Abstract
Leishmaniasis is one of the most neglected parasitic infections of the world and current therapeutic options show several limitations. In the search for more effective drugs, plant compounds represent a powerful natural source. Artemisinin is a sesquiterpene lactone extracted from Artemisia annua L. leaves, from which dihydroartemisinin (DQHS) and artesunic acid (AA)/artesunate are examples of active derivatives. These lactones have been applied successfully on malaria therapy for decades. Herein, we investigated the sensitivity of Leishmania braziliensis, one of the most prevalent Leishmania species that cause cutaneous manifestations in the New World, to artemisinin, DQHS, and AA. L. braziliensis promastigotes and the stage that is targeted for therapy, intracelular amastigotes, were more sensitive to DQHS, showing EC50 of 62.3 ± 1.8 and 8.9 ± 0.9 μM, respectively. Cytotoxicity assays showed that 50% of bone marrow-derived macrophages cultures were inhibited with 292.8 ± 3.8 μM of artemisinin, 236.2 ± 4.0 μM of DQHS, and 396.8 ± 6.7 μM of AA. The control of intracellular infection may not be essentially attributed to the production of nitric oxide. However, direct effects on mitochondrial bioenergetics and H2O2 production appear to be associated with the leishmanicidal effect of DQHS. Our data provide support for further studies of artemisinin and derivatives repositioning for experimental leishmaniasis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Leishmaniasis is among the top ten neglected tropical diseases with more than 12 million infected people and 0.9 to 1.6 million new cases each year. Also, 350 million people are at risk of infection worldwide (Alvar et al. 2012; WHO 2020a). Cutaneous leishmaniasis (CL) is a typical manifestation mostly caused by Leishmania amazonensis, L. mexicana, L. braziliensis, L. guyanensis, L. panamensis, and L. peruviana in the New World. Localized skin lesions that may disappear spontaneously characterize the main clinical form; however, a spectrum of cutaneous manifestations is recognized, such as the mucocutaneous form caused by L. braziliensis and L. guyanensis, a condition that affects the oral and/or nasal mucosa (Amato et al. 2007).
There are no ideal drugs to treat leishmaniasis, especially for complicated CL manifestations. Current therapy is mainly based on intramuscular injections of pentavalent antimonials for at least 20 days, which may cause serious systemic adverse effects. In addition, treatment with pentavalent antimonials presents variable efficacy (Oliveira et al. 2011; Alcântara et al. 2018). Second-line treatment options (amphotericin B deoxycholate, pentamidine mesylate/isethionate, and paromomycin) also share several drawbacks with antimonials, including long periods of treatment and serious side effects (Alcântara et al. 2018). Besides that, no vaccines for humans are available to prevent leishmaniasis.
In this scenario, the search for new antileishmanial drugs is a pressing need. One of the sources for novel leishmanicidal agents is based on bioactive compounds from medicinal plants (Brito et al. 2013). Artemisia annua L. is an Asian herb that has been used in tea form for centuries in Chinese medicine to treat malaria (Klayman 1985; Ansari et al. 2013). Artemisinin (ART) is a sesquiterpene lactone with very low toxicity and no mutagenic effects that is obtained from A. annua leaves. Several ART derivatives, such as dihydroartemisinin (DQHS) and artesunic acid/artesunate (AA), have been extensively studied in terms of antiplasmodial activity. Their synthesis occurs from sodium borohydride reduction of ART, generating DQHS as a reaction intermediate. ART and its derivatives contain an endoperoxide group, which is crucial for the generation of free radical intermediates that may kill malarial parasites via oxidative stress (Luo and Shen 1987; Balint 2001; Pandey and Ray 2016).
In fact, ART and a number of analogs/derivatives are antimalarial drugs that have been used in the clinical practice for several years, representing a substantial impact in reducing malaria deaths worldwide (WHO 2020b). Their well-documented safety profiles (Ansari et al. 2013) propelled us to investigate whether ART, DQHS, and/or AA could be effective against L. braziliensis, the most relevant species that causes localized/muco-CL in the New World. Moreover, experiments using the active metabolite DQHS were also conducted in order to investigate possible leishmanicidal mechanisms of action involving the control of the parasite’s mitochondrial bioenergetics and reactive oxygen species generation.
Material and methods
Artemisinin extraction and dihydroartemisinin and artesunic acid’s synthesis
Artemisinin (ART) was extracted from the leaves of Artemisia annua L., grown in the Experimental Field of CPQBA-UNICAMP, Paulínia, São Paulo, Brazil (22° 47′ 52.4″ S 47° 06′ 48.4″ E 590 m.; voucher #1246) and used as raw material for derivatives semi-synthesis reactions. Dihydroartemisinin (DQHS) and artesunic acid (AA) were synthesized and characterized by spectroscopic methods as previously described (Li et al. 1981; Brossi et al. 1988; Haynes et al. 2002; Kardono et al. 2014). Purity analysis for DQHS and AA were performed as previously described (Boaventura-Júnior 2015), and minimum purity values obtained were 81 and 95%, respectively.
Parasites
Leishmania (Viannia) braziliensis (MHOM/BR/75/2903) promastigotes were cultured at 25 °C in M-199 culture medium supplemented with 10% heat-inactivated fetal bovine serum, 100 mM adenine, 40 mM Hepes, pH 7.4, 0.0001% biotin, 0.0005% hemin, 2% human male-sterile urine, 50 mg/mL penicillin, and 50 mg/mL streptomycin (Kapler et al. 1990). All reagents were purchased from Sigma-Aldrich - Merck KgaA, Darmstadt, Germany; CultiLab, Campinas, SP, Brazil; EconoLab, Sao Paulo, Brazil; ThermoFisher Scientific, Waltham, MA, USA.
L. braziliensis promastigotes in vitro assays
The MTT assay was performed as previously described (Miguel et al. 2011). Briefly, 2.5–5 × 106 log-phase promastigotes were grown in culture medium in the presence or absence (control) of increasing concentrations of ART or its derivatives DQHS and AA. After 24 h, 30 μL of 5 mg/mL MTT (3-[4,5-dimethyl-2-thiazolyl]-2,5-diphenyl-2H-tetrazolium bromide; Sigma-Aldrich - Merck KgaA) was added to each well and the 96-well plate was incubated for an additional 3 h. The reaction was stopped by the addition of 50 μL 20% sodium dodecyl sulfate (Bio-Rad Laboratories, Inc., Hercules, CA, USA) to each well. Absorbance of formazan, the product of MTT reduction, was determined in a spectrophotometer AgileReader™ Elisa Plate Reader (AvansBio, Taipei City, Taiwan) with a reference and test wavelength of 650 and 600 nm, respectively. The viability percentages were calculated in relation to untreated control cells (100%). Amphotericin B (Sigma-Aldrich – Merck KgaA) was used as leishmanicidal control drug and prepared as previously described (Minori et al. 2020). Fifty percent effective concentrations (EC50) and 95% confidence intervals were determined from sigmoidal regression of the concentration-response curves using the GraphPad Prism 8.0 Software (San Diego, CA, USA).
Host cells and infection assays
Bone marrow-derived macrophages (BMDM) were obtained as previously described (Miguel et al. 2011). Briefly, bone marrow contents, from both femur and tibia of BALB/c mice, were flushed out with 5.0 mL of RPMI 1640 medium (Sigma-Aldrich - Merck KgaA) supplemented with 20% L929 fibroblasts culture supernatant, 20% fetal bovine serum, penicillin (100 U/mL), and streptomycin (100 μg/mL) (Cultilab, Campinas, SP, Brazil). BMDM were plated in Petri dishes for 7 days at 37 °C, 5% CO2. After 3 days, 5.0 mL of RPMI (20% L-929 and 20% FBS) was added to each plate. In total, 5 × 105 BMDM were incubated in the presence of different concentrations of ART and derivatives for 24 h. Cell viability was determined by the MTT assay, essentially as described above. Cytotoxic concentrations (CC50) were calculated as described for the anti-promastigote assay. Fifty percent effective concentrations were used to determine the selectivity index (SI = CC50/EC50). The protocol was approved by the Ethics Committee on Animal Use of the University of Campinas (CEUA/UNICAMP #4535-1/2017).
In parallel, BMDM samples were infected with L. braziliensis stationary phase promastigotes (MOI = 5) at 34 °C for 24 h, 5% CO2. Upon removing non-internalized promastigotes with warm PBS 1X, fresh culture medium RPMI was added followed by the addition or not (control) of different concentrations of DQHS. After 24 and 48 h, infection rates (%) and the number of amastigotes were determined by counting at least 300 methanol-fixed and stained (InstantProv, Pinhais, PR, Brazil) BMDM per coverslip in triplicates using Leica LAS Core microscope system (Leica, Wetzlar, Germany).
Nitrite determination in infected and non-infected BMDM supernatants
About 150 μL of culture supernatants of BMDM infection assays, in the presence of distinct concentrations of DQHS for 24 and 48 h, were collected for nitrite quantification following the manufacturer’s Griess reagent protocol (Griess Reagent kit, Invitrogen - ThermoFisher Scientific). Optical densities at 548 nm were determined using a Multiskan Sky Microplate reader (ThermoFisher Scientific). Additional groups of BMDM incubated with LPS (BMDM + LPS) at 10 ng/mL were included in the experiments.
Plasma membrane permeabilization
Ethidium bromide (EtBr) was used to assess plasma membrane permeabilization as previously described (Cohen et al. 1990; Mendes et al. 2019). In total, 5 × 107 L. braziliensis promastigotes were incubated with 0, 31.15 (EC50/2), and 62.30 μM (EC50) of DQHS. After 24 h, parasites were washed in PBS 1X (pH 7.2) and resuspended in this buffer in the presence of 100 μM EtBr. Digitonin (1.6 mM) was added in order to allow total cell permeabilization and maximal EtBr incorporation. Fluorescence was monitored at the excitation and emission wavelengths of 590 and 560 nm, respectively, using a Hitachi F2500 fluorescence spectrophotometer (Chiyoda, Tokyo, Japan). EtBr and digitonin were purchased from Sigma-Aldrich - Merck KgaA.
O2 consumption rates
O2 consumption rates were determined in a computer-interfaced Clark-type oxygen electrode with continuous stirring at 28 °C (Hansatech® Systems Inc., Norfolk, UK) as described (Peloso et al. 2011). Promastigotes (107/mL) were previously incubated in culture medium or with EC50/2 and EC50 DQHS for 24 h. After this period, viable parasites were counted and the same number of promastigotes for each condition was resuspended in a standard intracellular reaction medium (SRM) (125 mM sucrose, 65 mM KCl, 2 mM KH2PO4, 0.5 mM MgCl2, 10 mM HEPES pH 7.2, 1 mM EGTA, and 1 mg/mL BSA; Sigma-Aldrich - Merck KgaA) in the presence of 27.5 μM digitonin and 5 mM succinate. Respiratory control ratio (RCR) (state 3/state 4) was determined by the addition of 400 μM ADP (state 3) followed by 1 μg/mL oligomycin (state 4); Sigma-Aldrich - Merck KgaA).
Determination of H2O2 production
Approximately, 107 promastigotes/mL were incubated in culture medium alone or in the presence of EC50/2 and EC50 DQHS for 24 h. Next, cells were resuspended in PBS/1 mM MgCl2 in the presence of 5 mM succinate, 27.5 μM digitonin, 1 U/mL horseradish peroxidase (HRP); Sigma-Aldrich - Merck KgaA), and 25 μM Amplex Red (Molecular Probes®; Eugene, OR, USA). Fluorescence was monitored at the excitation and emission wavelengths of 563 nm and 587 nm, respectively, using a Hitachi F2500 fluorescence spectrophotometer. Correlation between fluorescence and concentration of H2O2 was determined through a calibration curve were known quantities of a freshly prepared H2O2 solution was added in the presence of Amplex Red and HRP (Peloso et al. 2011).
Superoxide production
Superoxide production was assayed using a mitochondrial-targeted probe, MitoSOX (3,8-phenanth-ridinediamine, 5-(60-triphenylphosphoniumhexyl)-5,6-dihydro-6-phenyl; Molecular Probes®) as described (Peloso et al. 2012). Briefly, 108 promastigotes/mL that was previously exposed to medium alone, EC50/2 or EC50 DQHS for 24 h were loaded with 5 μM MitoSOX in Krebs-Henseleit buffer (KH buffer: 15 mM NaCO3, 5 mM KCl, 120 mM NaCl, 0.7 mM Na2HPO4, 1.5 mM NaH2PO4, Sigma-Aldrich - Merck KgaA) at 28 °C for 10 min. Next, parasites were washed and resuspended in KH buffer. The detection of oxidized MitoSOX (oxMitoS-OX) was performed in this buffer in the presence of 27.5 μM digitonin and 5 mM succinate. The fluorescence was detected using a Hitachi F2500 fluorescence spectrophotometer with excitation and emission wavelengths of 510 and 580 nm, respectively.
Statistical analysis
Data represent the average ± standard errors (SE) or standard deviation (SD) of at least three independent experiments performed in duplicates or triplicates. Statistical analysis were performed using the appropriate test for each experiment with the GraphPad Prism 8.0 Software. A p value of less than 0.05 was considered statistically significant.
Results
Anti-promastigote effect of ART and derivatives
Aliquots of ART, DQHS, and AA were diluted in DMSO and used in 96-well plate assays for evaluation of logarithmic-phase promastigotes’ viability after 24 h. Leishmania braziliensis was more sensitive to DQHS (EC50: 62.3 ± 1.8 μM) than to ART and AA, as their EC50 values were approximately 4 to 4.5 times higher (Fig. 1). Cells that received amphotericin B were used as a positive control, showing low EC50 values (0.33 ± 0.13 μM). In parallel, cytotoxicity was determined for BALB/c bone marrow-derived macrophages (BMDM), in order that ideal concentrations could be defined for subsequent in vitro infection assays. Cytotoxic concentrations (CC50) ranged from 292.8 to 396.0 μM for ART and derivatives (Fig. 1). Selectivity indexes were calculated [CC50/EC50], for which DQHS showed the highest index among all compounds: ~ 4.7 versus 1.4 and 1.3 for ART and AA, respectively.
Dose-response curves of L. braziliensis promastigotes and BALB/c BMDM viability after ART and derivatives exposure. In total, 5 × 106 promastigotes (solid lines) and 5 × 105 BMDM (dashed lines) were incubated with ART (black triangle), DQHS (red square), and AA (blue circle) at increasing concentrations for 24 h at 25 °C and 37 °C, respectively. Cell viability was determined by the MTT method and percentages were calculated in relation to untreated control cells (100%). EC50 value ± standard error and (95% confidence interval) is shown for each compound. Three independent experiments were performed in triplicates
DQHS activity against intracelular amastigotes and nitrite quantification
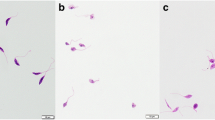
Despite observing moderate in vitro selectivity to primary macrophages, clinical safety data for ART and derivatives encouraged us to move forward with BMDM infection assays. Thereby, infections were performed in the presence of DQHS, the most effective ART metabolite at 0; 12.5; 25; 50; 100; and 200 μM for 24 and 48 h. Infection rates and numbers of intracellular amastigotes were significantly reduced in a dose-dependent manner for both time points (Fig. 2). Approximately 90 to 99% reduction of the infection rates were detected for 50, 100, and 200 μM DQHS after 24 h (Fig. 2a). Infection rates after 48 h were slightly higher, but still significantly lower in the presence of 50, 100, and 200 μM DQHS (Fig. 2b). In untreated control cells, amastigotes were observed (Fig. 2c–e), which were rarely seen for infections that received > 100 μM DQHS for 24 h and 200 μM DQHS for 48 h (Fig. 2c–f). EC50 of 8.9 ± 0.9 μM was calculated for intracellular amastigotes, about sevenfold lower than that calculated for promastigotes.
In vitro activity of DQHS against intracellular amastigotes of L. braziliensis. BALB/c BMDM were infected with stationary-phase L. braziliensis promastigotes (MOI = 5). After 24 h, cells were incubated with culture medium alone; 12.5; 25; 50; 100, and 200 μM DQHS for 24 (a) and 48 h (b). Cell monolayers were fixed with methanol and stained with Instant Prov for counting of infected cells every 300 cells per coverslip per condition. The numbers above each bar indicate the percentage of infection (a, b). (c, d): Number of amastigotes per 100 BMDM for 24 and 48 h, respectively. Results are representative of two independent experiments carried out in triplicates. (e) and (f): Examples of photomicrographs showing untreated infected BMDM, in which arrows point to intracellular amastigotes and infected cells incubated with 200 μM of DQHS for 48 h, respectively. Bar = 8 μm. Student’s t test was applied comparing each condition with the untreated control: *p < 0.05; **p < 0.005); ****p < 0.0001
Some studies demonstrated that nitric oxide (NO) production is inhibited by DQHS and AA in macrophages (Konkimalla et al. 2008; Yu et al. 2012), which probably indicates that the leishmanicidal effect observed in our in vitro infections is not due to NO generation. In this case, supernatants of infected- and uninfected-BMDM were collected for nitrite (a product of NO oxidation) dosage by Griess reagent protocol. Nitrite levels of infected BMDM exposed to concentrations lower than 200 μM DQHS were similar to those detected in untreated BMDM supernatants after 24 and 48 h. The only significant increase of nitrite was observed for DQHS incubation at 200 μM, for which levels of nitrite were comparable to BMDM previously stimulated with LPS (Fig. 3).
Nitrite quantification in supernatants of BMDM infected with L. braziliensis. BALB/c BMDM were infected with stationary-phase L. braziliensis promastigotes at MOI = 5. After 24 h, cells were incubated with culture medium alone (0); 12.5; 25; 50; 100; and 200 μM DQHS for 24 and 48 h. Nitrite levels (μM) were quantified in cultures’ supernatants for each condition using the Griess reaction protocol and are presented as mean ± standard deviation. “BMDM+LPS”: positive control for NO production. Student’s t test was applied comparing each condition with the untreated control and significant differences are indicated with asterisks
Investigation of plasma membrane integrity upon DQHS incubation
Based on our findings, the antileishmanial activity of DQHS could not be attributed solely to BMDM activation. In this sense, our next experiments focused on the investigation of the direct effects of DQHS on promastigotes. We assessed whether DQHS would be able to permeate the plasma membrane and interfere with its integrity in two sub-lethal doses (EC50/2 and EC50) (Fig. 4). Incubation with DQHS for 24 h did not trigger changes in the permeabilization of the parasites, since there was no change in the fluorescence signals related to the uptake of ethidium bromide in relation to untreated promastigotes. In this case, this ART metabolite is probably capable of being internalized without altering cell permeability at concentrations ≤ EC50, causing intracellular effects that could explain its leishmanicidal activity.
Plasma membrane permeability of L. braziliensis promastigotes incubated with DQHS. Approximately, 5 × 107 cells/mL were incubated with 0, 31.15 (EC50/2), and 62.3 μM (EC50) of DQHS for 24 h. Ethidium bromide (EtBr) at 100 μM was added to each group while fluorescence signal was acquired in a fluorescence spectrophotometer (Hitachi F-2500) for up to 600 s. A total of 1.6 mM digitonin was added after 550 s to obtain the maximum signal of fluorescence after cellular permeabilization. This is a representative assay of two independent experiments performed in duplicates
Interference of DQHS on mitochondrial bioenergetics of promastigotes
Next, we decided to evaluate whether DQHS could alter basal L. braziliensis functions, such as the mitochondrial bioenergetics of promastigotes. Parasite’s in situ mitochondrial O2 consumption was evaluated after incubation with EC50/2 and EC50 DQHS for 24 h. To stimulate the functioning of the mitochondrial electron transport chain and, consequently, measure the oxygen consumption rates of treated and untreated parasites, succinate was added in the experiments as an electron donor for complex II of the respiratory chain. Digitonin was also added to enable the permeabilization of the plasma membrane and allow the substrates to access the organelle. Figure 5a shows that promastigotes exposed to EC50/2 or EC50 for 24 h present an increase of > 83% on O2 consumption rates (p < 0.05). In order to determine the coupling between oxidative phosphorylation and the mitochondrial respiratory chain, the respiratory control rate (RCR) was determined by addition of ADP (state 3) followed by oligomycin, an inhibitor of FoF1-ATPase (state 4) (Fig. 5b). Exposure to DQHS at EC50 led to a decrease of ~ 30% on RCR (p < 0.05), as a result of an increase in state 4, indicating uncoupling of mitochondria, which leads to a decrease in ATP production. The increase in oxygen consumption rates observed is probably a result of an attempt of mitochondria to return to the resting potential in order to bring ATP production to its basal levels.
Leishmania braziliensis’ mitochondrial bioenergetics interference and ROS production caused by DQHS. (a, b) Oxygen consumption and respiratory control of L. braziliensis promastigotes incubated with 0, 31.15 (EC50/2), and 62.3 μM (EC50) of DQHS for 24 h, respectively. Oxygen consumption was determined as described in the “Material and Methods” section. Respiratory control (state 3/state 4) was measured in the presence of 400 μM ADP (state 3) and 1 μg/mL of oligomycin (state 4). (c) Detection of H2O2 production after incubation with DQHS. In total, 5 × 107 untreated and treated promastigotes were resuspended in PBS with 1 mM MgCl2 in the presence of 27.2 μM digitonin, 5 mM succinate, 1 U/mL HRP, and 25 μM Amplex Red. (d) Production of mitochondrial superoxide assessed with the MitoSox fluorescent probe. Data represent the mean ± standard deviation of two independent experiments in duplicates. Student’s t test was applied comparing each condition with the untreated control; *p < 0.05 and **p < 0.005
Many compounds exert their cytotoxicity by inducing the production of reactive oxygen species (ROS), with mitochondria being an important source of O2∙- and H2O2, important intracellular signaling components. Increase in H2O2 levels plays an important role in the redox balance and can lead to deleterious effects in biological molecules (Barreiros et al. 2006). To verify possible changes in the generation of these ROS induced by DQHS, promastigotes were incubated with EC50/2 and EC50 for later quantification of H2O2 and O2∙- (Fig. 5c, d). Promastigotes incubated with half EC50 showed an increased production of H2O2 in relation to untreated cells (~ 46%), with a higher increase when exposed to EC50 (136%), perhaps explaining the decrease in promastigotes viability incubated with DQHS. Regarding the levels of superoxide generated, no significant difference between the control group and EC50/2 and EC50 DQHS-incubated promastigotes was detected.
Discussion
Combinatory therapies containing ART and derivatives have been widely used worldwide to treat malaria patients (Ansari et al. 2013). The importance of ART extraction and purification is undeniable, leading the researcher Youyou Tu to receive part of the Nobel Prize in Physiology or Medicine in 2015 (Callaway and Cyranoski 2015). The widespread and well-established clinical use allowed us to expand studies on the activity of ART and some derivatives, such as DQHS (its main active metabolite) and AA against Leishmania, envisioning the potential for repositioning these sesquiterpene lactones. In fact, the use of repositioned drugs for leishmaniasis has been a reality for many decades (Alcântara et al. 2018; Charlton et al. 2018).
In the present study, we demonstrated that L. braziliensis promastigotes are sensitive to ART, DQHS, and AA in the micromolar range (Fig. 1), in agreement with studies carried out with ART and derivatives that showed EC50 values ranging from 104 and 160 μM for different species that cause the visceral form of the disease (L. donovani and L. infantum) (Sen et al. 2010; Mutiso et al. 2011; Islamuddin et al. 2014; Cortes et al. 2015; Sarkar et al. 2018) and varying from 83.7 and 177 μM for L. major, a causative species of CL in the Old World (Ebrahimisadr et al. 2013; Esavand Heydari et al. 2013). It is worth mentioning that a study using A. annua L. leaf extracts showed clinical improvement in experimental treatment for L. panamensis, a very related species to the one studied by our group (Mesa et al. 2017). As DQHS proved to be more effective in relation to ART and AA, our next step focused on the inhibition tests of clinically relevant stages; intracellular amastigotes. In fact, this metabolite was significantly more active against amastigotes (EC50 = 8.9 ± 0.9 μM) than promastigotes (EC50 = 62.3 ± 1.8 μM) (Figs. 1, 2). More important, despite host cells being relatively sensitive, the required amount of DQHS to inhibit 50% of their population was > 32-fold greater than the concentration required to eliminate the same proportion of L. braziliensis amastigotes.
After demonstrating the anti-amastigote effect of DQHS, we evaluated the role of nitric oxide (NO) as a possible effector in the control of in vitro infections, as it is a molecule of paramount importance for cellular defense by helping to eliminate invading pathogens (Das et al. 2010). There was a significant increase in NO production (Fig. 3) only for 200 μM incubation after 24 and 48 h. DQHS did not increase NO production by host cells at lower concentrations. It has been reported in the literature that macrophages incubated with DQHS show no increase in NO production, but the release of TNF-alpha and IL-6, which may result in downregulation of iNOS (Yu et al. 2012). Aldiere and others also showed that ART inhibits the synthesis of NO in human cells after 24 h (Aldiere et al. 2003).
Despite presenting known antiparasitic effects (Li 2012; Loo et al. 2017), the mechanism of action of ART and derivatives is only well established for Plasmodium, for which it is known that these lactones are activated by different mechanisms, may be explained by the reductive scission and/or open peroxide models. The result will lead to the cleavage of the endoperoxide bridge, a conserved structure in these molecules—including DQHS—that generates free radical intermediates responsible for causing oxidative stress. This cascade of events impairs several biological functions, such as inhibition of parasite’s essential proteins, heme alkylation, membrane damage, and covalent interaction with biomolecules (Greenwood et al. 2008; Pandey and Ray 2016).
Only a few studies have advanced in investigating the mechanisms of action of ART for Leishmania parasites (Sen et al. 2007; Sarkar et al. 2019), being scarcer in relation to its main active metabolite DQHS. Therefore, we decided to investigate possible mechanisms that explain its activity against L. braziliensis. Sun and Zhou demonstrated that both ART and DQHS are permeable to cell plasma membrane (Sun and Zhou 2016). Our results showed that promastigotes incubated with 31.15 (EC50/2) and 62.3 μM (EC50) of DQHS for 24 h do not show changes in the incorporation of EtBr, pointing out that membrane integrity is not affected under these conditions (Fig. 4). Interference on biochemical intracellular events was associated with ART’s effects, such as the induction of apoptosis-like cell death in L. donovani promastigotes (Sen et al. 2007). Sarkar and collaborators have shown that the endoperoxide-mediated radical formation by ART may represent a crucial step for triggering its anti-promastigote activity (Sarkar et al. 2019). From that and in a cascade of events, they observed an increase in ROS generation, depolarization of the mitochondrial membrane potential, increase in cytosolic calcium concentration, and decrease in ATP levels that led to apoptotic-like cell death (Sarkar et al. 2019). Recently, taking a step further on the mechanism of ART activation in Leishmania, Geroldinger, and coworkers used L. tarentolae promastigotes to show that activation of ART is preferentially triggered by degradation of hemin-derived products containing Fe2+, rather than low molecular iron in the labile iron pool. Although no homologs of mammalian heme oxygenase have been identified in the Leishmania genome so far, the hypothesis of the presence of a heme oxygenase-like system in these parasites has been raised (Geroldinger et al. 2020). Apart from this enzymatic mechanism, ART activation in Leishmania can also occur via interaction with hemin and various cellular reductants (Geroldinger et al. 2020).
In this context, we assessed the impairment of mitochondrial activity in L. braziliensis promastigotes exposed to DQHS. Only one mitochondrion is present per Leishmania, which provides ATP through coupling between the mitochondrial respiratory chain and oxidative phosphorylation. Digitonin was used to selectively permeabilize the plasma cell membrane to allow the study of mitochondrial functions in situ, i.e., within its own intracellular environment. Results presented in Fig. 5 led us to conclude that the increase in succinate-supported respiration in DQHS-treated cells indicates an attempt of mitochondrion to reestablish the membrane potential in order to restore ATP production to its normal levels. In this sense, respiratory control was shown to be compromised for parasites incubated with EC50 DQHS, due to a higher respiratory state 4, resulting from an uncoupling of the mitochondrial respiratory chain and oxidative phosphorylation and a probable depolarization of the mitochondrial inner membrane, which leads to a decrease in ATP production as previously demonstrated (Sarkar et al. 2019; Sun and Zhou 2016). Besides, an increase in H2O2 generation was also observed. These results are in accordance with the ones obtained for multiple myeloma cells where DQHS induced mitochondria-dependent apoptosis, which resulted in increased rates of oxygen consumption and ROS production, explaining cell death (Chen et al. 2020).
Finally, our study demonstrated that L. braziliensis promastigotes and amastigotes are sensitive to DQHS, being the intracellular forms more sensitive than promastigotes. In addition, DQHS promotes H2O2 production and mitochondrial dysfunction that may explain parasite’s loss of viability.
References
Alcântara LM, Ferreira TCS, Gadelha FR, Miguel DC (2018) Challenges in drug discovery targeting TriTryp diseases with an emphasis on leishmaniasis. Int J Parasitol Drugs Drug Resist 8(3):430–439. https://doi.org/10.1016/j.ijpddr.2018.09.006
Aldiere E, Atragene D, Bergandi L, Riganti C, Costamagna C, Bosia A, Ghugo D (2003) Artemisinin inhibits inducible nitric oxide synthase and nuclear fator NF-kB activation. FEBS Lett 552(2–3):141–144. https://doi.org/10.1016/S0014-5793(03)00905-0
Alvar J, Vélez ID, Bern C, Herrero M, Desjeux P, Cano J, Jannin J, Boer M, the WHO Leishmaniasis Control Team (2012) Leishmaniasis worldwide and global estimates of its incidence. PLoS One 7(5):e35671. https://doi.org/10.1371/journal.pone.0035671
Amato VS, Tuon FF, Siqueira AM, Nicodemo AC, Neto VA (2007) Treatment of mucosal leishmaniasis in Latin America: systematic review. Am J Trop Med Hyg 77:266–274. https://doi.org/10.4269/ajtmh.2007.77.266
Ansari MT, Saify ZS, Sultana N, Ahmad I, Saeed-Ul-Hassan S, Tariq I, Khanum M (2013) Malaria and artemisinin derivatives: an updated review. Mini Rev Med Chem 13(13):1879–1902. https://doi.org/10.2174/13895575113136660097
Balint GA (2001) Artemisinin and its derivatives: an important new class of antimalarial agents. Pharmacol Ther 90(2–3):261–265. https://doi.org/10.1016/S0163-7258(01)00140-1
Barreiros ALBS, David JM, David JP (2006) Estresse oxidativo: relação entre gerações de espécies reativas e defesa do organismo. Química Nova 29(1):113–123. https://doi.org/10.1590/S0100-40422006000100021
Brito AMG, Santos D, Rodrigues AS, Brito R, Xavier-Filho L (2013) Plants with anti-Leishmania activity: integrative review from 2000 to 2011. Pharmacogn Rev 7(13):34–41. https://doi.org/10.4103/0973-7847.112840
Brossi A, Venugolapan B, Gerpe LD, Yeh HJC, Flippen-Anderson JL, Buchs P, Luo XD, Milhous W, Peters W (1988) Arteether, a new antimalarial drug: synthesis and antimalarial properties. J Med Chem 31(3):645–650. https://doi.org/10.1021/jm00398a026
Callaway E, Cyranoski D (2015) Anti-parasite drugs sweep Nobel prize in medicine 2015. Nature. 526(7572):174–175. https://doi.org/10.1038/nature.2015.18507
Charlton R, Rossi-Bergmann DPW, Steel PG (2018) Repurposing as a strategy for the discovery of new anti-leishmanials: the state of the art. Parasitology. 145(2):219–236. https://doi.org/10.1017/S0031182017000993
Chen Y, Li R, Zhu Y, Zhong S, Qian J, Yang D, Jurczyszyn A, Beksac M, Gu C, Yang Y (2020) Dihydroartemisinin induces growth arrest and overcomes dexamethasone resistance in multiple myeloma. Front Oncol 15(10):767. https://doi.org/10.3389/fonc.2020.00767
Cohen BE, Benaim G, Ruiz M, Michelangeli F (1990) Increased calcium permeability is not responsible for the rapid lethal effects of amphotericin B on Leishmania sp. FEBS Lett 259(2):286–288. https://doi.org/10.1016/0014-5793(90)80028-H
Cortes S, Albuquerque A, Cabral LIL, Lopes L, Campino L, Cristiano MLS (2015) In vitro susceptibility of Leishmania infantum to artemisinin derivatives and selected trioxolanes. Antimicrob Agents Chemother 59(8):5032–5035. https://doi.org/10.1128/AAC.00298-15
Das P, Lahiri A, Lahiri A, Chakravortty D (2010) Modulation of the arginase pathway in the context of microbial pathogenesis: a metabolic enzyme moonlighting as an immune modulator. PLoS Pathog 6(6):e1000899. https://doi.org/10.1371/journal.ppat.1000899
Ebrahimisadr P, Ghaffarifar F, Mohammad Hassan Z (2013) In-vitro evaluation of antileishmanial activity and toxicity of artemether with focus on its apoptotic effect. Iran J Pharm Res 12(4):903–909. https://doi.org/10.22037/IJPR.2013.1406
Esavand Heydari F, Ghaffarifar F, Soflaei S, Dalimi A (2013) Comparison between in vitro effects of aqueous extract of Artemisia seiberi and artemisinin on Leishmania major. Jundishapur J Nat pharm prod. 8(2):70-5. https://doi.org/10.17795/jjnpp-9513
Geroldinger G, Tonner M, Quirgst J, Walter M, De Sarkar S, Machín L, Monzote L, Bein H, Stolze K, Duvigneau K, Staniek K, Chatterjee M, Gille L (2020) Activation of artemisinin and heme degradation in Leishmania tarentolae promastigotes: a possible link? Biochem Pharmacol 173:113737. https://doi.org/10.1016/j.bcp.2019.113737
Greenwood BM, Fidock DA, Kyle DE, Kappe SHI, Alonso PL, Collins FH, Duffy PE (2008) Malaria: progress, perils, and prospects for eradication. J Clin Invest 118(4):1266–1276. https://doi.org/10.1172/JCI33996
Haynes RK, Chan HW, Cheung MK, Lam WL, Soo MK, Tsang HW, Voerste A, Williams ID (2002) C-10 ester and ether derivatives of dihydroartemisinin – 10-α artesunate, preparation of authentic 10-β artesunate, and of other ester and ether derivatives bearing potential aromatic intercalating groups at C-10. Eur J Org Chem. (1):113–132. https://doi.org/10.1002/1099-0690(20021)2002
Islamuddin M, Chouhan G, Tyagi M, Abdin MZ, Sahal D, Afrin F (2014) Leishmanicidal activities of Artemisia annua leaf essential oil against visceral leishmaniasis. Front Microbiol 5:626. https://doi.org/10.3389/fmicb.2014.00626
Kapler GM, Coburn CM, Beverly SM (1990) Stable transfection of the human parasite Leishmania major delineates a 30-kilobase region suficiente for extrachromosomal replication and expression. Mol Cell Biol 10(3):1084–1094. https://doi.org/10.1128/MCB.10.3.1084
Kardono LBS, Wikara T, Tursiloadi H, Tursiloadi S (2014) Synthesis of dihydroartemisinin using Ni/TiO2 catalyst prepared by sol gel method. J Appl Pharm Sci 4(01):1–8. https://doi.org/10.7324/JAPS.2014.40101
Klayman DL (1985) Qinghaosu (artemisinin): an antimalarial drug from China. Science. 228(4703):1049–1055. https://doi.org/10.1126/science.3887571
Konkimalla VB, Blunder M, Korn B, Soomro A, Jansen H, Chang W, Posner G, Bauer R, Efferth T (2008) Effect of artemisinins and other endoperoxides on nitric oxide-related signaling pathway in RAW 264.7 mouse macrophage cells. Nitric Oxide-Biol Ch 19(2):184–191. https://doi.org/10.1016/j.niox.2008.04.008
Li Y (2012) Qinghaosu (artemisinin): chemistry and pharmacology. Acta Pharmacol Sin B. 33(9):1141-1146. 10.1038%2Faps.2012.104
Li Y, Yu PL, Chen YX, Li LQ, Gai YZ, Wang DS, Zheng YP (1981) Studies on analogs of artemisinin. I. The synthesis of ethers, carboxylic esters and carbonates of dihydroartemisinin. Acta Pharm Sin B 16(6):429–439
Loo CS, Lam NS, Yu D, Su XZ, Lu F (2017) Artemisinin and its derivatives in treating protozoan infections beyond malaria. Pharmacol Res 117:192–217. https://doi.org/10.1016/j.phrs.2016.11.012
Luo XD, Shen CC (1987) The chemistry, pharmacology, and clinical applications of qinghaosu (artemisinin) and its derivatives. Med Res Rev 7(1):29–52. https://doi.org/10.1002/med.2610070103
Mendes B, Almeida JR, Vale N, Gomes P, Gadelha FR, Silva SL, Miguel DC (2019) Potential use of 13-mer peptides based on phospholipase and oligoarginine as leishmanicidal agents. Comp Biochem Physiol C Toxicol Pharmacol 226:108612. https://doi.org/10.1016/j.cbpc.2019.108612
Mesa EL, Vasquez D, Lutgen P, Vélez ID, Restrepo AM, Ortiz I, Robledo SR (2017) In vitro and in vivo antileishmanial activity of Artemisia annua L leaf powder and its potential usefulness in the treatment of uncomplicated cutaneous leishmaniasis in humans Rev Soc Bras Med Trop 50(1). https://doi.org/10.1590/0037-8682-0457-2016
Miguel DC, Zauli-Nascimento RC, Yokoyama-Yasunaka JKU, Pereira LIA, Jerônimo SMB, Ribeiro-Dias F, Dorta ML, Uliana SRB (2011) Clinical isolates of New World Leishmania from cutaneous and visceral leishmaniasis patients are uniformly sensitive to tamoxifen. Int J Antimicrob Agents 38(1):93–94. https://doi.org/10.1016/j.ijantimicag.2011.03.012
Minori K, Rosa LB, Bonsignore R, Casini A, Miguel DC (2020) Comparing the antileishmanial activity of gold(I) and gold(III) compounds in L. amazonensis and L. braziliensis in vitro. ChemMedChem. DOI: https://doi.org/10.1002/cmdc.202000536
Mutiso JM, Macharia JG, Barasa M, Taracha E, Bourdichon AJ, Gicheru MM (2011) In vitro and in vivo antileishmanial efficacy of a combination therapy of diminazene and artesunate against Leishmania donovani in BALB/c mice. Rev Inst Med Trop Sao Paulo 53(3):129–132. https://doi.org/10.1590/S0036-46652011000300003
Oliveira LF, Schubach AO, Martins MM, Passos SL, Oliveira RV, Marzochi MC, Andrade CA (2011) Systematic review of the adverse effects of cutaneous leishmaniasis treatment in the New World. Acta Trop 118:87–96. https://doi.org/10.1016/j.actatropica.2011.02.007
Pandey N, Ray SP (2016) Updates on artemisinin: an insight to mode of actions and strategies for enhanced global production. Protoplasma. 253(1):15–30. https://doi.org/10.1007/s00709-015-0805-6
Peloso EF, Vitor SC, Ribeiro LHG, Piñeyro MD, Robello C, Gadelha FR (2011) Role of Trypanosoma cruzi peroxiredoxins in mitochondrial bioenergetics. J Bioenerg Biomembr 43:419–424. https://doi.org/10.1007/s10863-011-9365-4
Peloso EF, Gonçalves CC, Silva TM, Ribeiro LHG, Piñeyro MD, Robello C, Gadelha FR (2012) Tryparedoxin peroxidases and superoxide dismutases expression as well as ROS release are related to Trypanosoma cruzi epimastigotes growth phases. Arch Biochem Biophys 520:117–122. https://doi.org/10.1016/j.abb.2012.02.020
Sarkar SD, Sarkar D, Sarjar A, Dighal A, Chakrabarti S, Staniek K, Gille L, Cratterjee (2019) The leishmanicidal activity of artemisinin is mediated by cleavage of the endoperoxide bridge and mitochondrial dysfunction. Parasitology 146(4):511–520. https://doi.org/10.1017/S003118201800183X
Sen R, Bandyopadhyay S, Dutta A, Mandal G, Ganguly S, Saha P, Chatterjee M (2007) Artemisinin triggers induction of cell-cycle arrest and apoptosis in Leishmania Donovani promastigotes. J Med Microbiol 56(9):1213–1218. https://doi.org/10.1099/jmm.0.47364-0
Sen R, Ganguly S, Saha P, Chatterjee M (2010) Efficacy of artemisinin in experimental visceral leishmaniasis. Int J Antimicrob Agents 36(1):43–49. https://doi.org/10.1016/j.ijantimicag.2010.03.008
Sun C, Zhou B (2016) The molecular and cellular action properties of artemisinins: what has yeast told us? Microb Cell 14;3(5):196–205. https://doi.org/10.15698/mic2016.05.498
World Health Organization - WHO (2020a) Leishmaniasis (cutaneous, mucosal and visceral forms) https://www.who.int/ith/diseases/leishmaniasis/en/. Acessed 06 july 2020
World Health Organization - WHO (2020b) Malaria: retreat of a centuries-old scourge. https://www.who.int/publications/10-year-review/malaria/en/index6.html. Acessed 06 july 2020
Yu WY, Kan WJ, Yu PX, Li MM, Song JS, Zhao F (2012) Anti-inflammatory effect and mechanism of artemisinin and dihydroartemisinin. Zhongguo Zhongyao Zazhi 37(17):2618–2621
Funding
N.G. received a master scholarship from CAPES (Programa Demanda Social). D.C.M. is supported by a FAPESP Young Investigator Award (#2014/21129-4) and FAEPEX-Pesquisa, Pró-Reitoria de Pesquisa – UNICAMP (#519.292).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Handling Editor: Julia Walochnik
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Grazzia, N., Boaventura, S., Garcia, V.L. et al. Dihydroartemisinin, an active metabolite of artemisinin, interferes with Leishmania braziliensis mitochondrial bioenergetics and survival. Parasitol Res 120, 705–713 (2021). https://doi.org/10.1007/s00436-020-07019-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-020-07019-1