Abstract
Filarioid nematodes are parasites of the tissues and tissue spaces of all vertebrates except fish. Females produce microfilariae that enter the host’s blood circulation or skin and may cause ocular and neurological pathology, leading to important implications in veterinary and public health. The present work is the first investigation on Setaria labiatopapillosa conducted in Morocco to characterize the morphological features of both adult and microfilaria forms. Two adult female nematodes were found free in the thoracic cavity of a slaughtered 3.5-year-old (6 teeth) Moroccan enhanced cross-breed bull which was born and raised in Morocco. The worms were identified as S. labiatopapillosa by light microscope (LM) and scanning electron microscopy (SEM) on the basis of their characteristic features of the anterior and posterior parts of the worms. The two S. labiatopapillosa worms measured 90 mm and 105 mm in length and 0.55 and 0.64 mm in width, respectively. Microfilariae were detected in the fully developed eggs contained in the uterus of both nematodes. A detailed morphology of both the adult females and larvae of S. labiatopapillosa is described using LM and SEM. Although the origin of S. labiatopapillosa analyzed in the present study is unknown and there is currently no evidence that Setaria spp. have invaded Morocco, further surveillance is warranted to determine the incidence of setariasis, identify its vectors, and take appropriate measures to protect the livestock and cattle industry of the country.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Setaria spp. (Filarioidea, Nematoda) are vector-borne filarial nematodes transmitted through the bites of mosquitoes (Aedes, Anopheles, Culex, and Mansonia) and flies (Haematobia stimulans) (Azari-Hamidian et al. 2019). The mature adult roundworms measure between 5 and 10 cm and dwell primarily in the abdominal cavity of domesticated ungulates, such as cattle, camels, horses, goats, and sheep, as well as in wild mammals (Singh et al. 2014; Singla et al. 2014). Adult females produce larval infective forms, called microfilariae, that enter the blood stream of the infected host animal (i.e., microfilariosis). Many species of Setaria do not show any microfilarial periodicity, and only a few microfilariae are present in the blood. The microfilariae are ingested by insect vectors during blood meal, and the development of infective larvae (L3) occurs in the thoracic muscles of the vector (Shoho and Nair 1960). The infective stage microfilariae are transmitted through insect bites to other host animals and reach sexual maturity within 8–10 months, completing the life cycle (Nelson 1962).
Among Setaria worms, S. labiatopapillosa, S. digitata, S. cervi, S. marshalli, S. equina, and S. tundra have been reported worldwide. Several Setaria species infect cattle and buffaloes, including S. labiatopapillosa, S. marshalli, S. cervi, S. nelsoni, S. leichungwingi, S. equina, S. tundra, and S. digitata, the latter being the most common (Shoho 1958, 1976a; Becklund and Walker 1969; Kaur et al. 2015; Sundar and D’Souza 2015; Alborzi et al. 2020). Setaria digitata, S. marshalli, and S. labiatopapillosa are common nematodes found in cattle in Asia (Rhee et al. 1994; Tung et al. 2003; Nakano et al. 2007; Bazargani et al. 2008; Khedri et al. 2014). Setaria labiatopapillosa is a heteroxenous parasite of cattle with several species of mosquitoes belonging to the genus Culex and Anopheles acting as intermediate hosts. Adult worms in the peritoneal cavity are generally considered to be non-pathogenic in their natural host although they may cause a mild, clinically insignificant fibrinous peritonitis. The presence of microfilariae (L1) in the blood stream of bovine natural host leads to microfilariosis with little clinical consequence (Sigraskar et al. 1999). However, microfilariae may migrate into the eyes or to the central nervous system in unusual hosts, such as sheep, goats, horses, and humans, where ocular pathology, neurological disease, and even death may ensue (Rhee et al. 1994; Shin et al. 2002; Shin et al. 2017). Cerebrospinal nematodiasis may occur in epizootic proportions, leading to death in horses, sheep, and goats (Bazargani et al. 2008). The ubiquity and economic losses associated with Setaria spp. have rendered them among the most important parasites in unusual animal hosts. Rare occurrences of zoonotic transmission of S. equina (from horses and donkeys), S. labiatopapillosa, and S. digitata have also been reported, causing subconjunctival eye infection (Panaitescu et al. 1999; Ţălu et al. 2012; Nabie et al. 2017).
Setaria adult worms and their microfilariae occupy an important place in parasitological research as they are extensively used in diagnostic assays and are laboratory models used in drug studies of human filariasis due to their morphological, histological, and antigenic similarities (Murugananthan et al. 2010; Perumal et al. 2016). Investigations on S. labiatopapillosa have not been conducted in Morocco. The present study was carried out with the aim to identify and describe the morphological features of this nematode revealed by light microscopy (LM) and scanning electron microscopy (SEM).
Materials and methods
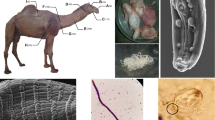
Female Setaria worms were found and collected from the thoracic cavity of a 3.5-year-old (6 teeth) cross-bred bull slaughtered at Sidi Yahia El Gharb slaughterhouse in May 2011 in Morocco and transported to the laboratory in 10% formalin. The bull was not imported. It was born and raised locally in a small town called Kceibya, located about 7 km from the city of Sidi Yahya El Gharb, northern Morocco. The worms were washed several times in physiological saline solution to remove host material and blood contamination. The anterior and posterior ends of the worms were cut and suspended in lactophenol solution for identification of the worm under light microscope. The dimensions of the worms, microfilariae, and eggs were measured using a Nikon Alphaphot YS microscope (Tokyo, Japan) equipped with an eyepiece micrometer, and the images were photographed using an Olympus μ700 digital camera (Olympus Corporation, Tokyo, Japan).
Unlike in the work of Ghahvei et al. (2020), which involved the preparation of the nematodes fixed with glutaraldehyde and osmium tetroxide, the adult worms were simply suspended in lactophenol solution before observation. The eggs and microfilariae were expelled spontaneously from the vulva of the female adult worms. They were suspended in lactophenol solution for further observation. Different parts of the nematodes and microfilariae were analyzed using a scanning electron microscope (SEM; model Quanta field emission gun [FEG] 200; ThermoFisher Scientific, Illkirch-Graffenstaden, France), at the National Center for Technical and Scientific Research (CNRST), Rabat, Morocco.
Results
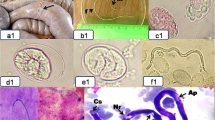
The milky white, threadlike nematodes with rounded anterior end and coiled posterior end collected alive from the thoracic cavity of a slaughtered bull were tentatively identified as S. labiatopapillosa female worms. The worms were 90 mm and 105 mm long and 0.55 mm and 0.64 mm wide, respectively (Fig. 1A). Light microscopy revealed clearly visible dorsal and ventral projections on the peribuccal crown from the lateral view. The distance between the dorsal and ventral projection was narrow (Fig. 1B). Symmetrical, posterior papillae were observed to be located approximately at the beginning of the glandular esophagus (Fig. 1C). The shape of the vagina was distinctive with a simple, straight vaginal canal. The ovejector comprised of a large, pyriform sphincter within which the genital lumen turns (Fig. 1D). The uteri of both worms were filled with numerous fully developed eggs and eggs in different developmental stages (Fig. 1E). The posterior end was characterized by a roughly furcated terminus (Fig. 1F).
Gross and light microscopy findings of adult female S. labiatopapillosa. (A) Two female adult worms of S. labiatopapillosa found in the thoracic cavity of a bull. (B) Light microscopy (LM) findings of the female; lateral view of the head region; lateral lips (circle); dorsal and ventral projections (arrow). (C) Ventral view; symmetrical papillae (arrow). (D) Vagina vera, lateral view; uterus (u), genital lumen (gl), ovijector (o), vaginal canal (vc). (E) Uterus full of eggs (u). (F) Latero-ventral view of posterior region; lateral appendage (arrow); terminal end (circle)
The eggs had an ovorectangular shape and contained embryonic mass (Fig. 2A). The embryonic mass slowly developed into a coiled larval form, i.e., microfilaria. Microfilaria inside the egg extended with round anterior and pointed posterior ends where the egg shell remained on the larvae forming a sheath around it (Fig. 2B–E). Microfilariae (220–260 μm × 5–6 μm) were detected in fully developed eggs and were observed under light microscope and SEM. The mean size of the eggs (n = 40) was 40 μm × 27 μm and ranged from 38 to 44 μm × 21 to 31 μm.
Light microscopy findings of the eggs and microfilariae in the eggs. (A) Ovorectangular-shaped eggs obtained from the uterus. (B) Microfilaria in the uterus. (C–D) Eggs from the uterus containing embryonic mass inside, Lugol’s iodine stain. (E) Microfilariae from the uterus, Lugol’s iodine stain. Scale bar: 20 μm
The SEM confirmed the characteristic feature of the peribuccal crown at the long, oval-shaped mouth opening in S. labiatopapillosa. The female did not have bifid lateral lips (Fig. 3A). Dorsal and ventral projections represented by two summits were found at the upper terminal end in both nematodes (Fig. 3A, B). A pair of lateral appendages was seen with the phasmididal pore situated at the upper armpit (Fig. 3C). Setaria labiatopapillosa had an obtusely ending tail with botryoidal tissue with many spiny projections, as shown by SEM (Fig. 3C). Very fine longitudinal striations were observed along the entire length of the worm (Fig. 3D) under the SEM, but not under light microscope. The irregularly arranged tubercles on the cuticle of posterior end (Fig. 3E) were also observed under the SEM. A peri-esophageal collar (necklace) was seen next to the front (Fig. 3F). Details of deirid and amphid of adult female S. labiatopapillosa are illustrated in Fig. 4A and B. The amphid showed a “rose-like” appearance. The observations of microfilariae under SEM showed sheathed microfilariae (L1) with a rounded anterior end and a pointed posterior end (Fig. 5A–C). Moreover, a succession of horizontal bands was seen along the entire length of microfilariae.
Scanning electron microscopy findings of adult female S. labiatopapillosa. (A) SEM photograph showing the oval mouth opening (M) flanked by lateral lips with non-furcated projection; dorsal and ventral projections (circle) of female S. labiatopapillosa. (B) A dorsal projection represented by two summits (s). (C) Photograph showing a pair of lateral appendages (arrow) and the phasmididal pore (f); an obtusely ending tail with a botryoidal with spiny projections (circle). (D) SEM photograph showing the very fine longitudinal striations on the cuticle of S. labiatopapillosa. (E) SEM photograph showing the irregularly arranged tubercles on the cuticle of posterior end of S. labiatopapillosa. (F) A collar (necklace) was seen next to the front (n)
SEM of microfilariae of S. labiatopapillosa. (A) The observations of microfilariae with SEM bearing sheathed (s) microfilariae (L1). (B) A round anterior end of microfilariae (a). (C) A pointed posterior end of microfilariae (p) and a succession of horizontal bands (hb) were seen along the entire length of microfilariae
Discussion
This is the first report on S. labiatopapillosa from Morocco. Setaria spp. have not been previously reported from neighboring countries bordering Morocco, i.e., Mauritania and Algeria. However, the presence of S. labiatopapillosa was reported from several sub-Saharan African countries, including Nigeria (Ogbogu et al. 1990), The Gambia (McFadzean 1955; Ndao et al. 1995), Burkina Faso (Gidel and Brengues 1972; Brengues and Gidel 1972), Senegal (Gueye et al. 1989), Chad (in a donkey) (Shoho 1976b), and Kenya (Nelson 1962). Another species, S. equina and its sub-species, seems to be more widespread in horses, donkeys, and zebras in sub-Saharan African countries, extending from west to east coast of the African continent, including south Africa (Shoho 1976b). At present, mosquito vectors that transmit Setaria spp. in Morocco are not known (Trari et al. 2003; El Joubari et al. 2014; Filali Mouatassem et al. 2019).
In our study, species other than S. labiatopapillosa were ruled out based on morphological features. Adult S. labiatopapillosa has the characteristic dorsal and ventral projections represented by two summits at the upper terminal end, whereas female S. digitata has lateral lips represented by one summit. Setaria labiatopapillosa is characterized by an obtusely ending tail with botryoidal tissue with many spiny projections. By contrast, the posterior end of S. digitata is a tapering terminus with a smooth knob. The female adult of S. labiatopapillosa does not have bifid lateral lips, and the distance between the dorsal and ventral projection is much smaller than in S. marshalli (Kim et al. 2010). In addition, amphid, deirid, postdeirid, and phasmidal pores are typically seen in S. labiatopapillosa (Shoho and Uni 1977).
In general, adults of Setaria spp. are found in the peritoneal cavity (Osipov 1972; Sundar and D’Souza 2015). However, immature Setaria worms or microfilariae may enter into blood circulation and may, in rare occasions, migrate erratically into the anterior chamber of the eye, abdominal cavity, pleural cavity, diaphragm, spinal cord, and brain where these worms or microfilariae can cause an inflammatory reaction (Rao 1941; Pelligrini et al. 1980; Shin et al. 2002; Tung et al. 2003; Nakano et al. 2007). Microfilariae of Setaria spp. and those of other filarial species, such as Deraiophoronema evansi (syn. Dipetalonema evansi), can also penetrate the placenta and migrate into the fetus, resulting in congenital infection (Fujii et al. 1995; Kim et al. 2010; Schuster et al. 2019). In this study, we found S. labiatopapillosa adult worms free in the thoracic cavity of a 3.5-year-old Moroccan bull. Other authors have found several worms embedded in the visceral walls of the pelvic peritoneum where inflammation occurred around the areas of worm attachment (Sundar and D’Souza 2015). Hemorrhagic inflammation was also observed in the peritoneal wall and reported in bulls. These findings are in accordance with earlier reports suggesting that these worms can also cause inflammatory reactions in their normal sites (Sarwar 1945; Chauhan and Pande 1980).
All morphological features of the anterior and posterior parts of S. labiatopapillosa encountered in the present study were in agreement with earlier description (Shoho and Uni 1977; Rhee et al. 1994; Nakano et al. 2007; Kim et al. 2010; Sundar and D’Souza 2015). Both nematodes were classified as fully developed female adults by using the criteria of the structure of the lateral lip, dorsal or ventral projection, tail, terminus, and embryonated eggs in the uterus. Most of the morphological findings of S. labiatopapillosa adult female worms and filarioids under SEM observed in our study confirmed earlier findings (Shoho and Uni 1977). Furthermore, our study on microfilariae (L1) of S. labiatopapillosa with SEM has shown a succession of horizontal bands limited by transverse striations along the entire length of microfilariae, similar to transverse annulation in microfilariae of Onchocerca jakutensis and O. fasciata (Bosch et al. 2016; Ghahvei et al. 2020).
Except for few “old” studies (Rao 1941; Sarwar 1946), cattle have been found to be less affected by S. labiatopapillosa in India. However, in a more recent Indian study, it was reported that close to 20% of the cattle were infected with this species (Sundar and D’Souza 2015). Reports from other countries indicated a higher infection rate: 75% in Australia (Green and Trueman 1971), 50% in France (Brengues and Gidel 1972), and 58.6% in Nigeria (Ogbogu et al. 1990). In the USA and Canada, S. labiatopapillosa was also reported to be the most common Setaria species in cattle (Becklund and Walker 1969). By contrast, only 2% (1 of 50) of the worms collected from the abdominal cavities of a cow in Japan were S. labiatopapillosa (Nakano et al. 2007). On the African continent, S. labiatopapillosa in cattle was reported from Senegal, The Gambia, Burkina Faso, Nigeria, Chad, and Kenya (McFadzean 1955; Nelson 1962; Brengues and Gidel 1972; Gidel and Brengues 1972; Shoho 1976b; Gueye et al. 1989; Ogbogu et al. 1990; Ndao et al. 1995).
In our study, S. labiatopapillosa was found in a bull that was born and raised in northern Morocco. Despite several visits to the slaughterhouse where the first case of S. labiatopapillosa was observed in Morocco in 2011, no other cow was found to be infected. One possibility that may explain this apparently isolated occurrence of setariosis in Morocco is transplacental infection, which was reported in some filarial species, including Setaria spp. (Fujii et al. 1995; Kim et al. 2010; Schuster et al. 2019). Microfilariae can penetrate the placenta, enter into the blood circulation of the fetus, and develop into adult worm. Adult S. marshalli parasites and microfilariae were detected in peritoneal cavities and in the blood of bovine fetuses, respectively, even though they were not detected in any of the mother cows (Fujii et al. 1995). Based on their observation that S. marshalli was not detected in cattle older than 2 years, these authors have hypothesized that while prenatal infection is common, post-natal infection of cattle occurs rarely.
At present, the origin of S. labiatopapillosa found in our study is unknown, and there is no evidence that Setaria spp. have invaded Morocco. However, further epizootiological surveillance and entomological studies are required to determine the incidence of setariasis in Moroccan cattle and aberrant animal hosts and identify the potential mosquito vectors in the country. The important implications of Setaria spp. for health of domesticated animals and their economic impact on the livestock and cattle industry should no longer be neglected.
References
Alborzi A, Haddadmolayan P, Tabandeh M, Ghorbanpoor M (2020) Ultrastructural and molecular characteristics of Setaria species based on sequence analysis of genomic and mitochondrial gene markers in cattle (Bos taurus) and buffaloes (Bubalus bubalis) from Iran. J Hellenic Vet Med Soc 70:1777–1788
Azari-Hamidian S, Norouzi B, Harbach RE (2019) A detailed review of the mosquitoes (Diptera: Culicidae) of Iran and their medical and veterinary importance. Acta Trop 194:106–122
Bazargani T, Eslami A, Gholami GR, Molai A, Ghafari-Charati J, Dawoodi J, Ashrafi J (2008) Cerebrospinal nematodiasis of cattle, sheep and goats in Iran. Iran J Parasitol 3:16–20
Becklund WW, Walker ML (1969) Taxonomy, hosts, and geographic distribution of the Setaria (Nematoda: Filarioidea) in the United States and Canada. J Parasitol 55:359–368
Bosch F, Manzanell R, Mathis A (2016) First description of Onchocerca jakutensis (Nematoda: Filarioidea) in red deer (Cervus elaphus) in Switzerland. Int J Parasitol Parasites Wildl 5:192–197
Brengues J, Gidel R (1972) Research on Setaria labiatopapillosa (Perroncito, 1882) in western Africa. II. Dynamics of this filariasis under natural conditions. Ann Parasitol Hum Comp 47:597–611
Chauhan PPS, Pande BP (1980) On the occurrence of Setaria labiatopapillosa in the intestinal lining of buffalo calf. Indian J Parasitol 4:89–91
El Joubari M, Louah A, Himmi O (2014) Mosquitoes (Diptera, Culicidae) of Smir marshes (northwest of Morocco): inventory and biotypology. Bull Soc Pathol Exot 107:48–59
Fujii T, Hayashi T, Ishimoto A, Takahashi S, Asano H, Kato T (1995) Prenatal infection with Setaria marshalli (Boulenger 1921) in cattle. Vet Parasitol 56:303–309
Filali Mouatassem T, Faraj C, Guemmouh R, Rais N, El Ouali LA (2019) Quantitative inventory of mosquito larvae (Diptera: Culicidae) and physicochemical analysis of aquatic habitats in the region of Fez, Morocco. Bull Soc Pathol Exot 112:105–113
Ghahvei Y, Mirzaei M, Hashemnia S, Golchin M, Kheirandish R, Uni S, Mendoza-Roldan JA, Otranto D, Sazmand A (2020) Scanning electron microscopy of Onchocerca fasciata (Filarioidea: Onchocercidae) adults, microfilariae and eggs with notes on histopathological findings in camels. Parasit Vectors 13:249
Gidel R, Brengues J (1972) Research on Setaria labiapapillosa (Perroncito, 1882) in Western Africa. III. Experimental infection of the normal host and of different abnormal hosts. Ann Parasitol 47:613–630
Green PE, Trueman KF (1971) The occurrence of Setaria labiatopapillosa in Queensland. Aust Vet J 47:624
Gueye A, Mbengue M, Diouf A (1989) Ticks and hemoparasitoses of livestock in Senegal. III. The Northern Sudan area. Rev Elev Med Vet Pays Trop 42:411–420
Kaur D, Ganai A, Parveen S, Borkataki S, Yadav A, Katoch R, Godara R (2015) Occurrence of Setaria digitata in a cow. J Parasit Dis 39:477–478
Khedri J, Radfar MH, Borji H, Azizzadeh M (2014) An epidemiological survey of Setaria in the abdominal cavities of Iranian sistani and brahman cattle in the southeastern of Iran. Iran J Parasitol 9:249–253
Kim NS, Kim HC, Sim C, Ji JR, Kim NS, Park BK (2010) Congenital infection with Setaria digitata and Setaria marshalli in the thoracic cavity of a Korean calf: a case report. Vet Med 6:275–280
McFadzean JA (1955) Setarial infections in The Gambia, British West Africa. Ann Trop Med Parasitol 49:417–418
Murugananthan A, Karunanayake EH, Tennekoon KH (2010) Cloning and characterisation of alkali myosin light chain gene (MLC-3) of cattle filarial parasite Setaria digitata. IIOAB J 1:1–10
Nabie R, Spotin A, Rouhani S (2017) Subconjunctival setariasis due to Setaria equina infection; a case report and a literature review. Parasitol Int 66:930–932
Nakano H, Tozuka M, Ikadai H, Ishida H, Goto R, Kudo N, Katayama Y, Muranaka M, Anzai T, Oyamada T (2007) Morphological survey of bovine Setaria in the abdominal cavities of cattle in Aomori and Kumamoto prefectures, Japan. J Vet Med Sci 69:413–415
Ndao M, Pandey VS, Zinsstag J, Pfister K (1995) Helminth parasites and hypobiosis of nematodes in N’Dama cattle during the dry season in The Gambia. Vet Parasitol 60:161–166
Nelson GS (1962) Observations on the development of Setaria labiatopapillosa using new techniques for infecting Aedes aegypti with this nematode. J Helminthol 36:281–296
Ogbogu VC, Bablis JM, Ajanusi OJ (1990) Prevalence of microfilariae in cattle at slaughter in Zaria, Nigeria. Vet Parasitol 36:171–175
Osipov AN (1972) Observations on the microfilarial numbers and on the life span of Setaria labiatopapillosa in cattle. Bylleten vsesoyuzwogo Gelmintologii im ki Stryabina 9:52–54
Panaitescu D, Preda A, Bain O, Vasile-Bugarin A. C (1999) Four cases of human filariosis due to Setaria labiatopapillosa found in Bucharest, Romania. Roum Arch Microbiol Immunol 58:203–207
Pelligrini N, Ramboli B, Poli A, Renzoni G, Gioli G (1980) Eosinophilic granuloma caused by adult Setaria labiatopapillosa in the bovine diaphragm. Anatamo-histopathological findings and pathogenic considerations. Annali Facolta di Medicina Veterinari di pisa 33:103–112
Perumal ANI, Gunawardene YINS, Dassanayake RS (2016) Setaria digitata in advancing our knowledge of human lymphatic filariasis. J Helminthol 90:129–138
Rao KSP (1941) Worm in the anterior chamber of the eye of a bullock. Indian Vet J 18:35
Rhee JK, Choi EY, Park BK, Jang BG (1994) Application of scanning electron microscopy in assessing the prevalence of some Setaria species in Korean cattle. Korean J Parasitol 32:1–6
Sarwar MM (1945) On the pathogenicity of Setaria cervi (Rud. 1819) in buffaloes. Curr Sci 14:107
Sarwar MM (1946) Two species of the nematode genus Setaria Vibrog. Indian Vet J 22:405–409
Schuster RK, Wibbelt G, Maio E, Wernery U, Sivakumar S (2019) Diaplacental infection of a bactrian camel (Camelus bactrianus) with the filarial worm Dipetalonema evansi: a case report. J Camel Pract Res 26:231–235
Shin J, Ahn KS, Suh GH, Kim HJ, Jeong HS, Kim BS, Choi E, Shin SS (2017) First blindness cases of horses infected with Setaria digitata (Nematoda: Filarioidea) in the Republic of Korea. Korean J Parasitol 55:667–671
Shin SS, Cho KO, Wee SH (2002) Ocular infection of cattle with Setaria digitata. J Vet Med Sci 64:7–10
Shoho C (1958) Studies on cerebrospinal nematodiasis in Ceylon. Ceylon Vet J 6:10–15
Shoho C (1976a) Setaria species from water buffalo of Southeast Asia and from Caffer Buffalo of Africa: comparative study with Setaria spp. from cattle of the respective areas. Ann Parasitol Hum Comp 51:577–588
Shoho C (1976b) On Setaria spp. (Nematoda, Filarioidea, Setariidae) from the peritoneal cavity of equine spp.: two new sub-species, Setaria equina theilerae from wild zebra of Africa, and Setaria equina dafaallai from horses and donkeys of southern Sahara area. Ann Parasitol Hum Comp 51:589–599
Shoho C, Nair VK (1960) Studies of cerebrospinal nematodiasis in Ceylon. VII. Experimental production of cerebrospinal nematodiasis by the inoculation of infective larvae of Setaria digitata into susceptible goats. Ceylon Vet J 8:1–11
Shoho C, Uni S (1977) Scanning electron microscopy (SEM) of some Setaria species (Filarioidea, Nematoda). Zeitschrift für Parasitenkunde 53:93–104
Sigraskar SU, Chouduri PC, Singhari NA (1999) Bubaline microfilariasis: epizootiological studies. Buffalo Bull 18:64–66
Singh ST, Malhotra P, Singla LD (2014) Fatal natural infection with microfilariae of Setaria species in a cattle bull. Prog Res 9:355–356
Singla LD, Moudgil AD, Sood NK, Deshmukh S, Turkar S, Uppal SK (2014) A unique case report on Setaria species microfilariosis in adult cattle in Punjab (India). Int Sci J 1:1–3
Sundar ST, D’Souza PE (2015) Morphological characterization of Setaria worms collected from cattle. J Parasit Dis 39:572–576
Ţălu S, Ştefănuţ A, Mihalca A, Coroiu Z (2012) Subconjunctival infestation with Setaria. Helminthologia 49:119–121
Trari B, Dakki M, Himmi O, El Agbani MA (2003) Mosquitoes (Diptera: Culicidae) of Morocco. Bibliographic review (1916–2001) and inventory of the species. Bull Soc Pathol Exot 96:329–334
Tung KC, Lai CH, Ooi HK, Yang CH, Wang JS (2003) Cerebrospinal setariosis with Setaria marshalli and Setaria digitata infection in cattle. J Vet Med Sci 65:977–983
Acknowledgments
The authors thank the farmers and breeders in Kénitra region (Morocco), the director of municipal slaughterhouse in Kénitra for collection of parasites, and Unité d’appui technique à la recherche scientifique of Centre National pour la Recherche Scientifique et Technique (CNRST, Rabat, Morocco) for technical support and use of scanning electronic microscope.
Author information
Authors and Affiliations
Contributions
This work is part of PhD thesis of RM under the guidance of his thesis director KEK. RM, KEK, and DB conceived and designed the experiments. RM performed the experiments. RM, MAL, KEK, and DB analyzed the data. RM, DB, and LB wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Section Editor: Abdul Jabbar
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mrifag, R., Lemrabott, M.A., El Kharrim, K. et al. Setaria labiatopapillosa (Filarioidea, Nematoda) in Moroccan cattle: atypical localization and morphological characterization of females and microfilariae by light and scanning electron microscopy. Parasitol Res 120, 911–918 (2021). https://doi.org/10.1007/s00436-020-06966-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-020-06966-z