer group (0.17 ± 0.04 × 104) (p < 0.05). The group receiving passive transfer of anti-L. major serum-while having higher parasitemia than the L. major-recovered group (0.85 ±0 .03 × 104), the value was not statistically greater in the spleens (p > 0.05).
Parasite burden in the spleen four weeks post L. infantum infection. Tissue parasite burden of mice (n = 6/treatment) challenged with 107 L. infantum after recovery from L. major infection (Lm recovered); passive transfer of naive serum 48 h pre-infection (Naive serum); Passive transfer of L. major antiserum 48 h pre-infection (Lm serum). ANOVA < .05 Tukey’s comparison: Lm recovered vs. Naïve serum: p < .05, Lm recovered vs. Lm serum: p > .05, Lm serum vs. Naïve serum: p < .001
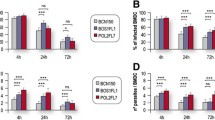
In vitro analysis using the BALB/c phagocytic cell line J774A.1 was carried out to determine whether L. major antiserum increased rates of parasite uptake. J774 cells were combined with L. major promastigotes in the presence of anti-L. major serum, control naive serum, or no serum. Samples were analyzed via flow cytometry at 0, 12, 18, and 24 h post co-culturing of macrophages and parasites. The percent of unbound/uninternalized parasites was measured using flow cytometry. Progression of infection and development of amastigotes was verified using fluorescent microscopy from 0 h to 24 h (Fig. 4). Fig 4a through d shows a visual change in size of the monocytes as they get infected with parasites. Although no measurements were made, it is obvious from these images that monocyte sizes changed from in 24 h after exposure to parasites. Figs. 5a–d are samples flow cytometry data that were used to plot Fig. 5e and f. For example, Fig. 5a is a scatter plot from a sample of J774 cells and L. major promastigotes incubated in the presence of control (non-immune) serum for 24 hours. The cells were run on the flow cytometer to count the number of events and analyzed for complexity and fluorescence (propidium iodide). FlowJoTM was used to set standard gates around the promastigotes and the J774 cells to give the percentage of events within each gate. These same gates were applied to all the samples. The gates provided the number of events i.e. number of J774 cells and promastigotes incubated for 24 h in the presence of control serum (Fig. 5a) or L. major serum (Fig. 5b). Figure panels C and D are histogram data showing the corresponding peaks from the gates in the scatter graph above, dark grey for the free promastigotes gate and light grey for the J774 gated cells. J774 cells that had phagocytosed promastigotes could not be distinguished from the uninfected cells, but the number of free promastigotes could be easily detected by flow cytometry and was used as the main parameter to assess phagocytosis in the presence of sera tested. The percentage of events corresponding to free parasites was significantly lower at 24 h in the presence of L. major serum with 18.73 ± 0.869, when compared to control serum or no serum at 30.43 ± 0.584 and 26.8 ± 0.493 respectively (Fig. 5e). This difference between percentages of free in the presence of L. major serum and other sera was statistically significant (No serum vs. L major serum: p < .01, Control serum vs. L major serum: p < .01)An identical experiment was carried out using L. infantum in the presence L. major antiserum to simulate heterologous re-exposure. Under these same conditions, no significant increased L. infantum uptake was observed in the presence of L. major antiserum with 50.73% ± 0.869 of events corresponding to unbound parasite versus 52.63% ± 0.584 with control serum and 46.43% ± 0.493 with no serum (Fig. 5f).
Progression of L. major infection of J774A.1 cells. Cells where harvested from select flow cytometry experimental timepoints. Photos depict- a. an uninfected cell at 0 hr, b. recently infected cell at 12 hr, c. heavily infected cells at 18 hr, d. and a heavily infected cells at 24 hr. Nuclei and kinetoplasts were stained red with propidium iodide. Membranes stained green with FITC-conjugated wheat germ agglutinin
Phagocytosis assays. a–d. Samples of raw data used to construct graphs shown in panels E and F. One of 3 scatter plots with gates for promastigotes and J774 monocytes for 24-hour control serum (a) and 24-hour L. major serum (b). Sample of histogram data showing peaks that correspond to free promastigotes and J774 from individual gates overlaying the ungated histogram for 24-hour control serum (c) and 24-hour L. major serum (d). Gates denote the percentage of events within the selected range and correspond to free parasites and macrophages. e–f. Graphs represent free/uninternalized L. major promastigotes at each timepoint with standard error bars. NS, no serum; CS, control serum; LmS, L. major serum. e. Free (non-phagocytosed) L. major in the presence of L. major serum, control serum, and no serum. * ANOVA: p = .0002, F = 51.68. Tukey’s comparison: NS vs. CS: p > .05, NS vs. LmS: p < .01, CS vs. LmS: p < .01 f. Free (non-phagocytosed) L. infantum promastigotes in the presence of L. major serum, control serum, and no serum. ANOVA: p = .1362, F = 2.831 CS, control serum; LmS, L. major serum
Discussion
Using ELISA, we demonstrated substantial cross reactivity of serum from L. major infected mice against L. infantum SLA (Fig. 1). This was to be expected as previous works have demonstrated not only significant cross reactivity between Leishmania species, but even between other species of Trypanosoma genus (Malchiodi et al. 1994; Vexenat et al. 1996; Vale et al. 2009). Having established the basic premise of the experiment, we examined if immune serum conferred any immune enhancement or exacerbation in vivo. Our results were consistent with those of Nation et al. (2012), in that significantly higher parasite burden was observed in the L. major-recovered group. Interestingly, even higher parasitemia was observed in the group passively transferred with L. major serum (Figs. 2 and 3). In the draining lymph nodes collected from the L. major-serum group, there was significantly higher parasitemia than the L. major recovered group (Fig. 3). This observation supports the idea that antibody dependent enhancement (ADE) may be playing a role.
ADE is known to occur by two distinct mechanisms, extrinsic vs. intrinsic. Extrinsic ADE is generally defined as increasing rates of infection through opsonization. Antibody binding of the pathogen leads to increased uptake by phagocytes through Fc mediated internalization. Intrinsic ADE is characterized by host cells becoming more receptive and less hostile toward potential attackers. It is often mediated by changes induced by cell signaling, especially increased IL-10 produced as a result of Fcγ-FcγR signaling (Katzelnick et al. 2017).
Leishmania infection has been shown to induce production of detrimental cytokines and exacerbate disease to establish persistent infections (Kima 2007). This immune subversion can, in part, be attributed to alterations in cytokine expression of macrophages as a result of Fcγ signaling. Anderson et al. (2002) demonstrated that the presence of antibody-opsonized antigen can induce secretion of IL-4 and IL-10 by macrophages while reducing IFN-γ and IL-12 production, a disease-healing cytokine profile. They further showed that the altered cytokine profiles could then push CD4 T-cells toward a non-healing Th2 phenotype. Studies specific to Leishmania have produced results that support these findings. One such study using L. major showed that IgG knockout mice were far more resistant to infection than their wild-type counterparts (Miles et al. 2005). In this same study, administration of exogenous IgG abrogated this resistance. Miles et al. (2005) also noted that IgG-induced susceptibility correlated with increased IL-10 expression and blockage of IL-10R restored resistance to infection. Similar effects have been documented by Buxbaum (2008) with infection by L. mexicana. It was noted that production of IL-10 was observed to correspond with production of IgG. Furthermore, opsonized amastigotes caused increased secretion of IL-10 and suppressed IL-12 production (Buxbaum 2008). This effect is not necessarily specific to IgG. Increased levels of IL-10 and susceptibility to L. infantum infection have also been associated with presence of IgM (Deak et al. 2010).
Previous research in our lab noted that mice which had been exposed to a low, self-healing dose of L. major, then challenged with L. infantum, developed higher parasitemia than control non-primed mice (Nation et al. 2012). When the cytokine profile was examined post infection there was a trend toward increased Th2 cytokines, however only IL-4 was significantly increased. There was no significant change in Th1 or Th17 cytokines. The lack of a robust cytokine shift leads us to ask if ADE might be playing a role in the disease exacerbation.
To further investigate if extrinsic ADE was inducing the increased parasitemia seen in vivo, we carried out phagocytosis assays to measure uptake of parasites by J774A.1 monocytes in vitro. While we were unable to definitively differentiate infected from uninfected monocytes, we could easily measure the number of unbound/uninternalized promastigotes using flow cytometry. Uptake of L. major promastigotes in the presence of L. major antiserum was enhanced, showing lower numbers of free parasites compared to those treated with naïve serum and no serum (Fig. 5A). This technique was repeated to measure the number of free L. infantum promastigotes exposed to J774 monocytes under the same conditions. There was no measurable decrease in the number of free L. infantum promastigotes exposed to L. major antiserum relative to controls (Fig. 5B). This experiment was repeated multiple times. L. major serum decreased the number of free L. major promastigotes, but not the number of free L. infantum promastigotes relative to control serum or no serum in each experiment. This result would suggest that the heterologous disease exacerbation observed in vivo cannot be attributed to extrinsic ADE (increased infection of phagocytes due to opsonization).
We were unable to demonstrate extrinsic ADE using J774 monocytes which are known to be competent in antibody dependent phagocytosis (Ralph and Nakoinz 1975. The heterologous disease exacerbation observed in this study as well as that of Nation et al. (2012) has two possible explanations). The first would be intrinsic ADE. In short, the circulating antibody opsonized the parasites, attracting macrophages which bound the antibody, inducing IL-10 production through Fcγ signaling (Anderson et al. 2002). IL-10 potentially in turn down-regulated the microbicidal response of macrophages allowing for easier entry and more hospitable conditions for L. infantum (Kane and Mosser 2001). A second possibility would be a lack of concomitant antigen-specific CD4 T cells to reinforce effector cytokine functions.
The assumption that intrinsic ADE is at play fails to address the findings of Romano et al. (2015), who observed protective effects against L. infantum after immunization with live L. major. These authors opted to use the C57BL6 model, known for its predisposition for the Th1 adaptive response and relative resistance to Old World CL (Scott et al. 1988; Scott 1989; Heinzel et al. 1991). Knowing this, it may come as no surprise that C57BL6s are able to quell leishmaniasis, given their propensity toward the typically protective cytokines IFN-γ and TNFα (Heinzel et al. 1989; Wilhelm et al. 2001). However, in consideration of our current results and the observations of others, we hypothesize that biased memory T cell responses can account for these seemingly conflicting observations. It has been shown that macrophages are capable of activating CD4 cells and influencing whether they will be directed toward the Th1 or Th2 phenotype (Anderson et al. 2002). Resistant and vulnerable mouse strains have correspondingly biased macrophages (Buchmüller-Rouiller and Mauël 1986). These biased macrophages are classified as M1 and M2 and have cytokine profiles analogous to those of Th1 and Th2 CD4 cells respectively (Mills et al. 2000). Romano et al. (2017) demonstrated that in immune C57/BL6 mice secondary infection resulted in parasite killing by dermal monocytes in an IFN-γ dependent manner. The intra-phagosomal pathogen Leishmania must contend with the robust inflammatory response initiated by previous infection. Inflammatory monocytes are recruited to the site of infection, which reduced the parasite burden via nitric oxide and reactive oxygen species induced by skin-resident memory CD4+ T cells (Glennie et al. 2017). Mandell and Beverley (2017) have shown the importance of persistent parasites in protective immunity. The parasites reside in macrophages and DCs, the majority of which are iNOS+. Infected macrophages both maintain the infection and likely also provides a reservoir of antigens for immune stimulation and the maintenance of protective immunity. The concomitant immunity that is generated against the same species of Leishmania, may not be strong enough to provide cross protection in the BALB/c mouse. In addition, Anderson et al. (2002) have shown that CD4 cells will retain the bias of the macrophages which originally activated them. Furthermore, CD4 cells will react to repeated exposures by presented antigen in the same manner they did during first exposure, even in the absence of biasing conditions, and even when activated by different types of APC (Anderson et al. 2002). In light of this information, we postulate that the heavily biased adaptive responses of the BALB/c and C57BL6 models will give rise to correspondingly biased T cell populations after first exposure to L. major. These T cells will then retain their biases, whether protective or detrimental, and react in the same manner upon subsequent exposure to similar antigens. In short, the systems are biased to begin with, and these biases are only reinforced with subsequent exposures. Further inquiry using in vitro studies with CD4 involvement are needed to further elucidate these phenomena.
References
Aguilar-Be I, da Silva ZR, Paraguai de Souza E, Borja-Cabrera GP, Rosado-Vallado M, Mut-Martin M, Garcia-Miss M, Palatnik de Sousa CB, Dumonteil E (2005) Cross-protective efficacy of a prophylactic Leishmania donovani DNA vaccine against visceral and cutaneous murine leishmaniasis. Infect Immun 73:812–819. https://doi.org/10.1128/IAI.73.2.812-819.2005
Ahmed S, Colmenares M, Soong L, Goldsmith-Pestana K, Munstermann L, Molina R, McMahon-Pratt D (2003) Intradermal infection model for pathogenesis and vaccine studies of murine visceral leishmaniasis. Infect Immun 71:401–410. https://doi.org/10.1128/IAI.71.1.401-410.2003
Akhoundi M, Kuhls K, Cannet A, Votýpka J, Marty P, Delaunay P, Sereno D (2016) A historical overview of the classification, evolution, and dispersion of Leishmania parasites and sandflies. PLOS Neglect Trop D 10:e0004349
Alvar J, Vélez ID, Bern C, Herrero M, Desjeux P, Cano J, Jannin J, Boer M (2012) Leishmaniasis worldwide and global estimates of its incidence. PLoS One 7:e35671
Anderson CF, Gerber JS, Mosser DM (2002) Modulating macrophage function with IgG immune complexes. J Endotoxin Res 8:477–481. https://doi.org/10.1179/096805102125001118
Bardina SV, Bunduc P, Tripathi S, Duehr J, Frere JJ, Brown JA, Nachbagauer R, Foster GA, Krysztof D, Tortorella D, Stramer SL, García-Sastre A, Krammer F, Lim JK (2017) Enhancement of Zika virus pathogenesis by preexisting antiflavivirus immunity. Science 356:175–180. https://doi.org/10.1126/science.aal4365
Buchmüller-Rouiller Y, Mauël J (1986) Correlation between enhanced oxidative metabolism and leishmanicidal activity in activated macrophages from healer and nonhealer mouse strains. J Immunol 136:3884–3890
Buxbaum LU (2008) A detrimental role for IgG and FcgammaR in Leishmania mexicana infection. Immunol Res 42:197–209
Deak E, Jayakumar A, Cho KW, Goldsmith-Pestana K, Dondji B, Lambris JD, McMahon-Pratt D (2010) Murine visceral leishmaniasis: IgM and polyclonal B-cell activation lead to disease exacerbation. Eur J Immunol 40:1355–1368
Dey R, Natarajan G, Bhattacharya P, Cummings H, Dagur PK, Terrazas C, Selvapandiyan A, McCoy JP Jr, Duncan R, Satoskar AR, Nakhasi HL (2014) Characterization of cross-protection by genetically modified live-attenuated Leishmania donovani parasites against Leishmania mexicana. J Immunol 193:3513–3527. https://doi.org/10.4049/jimmunol.1303145
Dondji B, Pérez-Jimenez E, Goldsmith-Pestana K, Esteban M, McMahon-Pratt D (2005) Heterologous prime-boost vaccination with the LACK antigen protects against murine visceral leishmaniasis. Infect Immun 73:5286–5289
Dondji B, Deak E, Goldsmith-Pestana K, Perez-Jimenez E, Esteban M, Miyake S, Yamamura T, McMahon-Pratt D (2008) Intradermal NKT cell activation during DNA priming in heterologous prime-boost vaccination enhances T cell responses and protection against Leishmania. Eur J Immunol 38:706–719
Gicheru MM, Olobo JO, Anjili CO (1997) Heterologous protection by Leishmania donovani for Leishmania major infections in the vervet monkey model of the disease. Exp Parasitol 85:109–116. https://doi.org/10.1006/expr.1996.4117
Glennie ND, Volk SW, Scott P (2017) Skin-resident CD4+ T cells protect against Leishmania major by recruiting and activating inflammatory monocytes. PLoS Pathog 13(4):e1006349. https://doi.org/10.1371/journal.ppat.1006349
Gould EA, Buckley A (1989) Antibody-dependent enhancement of yellow fever and Japanese encephalitis virus neurovirulence. J Gen Virol 70:1605–1608
Heinzel FP, Sadick MD, Holaday BJ, Coffman RL, Locksley RM (1989) Reciprocal expression of interferon γ or interleukin 4 during the resolution or progression of murine leishmaniasis: evidence for expansion of distinct helper T cell subsets. J Exp Med 169:59–72
Heinzel FP, Sadick MD, Mutha SS, Locksley RM (1991) Production of interferon gamma, interleukin 2, interleukin 4, and interleukin 10 by CD4+ lymphocytes in vivo during healing and progressive murine leishmaniasis. P Natl Acad Sci USA 88:7011–7015
Kane MM, Mosser DM (2001) The role of IL-10 in promoting disease progression in leishmaniasis. J Immunol 166:1141–1147
Katzelnick LC, Gresh L, Halloran ME, Mercado JC, Kuan G, Gordon A, Harris E et al (2017) Antibody-dependent enhancement of severe dengue disease in humans. Science 358:929–932. https://doi.org/10.1126/science.aan6836
Kima PE (2007) The amastigote forms of Leishmania are experts at exploiting host cell processes to establish infection and persist. Int J Parasitol 37:1087–1096. https://doi.org/10.1016/j.ijpara.2007.04.007
Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, AlMazroa MA, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Barker-Collo S, Bartels DH, Bell ML, Benjamin EJ, Bennett D, Bhalla K, Bikbov B, Abdulhak AB, Birbeck G, Blyth F, Bolliger I, Boufous S, Bucello C, Burch M, Burney P, Carapetis J, Chen H, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahodwala N, de Leo D, Degenhardt L, Delossantos A, Denenberg J, Des Jarlais DC, Dharmaratne SD, Dorsey ER, Driscoll T, Duber H, Ebel B, Erwin PJ, Espindola P, Ezzati M, Feigin V, Flaxman AD, Forouzanfar MH, Fowkes FGR, Franklin R, Fransen M, Freeman MK, Gabriel SE, Gakidou E, Gaspari F, Gillum RF, Gonzalez-Medina D, Halasa YA, Haring D, Harrison JE, Havmoeller R, Hay RJ, Hoen B, Hotez PJ, Hoy D, Jacobsen KH, James SL, Jasrasaria R, Jayaraman S, Johns N, Karthikeyan G, Kassebaum N, Keren A, Khoo JP, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Lipnick M, Lipshultz SE, Ohno SL, Mabweijano J, MacIntyre MF, Mallinger L, March L, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGrath J, Memish ZA, Mensah GA, Merriman TR, Michaud C, Miller M, Miller TR, Mock C, Mocumbi AO, Mokdad AA, Moran A, Mulholland K, Nair MN, Naldi L, Narayan KMV, Nasseri K, Norman P, O'Donnell M, Omer SB, Ortblad K, Osborne R, Ozgediz D, Pahari B, Pandian JD, Rivero AP, Padilla RP, Perez-Ruiz F, Perico N, Phillips D, Pierce K, Pope CA III, Porrini E, Pourmalek F, Raju M, Ranganathan D, Rehm JT, Rein DB, Remuzzi G, Rivara FP, Roberts T, de León FR, Rosenfeld LC, Rushton L, Sacco RL, Salomon JA, Sampson U, Sanman E, Schwebel DC, Segui-Gomez M, Shepard DS, Singh D, Singleton J, Sliwa K, Smith E, Steer A, Taylor JA, Thomas B, Tleyjeh IM, Towbin JA, Truelsen T, Undurraga EA, Venketasubramanian N, Vijayakumar L, Vos T, Wagner GR, Wang M, Wang W, Watt K, Weinstock MA, Weintraub R, Wilkinson JD, Woolf AD, Wulf S, Yeh PH, Yip P, Zabetian A, Zheng ZJ, Lopez AD, Murray CJL (2012) Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380:2095–2128. https://doi.org/10.1016/S0140-6736(12)61728-0
Lum FM, Couderc T, Chia BS, Ong RY, Her Z, Chow A, Ng LF (2018) Antibody-mediated enhancement aggravates chikungunya virus infection and disease severity. Sci Rep-UK 8:1860. https://doi.org/10.1038/s41598-018-20305-4
Macura N, Zhang T, Casadevall A (2007) Dependence of macrophage phagocytic efficacy on antibody concentration. Infect Immun 75:1904–1915. https://doi.org/10.1128/IAI.01258-06
Malchiodi EL, Chiaramonte MG, Taranto NJ, Zwirner NW, Margni RA (1994) Cross-reactivity studies and differential serodiagnosis of human infections caused by Trypanosoma cruzi and Leishmania spp; use of immunoblotting and ELISA with a purified antigen (Ag163B6). Clin Exp Immunol 97:417–423
Mandell MA, Beverley SM (2017) Continual renewal and replication of persistent Leishmania major parasites in concomitantly immune hosts. PNAS 114(5):E801–E810. https://doi.org/10.1073/pnas.1619265114
Manson-Bahr PEC (1961) Immunity in Kala-Azar. T Roy Soc Trop Med H 55:550–555. https://doi.org/10.1016/0035-9203(61)90078-5
Markle WH, Makhoul K (2004) Cutaneous leishmaniasis recognition and treatment. Am Fam Physician 69:1455–1460
Mathers CD, Ezzati M, Lopez AD (2007) Measuring the burden of neglected tropical diseases: the global burden of disease framework. PLoS Neglect Trop D 1:e114. https://doi.org/10.1371/journal.pntd.0000114
Miles SA, Conrad SM, Alves RG, Jeronimo SM, Mosser DM (2005) A role for IgG immune complexes during infection with the intracellular pathogen Leishmania. The J Exp Med 201:747–754
Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM (2000) M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol 164:6166–6173
Mitchell GF, Handman E (1987) Heterologous protection in murine cutaneous leishmaniasis. Immunol Cell Biol 65:387–392. https://doi.org/10.1038/icb.1987.44
Nadim A, Javadian E, Mohebali M (1997) The experience of leishmanization in the Islamic Republic of Iran. E Mediterr Health J 3:284–289
Nation CS, Dondji B, Stryker GA (2012) Previous exposure to a low infectious dose of Leishmania major exacerbates infection with Leishmania infantum in the susceptible BALB/c mouse. Parasitol Res 111:1407–1415. https://doi.org/10.1007/s00436-012-2899-5
Nico D, Gomes DC, Alves-Silva MV, Freitas EO, Morrot A, Bahia D et al (2014) Cross-protective immunity to Leishmania amazonensis is mediated by CD4+ and CD8+ epitopes of Leishmania donovani nucleoside hydrolase terminal domains. Front Immunol 5. https://doi.org/10.3389/fimmu.2014.00189
Pigott DM, Bhatt S, Golding N, Duda KA, Battle KE, Brady OJ, Messina JP, Balard Y, Bastien P, Pratlong F, Brownstein JS, Freifeld CC, Mekaru SR, Gething PW, George DB, Myers MF, Reithinger R, Hay SI (2014) Global distribution maps of the Leishmaniases. ELife 3:e02851
Rachamim N, Jaffe CL (1993) Pure protein from Leishmania donovani protects mice against both cutaneous and visceral leishmaniasis. J Immunol 150:2322–2331
Ralph P, Nakoinz I (1975) Phagocytosis and cytolysis by a macrophage tumor and its cloned cell line. Nature 257(5525):393–394. https://doi.org/10.1038/257393a0
Romano A, Doria NA, Mendez J, Sacks DL, Peters NC (2015) Cutaneous infection with Leishmania major mediates heterologous protection against visceral infection with Leishmania infantum. J Immunol 195:3816–3827. https://doi.org/10.4049/jimmunol.1500752
Romano A, Carneiro MBH, Doria NA, Roma EH, Ribeiro-Gomes FL, Inbar E, Lee SH, Mendez J, Paun A, Sacks DL, Peters NC (2017) Divergent roles for Ly6C+CCR2+CX3CR1+ inflammatory monocytes during primary or secondary infection of the skin with the intra- phagosomal pathogen Leishmania major. PLoS Pathog 13(6):e1006479. https://doi.org/10.1371/journal.ppat.1006479
Scott P (1989) The role of TH1 and TH2 cells in experimental cutaneous leishmaniasis. Exp Parasitol 68:369–372. https://doi.org/10.1016/0014-4894(89)90120-3
Scott P, Natovitz P, Coffman RL, Pearce E, Sher A (1988) Immunoregulation of cutaneous leishmaniasis. T cell lines that transfer protective immunity or exacerbation belong to different T helper subsets and respond to distinct parasite antigens. J Exp Med 168:1675–1684
Silva VMG, Larangeira DF, Oliveira PRS, Sampaio RB, Suzart P (2011) Enhancement of experimental cutaneous leishmaniasis by Leishmania molecules is dependent on interleukin-4, serine protease/esterase activity, and parasite and host genetic backgrounds. Infect Immun 79:1236–1243. https://doi.org/10.1128/IAI.00309-10
Silva VMG, de Araújo CF, Navarro IC, Oliveira PRS, Pontes de Carvalho L (2015) Enhancement of experimental cutaneous leishmaniasis by Leishmania extract: identification of a disease-associated antibody specificity. BMC Res Notes 8:197. https://doi.org/10.1186/s13104-015-1158-0
Soong L, Duboise SM, Kima P, McMahon-Pratt D (1995) Leishmania pifanoi amastigote antigens protect mice against cutaneous leishmaniasis. Infect Immun 63:3559–3566
Streit JA, Recker TJ, Filho FG, Beverley SM, Wilson ME (2001) Protective immunity against the protozoan Leishmania chagasi is induced by subclinical cutaneous infection with virulent but not avirulent organisms. J Immunol 166:1921–1929
Titus RG, Marchand M, Boon T, Louis JA (1985) A limiting dilution assay for quantifying Leishmania major in tissues of infected mice. Parasite Immunol 7:545–555
Vale AM, Fujiwara RT, Neto AFDS, Miret JA, Alvarez DCC, Silva JCFD, Campos-Neto A, Reed S, Mayrink W, Nascimento E (2009) Identification of highly specific and cross-reactive antigens of Leishmania species by antibodies from Leishmania (Leishmania) chagasi naturally infected dogs. Zoonoses Public Hlth 56:41–48. https://doi.org/10.1111/j.1863-2378.2008.01183.x
Vexenat AC, Santana JM, Teixeira AR (1996) Cross-reactivity of antibodies in human infections by the kinetoplastid protozoa Trypanosoma cruzi, Leishmania chagasi and Leishmania (viannia) braziliensis. Rev I Med Trop 38:177–185
Wilhelm P, Ritter U, Labbow S, Donhauser N, Röllinghoff M, Bogdan C, Körner H (2001) Rapidly fatal Leishmaniasis in resistant C57BL/6 mice lacking TNF. J Immunol 166:4012–4019. https://doi.org/10.4049/jimmunol.166.6.4012
Acknowledgements
The authors would like to express their gratitude to Mark Young and Jonathon Betz for their technical assistance and support. We also wish to thank the Central Washington University College of the Sciences for partially funding this research. The work described here formed part of a thesis approved by Central Washington University for a Master of Sciences degree awarded to A. McNolty.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Section Editor: Sarah Hendrickx
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
McNolty, A., Anderson, H., Stryker, G.A. et al. Investigations on the effects of anti-Leishmania major serum on the progression of Leishmania infantum infection in vivo and in vitro – implications of heterologous exposure to Leishmania spp. Parasitol Res 120, 1771–1780 (2021). https://doi.org/10.1007/s00436-021-07130-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-021-07130-x