Abstract
Two digenean species, Infidum infidum Faria, 1910 (Dicrocoeliidae) and Travtrema stenocotyle Cohn, 1902 (Plagiorchiidae), were collected in the large pit viper Bothrops moojeni Hoge, 1966 from Reserva Particular do Patrimônio Natural Cisalpina, municipality of Brasilândia, Mato Grosso do Sul State, Brazil. In this study, we provide the first molecular characterisation using the 28S rDNA and phylogenetic position data of these two common digeneans from B. moojeni. The molecular framework revealed topologies with strongly supported clades using maximum likelihood and Bayesian inference methods, positioned I. infidum among Plagiorchiidae and not among Dicrocoeliidae as expected and T. stenocotyle (Plagiorchiidae) surprisingly grouped as a sister group to Allassogonoporidae, Microphallidae, Pleurogenidae, and Prosthogonimidae, not related to plagiorchids. Our molecular phylogenetic data showed that these species may not correspond to their assigned families and encourage future studies on the systematic of these understudied groups.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bothrops moojeni Hoge, 1966 (Viperidae) is a large pit viper known as “caiçaca”, with a wide distribution through Brazil, Paraguay, and Argentina (Almeida-Santos et al. 2017; Costa and Bérnils 2018). In Brazil, its distribution comprises the riparian forests and adjacent areas and wetlands in central and southeastern regions, over the Cerrado biome (Wallach et al. 2014; Almeida-Santos et al. 2017; Costa and Bérnils 2018; Uetz et al. 2020).
Some digenean species were previously reported infecting B. moojeni from South America, as follows: Infidum infidum Faria, 1910 (Dicrocoeliidae), Opisthogonimus artigasi Ruiz and Leão, 1942, Opisthogonimus fonsecai Ruiz and Leão, 1942, Opisthogonimus lecithonotus Lühe, 1900 (Opisthogonimidae), Renifer heterocoelium Travassos, 1921 (Reniferidae), Sticolecitha serpentis Prudhoe, 1945, Styphlodora condita Faria, 1911, and Travtrema stenocotyle Cohn, 1902 (Plagiorchiidae) (Fernandes and Kohn 2014).
Species of the genus Infidum Travassos, 1916 (Dicrocoeliidae) occur in the Neotropical realm and parasitize the gall bladder and bile duct of several species of reptiles (Travassos 1916; Lunaschi and Drago 2007, 2010; Martinez-Salazár et al. 2016). In South America, only three species were described for this genus: the type species I. infidum, Infidum luckeri McIntosh, 1939, and Infidum similis Travassos, 1916. Fourteen snakes species from Argentina, Bolivia, and Brazil were previously reported infected with these Infidum spp. In Brazil, the infection with I. infidum was detected in B. moojeni, Eunectes murinus Linnaeus, 1758 (Boidae), and Hydrodynastes gigas Duméril, Bibron and Duméril, 1854 (Colubridae). Additionally, this digenean was also found infecting the anuran Leptodactylus podicipinus Cope, 1862 (Leptodactylidae), however, as an accidental infection (Lunaschi and Drago 2007; Campião et al. 2009). The other congeneric species I. similis was found infecting Bothrops jararaca Wied, 1824 (Viperidae), Drymarchon corais Boie, 1827, Erythrolamprus miliaris Linnaeus, 1758, Erythrolamprus poecilogyrus Wied, 1824, and Mastigodryas bifossatus Raddi, 1820 (Colubridae) in Brazil (Fernandes and Kohn 2014).
Travtrema Pereira, 1929 (Plagiorchiidae) is also commonly found in reptiles and amphibians (Fernandes and Kohn 2014; Campião et al. 2014) and only T. stenocotyle occurs in these hosts in South America. The adult digenean was reported in the intestine of seventeen snake species from Argentina, Brazil, Paraguay, and Uruguay (Fernandes and Kohn 2014) and also in the anuran L. podicipinus in Brazil (Campião et al. 2009). Among the Brazilian snakes, T. stenocotyle was found in Bothrops neuwiedi Wagler in Spix, 1824, B. moojeni, Chironius fuscus Linnaeus, 1758, E. miliaris, E. poecilogyrus, M. bifossatus, Philodryas patagoniensis Girard, 1858, Thamnodynastes pallidus Linnaeus, 1758, Tomodon dorsatus Duméril, Bibron and Duméril, 1854, and Xenodon merremii Wagler in Spix, 1824 (Fernandes and Kohn 2014). Besides, metacercariae of this digenean were found infecting the pharynx, muscles, mesentery, and body cavity of Leptodactylus chaquensis Cei, 1950, Leptodactylus latinasus Jiménez de la Espada, 1875, Scinax nasicus Cope, 1862 (Hamann et al. 2006a, b; 2009, 2010), Pseudopaludicola boliviana Parker, 1927 (Duré et al. 2004), Elachistocleis bicolor Valenciennes in Guérin-Menéville, 1838, Odontophrynus americanus Duméril and Bibron, 1841, Physalaemus albonotatus Steindachner, 1864, and Physalaemus santafecinus Barrio, 1965 (Hamann et al. 2009), indicating that amphibians can act as intermediate hosts in the biological cycle of T. stenocotyle.
Integrative taxonomy was recently introduced as a comprehensive framework to delimit and describe taxa by integrating information from different types of data and methodologies (Pante et al. 2015). Few digenean parasites of Brazilian snakes were analysed at this viewpoint (Müller et al. 2018). In this study, we provide the first molecular characterisation using the 28S rDNA and phylogenetic position data of two common digeneans from B. moojeni in Brazil, which are I. infidum and T. stenocotyle.
Material and methods
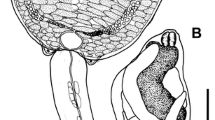
Three specimens of B. moojeni (Fig. 1) (one male, juvenile, snout-vent length (SLV) 49 cm and weight (W) 49.5 g; one female, adult, SLV 78 cm, W 140 g; and one female, juvenile, SVL 44 cm, W 37 g) were collected on February 2, 2018, at the Reserva Particular do Patrimônio Natural Cisalpina (21° 17′ 20.98″ S; 51° 55′ 2.76″ W), municipality of Brasilândia, Mato Grosso do Sul State, Brazil. During necropsy, we found digeneans in the gall bladder (I. infidum) (Fig. 2) and large intestine (T. stenocotyle) (Fig. 3). Infidum infidum was identified according to Faria (1910), Travassos (1916), and Travassos (1944) while T. stenocotyle identification followed Cohn (1902), Mane-Garzon and Gostari (1965), Pereira (1929), and Freitas and Dobbin Jr (1957). The parasites found were fixed in alcohol-formalin-acetic acid solution under the slight pressure of a coverslip for 10 min and transferred to 70% alcohol for further processing. Two specimens of each parasite were transferred to 96% ethanol for molecular study. Digenean specimens were stained with carmine, cleared with eugenol, analysed in a computerised system for image analysis (V3 Leica Application Suite, Leica Microsystems, Wetzlar, Germany) in a microscope with differential interface contrast for taxonomic identification. The digenean vouchers were deposited in the Helminthological Collection of the Instituto de Biociências, Universidade Estadual Paulista (UNESP), in the municipality of Botucatu, São Paulo State, Brazil, under #CHIBB 9038-90338 for I. infidum and #CHIBB 9039-9040 for T. stenocotyle. The snake hosts were deposited at the Herpetological Collection of the Universidade Regional do Cariri URCA-H, municipality of Crato, Ceará State, Brazil.
Specimen of Bothrops moojeni Hoge 1966 (Viperidae) from the Reserva Particular do Patrimônio Natural Cisalpina, municipality of Brasilândia, Mato Grosso do Sul State, Brazil
DNA was extracted using a DNeasy® Blood and Tissue Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions, with a final volume of 30 μl. PCR amplifications were performed in 25 μl reactions containing 5 μl of DNA extract with PureTaq™ Ready-to-Go PCR beads (GE Healthcare Life Sciences, UK). The primers and cycling conditions used to amplify and sequence the partial 28S ribosomal DNA (rDNA) (D1–D3) have been previously described (Mendoza-Palmero et al. 2015). Amplicons were visualised on GelRed (Biotium, Fremont, CA, USA) and purified using a QIAquick PCR Purification Kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s instructions. Sequencing reactions were performed directly on the purified PCR products using a BigDye v3.1 Terminator Cycle Sequencing Ready Reaction kit (Applied Biosystems, Foster City, CA, USA) and sequences were run on an ABI 3500 DNA genetic analyser (Applied Biosystems).
Contiguous sequences were assembled for phylogenetic analysis, in Sequencher™ 5.2.4 (Gene Codes, Ann Arbor, MI, USA). Sequences of partial 28S rDNA were aligned with representatives of the Plagiorchiida available in GenBank and the chosen outgroups were Gorgodera cygnoides (Zeder 1800) (Gorgoderidae) (AF1519382) and Nephrotrema truncatum (Leuckart, 1842) (Troglotrematidae) (AF151936) according to Tkach et al. (2000). Newly obtained sequences were aligned using MUSCLE (Edgar 2004) implemented in Geneious version 11.1.4 (Kearse et al. 2012). To evaluate the occurrence of substitution saturation, we estimated the Iss index in DAMBE 6 (Xia 2013). The best-fit model for nucleotide substitution in the resulting matrix was determined by the Akaike information criterion in jModelTest (Posada 2008). Phylogenetic analyses were performed using Bayesian inference and maximum likelihood (ML) using MrBayes and RaxML analysis was carried out using the computational resource CIPRES (Miller et al. 2010). The Bayesian analysis was run with the nucleotide substitution model GTR+I+G. To search with the Markov chain Monte Carlo method, chains were run with 10,000,000 generations, saving one tree every 1000 generations. On the burn-in, the first 25% of generations were discarded, and the consensus trees were estimated using the remaining trees. Bayesian posterior probabilities (BPP) cutoff was considered > 90%. The supports for ML were determined by performing 1000 bootstrap replicates. The trees were visualised in FigTree v1.3.1 (Rambaut 2009). Genetic divergence was calculated using the Kimura 2-parameter model in MEGA7.0.20 software (Kimura 1980; Tamura et al. 2013).
Results
Four partial sequences of the 28S rDNA gene (two from I. infidum 1268 bp accession number MW317230 and 1182 bp MW317227 and two from T. stenocotyle 1269 bp MW317229 and 1264 bp MW317228) were successfully sequenced and aligned with 34 digeneans belonging to Plagiorchiida. The final 28S rDNA alignment consisted of 1262 bp long. The Iss indicated no saturation in either transitions or transversions. Critical index of substitution saturation (Iss.c) values were greater than the Iss values. Genetic divergence among specimens of I. infidum presented no variation. Between I. infidum and I. similis, the divergence was 1%, and between I. infidum and Choledocystus hepaticus Lutz, 1928 (Plagiorchiidae), it was 2% (Supplementary Table 1). Both T. stenocotyle sequences were identical and varied from 11 to 16% among species from Plagiorchiida (Supplementary Table 1).
Bayesian and maximum likelihood (ML) inference methods resulted in identical topologies with supported values in the nodes in most clades (Fig. 4). The phylogenetic inference presented two main clades labeled A and B; main clade A represented members from Plagiorchioidea and main clade B, members from Microphalloidea (Fig. 4). Main clade A was strongly supported and divided into clades A1, A2, A3, and A4 (Fig. 4). Clade A1 was highly supported nodes and comprised species from Plagiorchiidae and Omphalometridae. Clade A2 not supported by bootstrap was represented by clades A3 and A4. Clade A3 (not supported) is formed by species from Plagiorchiidae, Brachycoeliidae, and Mesocoeliidae. Clade 4 (not supported) possesses the majority of species and is subdivided into clades A4.1 and A 4.2. Clade A4.1 is formed by species from Leptophaliidae and Macroderoididae and clade 4.2 is formed by members of the Glypthelminthidae, Haematoloechidae, Ochetosomatidae, Reniferidae, Telorchiidae, Plagiorchiidae, and Dicrocoeliidae (Fig. 4). Infidum infidum cluster as sister species to I. similis and closely related to C. hepaticus with high PP (posterior probability) and bootstrap support (Fig. 4).
Bayesian topology based on partial 28S ribosomal DNA sequences of Digenea. GenBank accession numbers are indicated next to species names. Numbers above nodes represent supported nodes by posterior probabilities for Bayesian analyses and bootstrap for maximum likelihood analyses respectively (posterior probabilities > 0.90 and bootstrap scores > 70). Branch length scale bar indicates number of substitutions per site. Coloured squares represent main clades
Clade B was composed of T. stenocotyle species closely related to species from Allassogonoporidae, Microphallidae, Pleurogenidae, Prosthogonimidae, and Plagiorchiidae, with high nodal support (clade B1) (Fig. 4).
Discussion
Infidum infidum and T. stenocotyle are digeneans commonly found in South American snakes, including some Brazilian species. Both species have their morphology well known since many studies had reported about them (Fernandes and Kohn 2014 and references therein). However, no molecular study was conducted with these parasite species.
According to morphological features, the genus Infidum belongs to the family Dicrocoeliidae, subfamily Leipertrematinae (Pojmańska 2008). However, Martinez-Salazár et al. (2016), in their phylogenetic studies using 28S rDNA and COI mtDNA, recovered specimens of I. similis clustering among plagiorchiids (closely related to Choledocystus hepaticus), which led those authors to infer that the phylogenetic placement of this species was not among dicrocoeliids. In our phylogenetic analyses, I. infidum was recovered as sister taxa of I. similis, as expected, and closely related to C. hepaticus, thus corroborating with the findings of Martinez-Salazár et al. (2016).
The taxonomic history of Infidum is controversial (Pojmańska 2008; Martinez-Salazár et al. 2016). Travassos (1944) included this genus in the subfamily Infidinae; later, Yamaguti (1958) considered this subfamily invalid and created 2 subfamilies inside Dicrocoeliidae (Leipertrematinae and Stromitrematinae) and placed this genus in the subfamily Leipertrematinae. The last classification and most recent consider the genus among the subfamily Leipertrematinae (Pojmańska 2008).
Molecular phylogenetic analyses provided a framework to discuss the interrelationships among trematodes and have defied classical morphological classification (Hernández-Mena et al. 2016). Clearly, the molecular findings do not agree with morphological classification and perhaps, as Martinez-Salazár et al. (2016) stated, Infidum spp. are not Dicrocoeliidae, instead a genus that belongs to Plagiorchiidae because of the close relationship to C. hepaticus. Despite that our study presents the phylogenetic position of the type species of the genus, I. infidum, we might not suggest any modification on the taxonomic classification of the genus by analysing just one marker (28S rDNA). The amplification of 28S rDNA gene is largely used in several taxa around the globe. Among trematodes is perhaps the most common marker used to infer phylogenies (Hernández-Mena et al. 2016; Martinez-Salazár et al. 2016; Müller et al. 2018) and exhibits a great resolution among different families. For example, Tkach et al. (2016) provided a broad diverse phylogeny using 28S rDNA with 80 species, representing 8 families and 40 genera of Echinostomatoidea, and they evaluated the morphological classification consistency with phylogeny based on molecular data.
However, we strongly recommend that, in future studies, more taxa and molecular markers should be added to the phylogeny of Infidum infidum, as well as more information on the life cycle and morphological characters, to accommodate these species in the non-monophyletic Plagiorchiidae or to assigned them to another taxonomic family. This is the first phylogenetic study of Travtrema using molecular data, and T. stenocotyle was first sequenced using 28S rDNA. Travtrema stenocotyle (Plagiorchiidae) was surprisingly grouped as a sister group to Allassogonoporidae, Microphallidae, Pleurogenidae, and Prosthogonimidae (Fig. 4) in the superfamily Microphalloidea (Tkach et al. 2000) and not within species from Plagiorchioidea as expected. According to Tkach et al. (2000), the suborder Plagiorchiata sensu stricto comprehends several clades, with two large clades composed with two superfamilies: Plagiorchioidea and Microphalloidea. Plagiorchioidea corresponds to the existing families: Brachycoeliidae, Telorchiidae, Plagiorchiidae, Haematoloechiidae, and Leptophaliidae (Tkach et al. 2000; Cribb et al. 2003).
Plagiorchiidae is the central family in the large superfamily Plagiorchioidea, and despite its systematic and practical importance, it is one of the least understood and understudied groups among Digenea (Ndiaye et al. 2013) and, therefore, there are still many questions and controversies related to the systematic position of several genera and their evolutionary interrelationships (Tkach et al. 2000; Tkach 2008; Ndiaye et al. 2013). The high level of homoplastic characters among Plagiorchiates, the extreme diversity of taxa placed in the paraphyletic Plagiorchiidae, and the lack of agreement of reliable diagnostic criteria might be some characteristics related to the confusion of plagiorchiids systematic (Tkach 2008; Zikmundova et al. 2014; Suleman et al. 2019). Poor morphological descriptions, unkown ranges of variability, and low host specificity might also have lead to systematics confusion (Zikmundova et al. 2014, Suleman et al. 2019). Several genera which once belonged to Plagiorchiidae have been placed in other families, e.g. Glyphthelminthidae and Omphalometridae (Tkach et al. 2001; Razo-Mendivil and Pérez-Ponce de Léon 2011), or have been waiting for the appropriate placement in another family, e.g. Haplometroides spp. (Müller et al. 2018). This might be the case of T. stenocotyle, in which more data (systematic, ecological, and evolutionary) and taxa are still necessary to accommodate this genus in a different family. The low support in the nodes recovered in some clades (Fig. 4) might correspond to the low number of available sequences; therefore, more taxa should be sequenced and added to improve these results in the future. In addition, Digenea systematics are controversial, which most of taxa are classified mainly on taxonomic schemes and phylogenetic inference based only on morphology, and some characters are homoplastic (Maldonado et al. 2001; Razo-Mendivil and Pérez-Ponce de Léon 2011; Müller et al. 2018).
Our study provides molecular phylogenetic position data of two trematodes species I. infidum, considered Dicrocoeliidae, and T. stenocotyle, considered Plagiorchiidae. Our results using molecular phylogenetic data using 28S rDNA showed that these species may not correspond to their assigned families and encourage future studies on the systematic of these understudied groups.
Data availability
Permission to collect and transport the snakes was given by the Brazilian Institute of Environment and Renewable Natural Resources (IBAMA) under registration 59230-1 (SISBIO).
References
Almeida-Santos SM, Barros VA, Rojas CA, Sueiro LR, Nomura RHC (2017) Reproductive biology of the Brazilian lancehead, Bothrops moojeni (Serpentes, Viperidae), from the State of São Paulo, Southeastern Brazil. South Am J Herpetol 12(2):174–181. https://doi.org/10.2994/SAJH-D-16-00047.1
Campião KM, Morais DH, Dias OT, Aguiar A, Toledo GM, Tavares LER, Silva RJ (2014) Checklist of helminth parasites of Amphibians from South America. Zootaxa 3843(1):001–093. https://doi.org/10.11646/zootaxa.3843.1.1
Campião KM, Silva RJ, Ferreira VL (2009) Helminth parasites of Leptodactylus podicipinus (Anura: Leptodactylidae) from south-eastern Pantanal, state of Mato Grosso do Sul, Brazil. J Helminthol 83:345–349. https://doi.org/10.1017/S0022149X09289358
Cohn L (1902) Zwei neue Distomen. Centralblatt für Bakteriologie 32(12):877–885
Costa HC, Bérnils RS (2018) Répteis do Brasil e suas Unidades Federativas: Lista de espécies. Herpetol Bras 7(1):11–57
Cribb TH, Bray RA, Olson PD, Littlewood DTJ (2003) Life cycle evolution in the Digenea: a new perspective from phylogeny. Adv Parasitol 54:197–254
Duré MI, Schaefer EF, Hamann MI, Kehr AI (2004) Consideraciones ecológicas sobre la dieta, la reprodución y el parasitismo de Psedopaludicola boliviana (Anura, Leptodactylidae) de Corrientes, Argentina. Phylomedusa 3(2):121–131
Edgar RC (2004) Muscle: a multiple sequence alignment method with reduced time and space complexity. BMC Bioinformatics 5(113). https://doi.org/10.1186/1471-2105-5-113
Faria G (1910) Contribuição para a sistemática helmintológica brasileira. II Dicrocoelium infidum n. sp. Parasito de vezicula biliar de Eunectes murina L. Mem do Inst Osw Cruz. 2(1):22–28
Fernandes BMM, Kohn A (2014) South American trematodes parasites of amphibians and reptiles Rio de Janeiro: Oficina de Livros, 228 p
Freitas JFT, Dobbin JE Jr (1957) Sobre Travtrema stenocotyle (Cohn, 1902). Bol do Museu Nac 170:1–25
Hamann MI, González CE, Kehr AI (2006a) Helminth community structure of the oven frog Leptodactylus latinasus (Anura, Leptodactylidae) from Corrientes, Argentina. Acta Parasitol 51(4):294–299. https://doi.org/10.2478/s11686-006-0045-1
Hamann MI, Kehr AI, González CE (2006b) Species affinity and infracommunity ordination of helminths of Leptodactylus chaquensis (Anura: Leptodactylidae) in two contrasting environments from Northeastern Argentina. J Parasitol 92(6):1171–1179. https://doi.org/10.1645/GE-862R1.1
Hamann MI, Kehr AI, González CE, Duré MI, Schaefer EF (2009) Parasite and reproductive features of Scinax nasicus (Anura: Hylidae) from a South American subtropical area. Interciencia 34(3):214–218
Hamann MI, Kehr AI, González CE (2010) Helminth community structure of Scinax nasicus (Anura: Hylidae) from South American subtropical area. Dis Aq Org 93:71–82. https://doi.org/10.3354/dao02276
Hernández-Mena DI, Mendoza-Garfias B, Ornelas-Garcia CP, Pérez-Ponde de León G (2016) Phylogenetic position of Magnivitellinum Kloss, 1966 and Perezitrema Barus & Moravec, 1967 (Trematoda: Plagiorchioidea: Macroderoididae) inferred from partial 28S rDNA sequences, with the establishment of Alloglossidiidae n. fam. Syst Parasitol 93:525–538. https://doi.org/10.1007/s11230-016-9645-9
Hoge AR (1966) Preliminary account on Neotropical Crotalinae (Serpentes: Viperidae). Mem do Inst Butantan 32:109–184
Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, Buxton S, Cooper A, Markowitz S, Duran C, Thierer T, Ashton B, Mentjies P, Drummond A (2012) Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28:1647–1649. https://doi.org/10.1093/bioinformatics/bts199
Kimura M (1980) A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. J Mol Evol 16:111–120
Lunaschi LI, Drago FB (2007) Checklist of digenean parasites of amphibians and reptiles from Argentina. Zootaxa 1476:51–68
Lunaschi LI, Drago FB (2010) Platyhelminthes, Trematoda, Digenea Carus, 1863: distribution extension in Argentina and new Anura and Ophidia hosts. CheckList 6:447–450
Maldonado JA, Loker ES, Morgan JAT, Rey L, Lanfredi RM (2001) Description of the adult worms of a new Brazilian isolate of Echinostoma paraensis (Platyhelminthes: Digenea) from its natural vertebrate host Nectomys squamipes by light and scanning electron microscopy and molecular analysis. Parasitol Res 87:840–848
Mane-Garzon F, Gostari AM (1965) Sobre alguns trematodos de ofídeos del Uruguay. Comunicaciones Zoologicas Museo Hist Nat Montevideo 8:1–21
Martinez-Salazár EA, Rosas-Valdez R, Gregory TR, Violante-González J (2016) Molecular phylogenetic analysis of Infidum similis, including morphological data and estimation of its genome size. J Parasitol 102(4):468–475. https://doi.org/10.1645/15-915
Mendoza-Palmero CA, Blasco-Costa I, Scholz T (2015) Molecular phylogeny of Neotropical monogeneans (Platyhelminthes: Monogenea) from catfishes (Siluriformes). Parasit Vectors 8:164. https://doi.org/10.1186/s13071-015-0767-8
Miller MA, Pfeiffer W, Schwartz T (2010) Creating the CIPRES Science Gateway for inference of large phylogenetic trees” in Proceedings of the Gateway Computing Environments Workshop (GCE), 14 nov. 2010, New Orleans, LA pp 1-8
Müller MI, Morais DH, Silva RJ (2018) Molecular phylogenetic postion of Haplometroides intercaecalis (Digenea, Plagiorchiidae). Acta Parasitol 63(6):522–526. https://doi.org/10.1515/ap-2018-0062
Ndiaye PI, Quilichini Y, Tkach VV, Greiman SE, Ba CT, Marchand B (2013) Ultrastructure of the spermatozoon of the digenean Plagiorchis elegans (Rudolphi, 1802) (Plagiorchioidea, Plagiorchiidae). J Morphol 274:965–972. https://doi.org/10.1002/jmor.20152
Pante E, Schoelinck C, Puillandre N (2015) From integrative taxonomy to species description: one step beyond. Syst Biol 64(1):152–160. https://doi.org/10.1093/sysbio/syu083
Pereira C (1929) Travtrema travtrema n.gen. e n. sp., trematoide parasite do intestine de cobra. Bol Biol 16:92–96
Pojmańska T (2008) Family Dicrocoeliide Loss, 1899. In: Keys to the Trematoda, Vol3 (eds) RA Bray, DI Gibson, A. Jones, CABI International, Wallingford, UK, The Natural History Museum, London p 233 – 260
Posada D (2008) jModelTest: phylogenetic model averaging. Mol Biol Evol 25:1253–1256. https://doi.org/10.1093/molbev/msn083
Rambaut A (2009) Molecular evolution, phylogenetics and epidemiology: Fig-Tree. Available at http//tree. bio.ed.ac.uk/software/figtree/ (accessed 10 Nov 2015)
Razo-Mendivil U, Pérez-Ponce de Léon G (2011) Testing the evolutionary and biogeographical history of Glyphtelmins (Digenea: Plagiorchiida) a parasite of anurans, through a simultaneous analysis of molecular and morphological data. Mol Phylogenet Evol 59(2):331–341. https://doi.org/10.1016/j.ympev.2011.02.018
Suleman JM, Khan MS, Tkach VV, Muhammad N, Zhang D, Zhu XQ (2019) Characterization of the complete mitochondrial genome of Plagiorchis maculosus (Digenea, Plagiorchiidae), representative of a taxonomically complex digenean family. Parasitol Int 71:99–105. https://doi.org/10.1016/j.parint.2019.04.001
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol 12:2725–2729
Tkach VV (2008) Family Plagiorchiidae Lühe, 1901. In: Bray RA, Gibson D, Jones A (eds) Keys to the Trematoda, vol 3. CAB International, Wallingford, pp 295–326
Tkach V, Pawlowski J, Mariaux J (2000) Phylogenetic analysis of the suborder plagiorchiata (Platyhelminthes, Digenea) based on partial lsrDNA sequences. Int J Parasitol 30(1):83–93. https://doi.org/10.1016/s0020-7519(99)00163-0
Tkach VV, Grabda-Kazuska B, Swiderski Z (2001) Systematic position and phylogenetic relationships of the family Omphalometridae (Digenea, Plagiorchiida) inferred from partial lsrDNA sequences. Int J Parasitol 31:81–85
Tkach VV, Kudlai O, Kostadinova A (2016) Molecular phylogeny and systematics of the Echinostomatoidea Looss, 1899 (Platyhelminthes: Digenea). Int J Parasitol 46(3):171–185. https://doi.org/10.1016/j.ijpara.2015.11.001
Travassos L (1916) Trematodos novos. Braz Medic 33:257–259
Travassos L (1944) Revisao da familia Dicrocoeliidae Odhner, 1910. Monog do Inst Osw Cruz 2:231–246
Uetz P, Freed P, Hošek J (2020) The Reptile Database. Accessible at http://www.reptile-database.org. Acessed: 27 Apr 2020
Wallach V, Williams KL, Boundy J (2014) Snakes of the world: a catalogue of living and extinct species. CRC Press, Boca Raton
Xia X (2013) DAMBE 5. A comprehensive software package for data analysis in molecular biology and evolution. Mol Biol Evol 30(7):1720–1728. https://doi.org/10.1093/molbev/mst064
Yamaguti S (1958) Systema Helminthum, the digenetic trematodes of vertebrates, Vol 1
Zikmundova J, Georgieva S, Faltynkova A, Soldanova M, Kostadinova A (2014) Species diversity of Plagiorchis Luhe, 1899 (Digenea: Plagiorchiidae) in lymnaeid snails from freshwater ecosystems in Central Europe revealed by molecules and morphology. Syst Parasitol 88:37–54. https://doi.org/10.1007/s11230-014-9481-8
Acknowledgements
We would like to acknowledge colleagues from the laboratory who helped us with fieldwork and Companhia Energética de São Paulo (CESP) for logistical support and authorization to work in Reserva Particular do Patrimônio Natural (RPPN) Cisalpina.
Funding
Financial support for this study was provided by Fundação de Amparo a Pesquisa do Estado de São Paulo (FAPESP), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). M.I.M. is supported by CAPES (grant number AUX-PE-PNPD #3005/2010), the Young Researcher Program (PROPE-UNESP #02/2016), and FAPESP: #2017/16546-3. L.P.U. is supported by FAPESP: #2018/00754-9. L.H.O is supported by FAPESP: #2018/09623-4. CNPq supported R.J.S. #309125/2017-0 and D.H.M. #313241/2018-0.
Author information
Authors and Affiliations
Contributions
Designed the study: MIM, RJS; collected data: EE, EPA, LPU, DHM, LHO, RJS; performed research: MIM, MBE, RJS; analyzed data: MIM, RJS; wrote the manuscript: MIM, RJS; revised and approved the final manuscript: MIM, EE, EPA, LPU, LHO, MBE, DHM, RJS
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Code availability
Not applicable
Additional information
Section Editor: Sven Klimpel
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
Table S1 Genetic divergence (p-distance, expressed in %) estimated for the 28SrDNA within species of Trematoda retrieved from GenBank and the species identified (in bold) in this study (XLSX 51 kb)
Rights and permissions
About this article
Cite this article
Müller, M.I., Emmerich, E., de Alcantara, E.P. et al. First molecular assessment of two digenean parasites of the lancehead snake Bothrops moojeni Hoge, 1966 (Serpentes, Viperidae) in Brazil. Parasitol Res 120, 971–977 (2021). https://doi.org/10.1007/s00436-020-07041-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-020-07041-3