Abstract
Alaria alata is known as a trematode with a complex life cycle. The trematode Alaria alata infects amphibians as second intermediate hosts. In the present study, we examined 390 amphibians—European water frogs Pelophylax esculentus complex (n = 335), common frogs Rana temporaria (n = 19), moor frogs Rana arvalis (n = 3), and common toads Bufo bufo (n = 30) collected from randomly selected wetland habitats in Latvia. Out of all examined specimens, 80 were tadpoles and 310 were adult amphibians. Mesocercariae of A. alata was detected in 108 specimens from all examined amphibian species, except the common toad, reaching the overall prevalence of 27.7%. Tadpoles were found to be more frequently infected with A. alata, when compared with adults, 58.8% and 22.4%, respectively. The results showed that mesocercariae accumulate in visceral membranes, different internal organs, and muscles in the head area. This is a comprehensive study to identify A. alata mesocercariae predilection sites in amphibians.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alaria alata is known as a common trematode in European canids. The adult trematode lives in the small intestine of definitive hosts. Some hosts, however, are able of harbor mesocercariae, including felids, mustelids, and procyonids (Szczęsna et al. 2008; Möhl et al. 2009; Castro et al. 2009; Tǎbǎran et al. 2013; Rentería-Solis et al. 2013; Takeuchi-Storm et al. 2015; Rodríguez-Ponce et al. 2016; Martinković et al. 2017; Ozoliņa et al. 2018; Ozoliņa et al. 2019). Scant information is available on the freshwater snail (1st intermediate host) and the amphibian (2nd intermediate host) roles in the A. alata life cycle (Shimalov and Shimalov 2000, 2001; Shimalov et al. 2000, 2001; Portier et al. 2012; Chikhlyaev and Ruchin 2014; Chikhlyaev et al. 2016; Patrelle et al. 2015; Voelkel et al. 2019; Huguenin et al. 2019). Therefore, more attention has been paid to paratenic hosts as potential reservoirs of alariosis. More frequently, wild boars were the object of study, and A. alata mesocercariae prevalence varied from 1.6 to 11.5% in Western Europe (Riehn et al. 2012; Paulsen et al. 2012, 2013; Berger and Paulsen 2014; Malešević et al. 2016). At the same time, significantly higher prevalence (44.3%) was observed in north-eastern Poland (Strokowska et al. 2020).
Amphibians are second intermediate hosts of the trematode. The mesocercariae can localize in different tissues of intermediate and paratenic hosts in many species of mammals and also, potentially, in humans (Skrjabin 1960; Borgsteede 1984; Shimalov and Shimalov 2000, 2001, 2003; Shimalov et al. 2000, 2001; Segovia et al. 2003; Craig and Craig 2005; Möhl et al. 2009). Different development stages of amphibians became infected when the A. alata cercariae actively penetrated their skin with a specific penetration apparatus after cercariae were released from the freshwater snail (1st intermediate host) and actively sought their next host in an aquatic environment (Galaktionov and Dobrovolskij 2003; Möhl et al. 2009; Portier et al. 2012). Cercariae that penetrate the 2nd intermediate host tissues develop to an additional mobile larval stage, mesocercariae, instead of developing into an encysted metacercaria which was typical in other trematodes (Pearson 1956; Möhl et al. 2009). Experimental studies with A. alata have not been done; however, North American Alaria species showed that mesocercariae developed in the second intermediate host tissues within 2 weeks (reviewed by Olsen 1974).
In Europe, several studies have shown the A. alata mesocercariae occurrence in different hosts. In particular, the prevalences of A. alata in amphibians and reptiles from Belarus were studied in the smooth newt Triturus vulgaris (10.7%), the northern crested newt Triturus cristatus (20.0%), the sand lizard Lacerta agilis (17.0%), the viviparous lizard Lacerta vivipara (9.1%), the grass snake Natrix natrix (21.2%), the smooth snake Coronella austriaca (20.0%), the adder Vipera berus (22.6%), the common toad Bufo bufo (8.0%), the natterjack Bufo calamita (36.4%), and the green toad Bufo viridis (67.9%) (Shimalov and Shimalov 2000, 2001; Shimalov et al. 2000, 2001). By gathering data over the past 30 years and supplemented with their own original study results, researchers found A. alata mesocercariae from Volga Basin, Russia in both the common brown frog, and the European common toad (Chikhlyaev and Ruchin 2014; Chikhlyaev et al. 2016). The previous studies in Europe had shown that A. alata mesocercariae might be located in different body parts of a frog, including its body cavity, internal organs, head, periorbital, hindlimb, and forelimb. The prevalence of A. alata mesocercariae ranges from 39% in the brown frog group (e.g., Rana dalmatina and R. temporaria) to 87% in the water frog group (Patrelle et al. 2015; Voelkel et al. 2019).
The aim of the present study was to estimate the prevalence of A. alata mesocercariae and to determine their predilection sites in amphibians in Latvia.
Material and method
During the period 2017–2019, amphibians were collected with a special permission that was granted by the Latvian authorities for the collecting and euthanizing of amphibians for scientific purposes (26/2017-E, 14/2018-E, 21/2019-E – Nature Conservation Agency of Latvia).
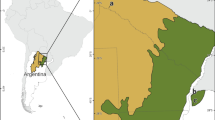
Overall, 390 specimens were collected in 86 different randomly selected wetlands (Fig. 1), permanently full of water. The geographical coordinates of the collection sites were converted to a map layer and plotted on a base map of Latvia (divided into planning regions) using a Geographic Information System (GIS) Program, the ArcGIS version 10 (ESRI 2011).
Adult frogs and tadpoles were gathered from shallow portions using a standard O-frame net with a diameter of 0.6 m, 5-mm mesh size and a handle length of 1.5 m. The collected samples were placed in a disposable box with water (300 ml) and transported to the laboratory within 8 h and kept at + 4 °C until further procedures. Euthanasia was performed in the laboratory by a blow to the head as per European Union requirements and the Federation of European Laboratory Animal Science Association regulations (FELASA) (Guillen 2012), under the supervision of a FELASA-certified specialist.
The species of all collected amphibians and tadpoles were identified based on morphological criteria (Bannikov et al. 1977) as the European common frog Rana temporaria, the European moor frog Rana arvalis, the European common toad Bufo bufo, and the water frog group Pelophylax esculentus complex (Pelophylax ridibundus, Pelophylax lessonae, and the hybrid Pelophylax esculentus). The development stage (adult frog or tadpole) and adult sex were noted. Weight (g) was measured using calibrated scales with precision ± 0.01 g. Body length (cm) was measured from the nose to the cloaca.
Amphibian skin was peeled off and divided into two parts (head and torso) and rinsed with distilled water. Next, the head, torso, internal organs (lungs, liver, kidneys, and intestinal wall) and their visceral membranes, forelimbs, and hindlimbs musculature were examined by using the compression method (Justine et al. 2012; Khalil et al. 2014). Dissected parts were compressed between two slides and analyzed with a stereomicroscope. All mesocercariae were separated from the muscle tissue and analyzed with a microscope (× 100–× 400). Alaria alata mesocercariae identification was based on morphological characteristics, such as the number of glandular cells, body shape, and size (Skrjabin 1960; Möhl et al. 2009; Patrelle et al. 2015). All of the found mesocercariae met the criteria and were identified as Alaria alata.
For each amphibian species, the prevalence (percentage of infected animals from all analyzed animals), median intensity (median number of a parasite considering only the infected members of said host species), and mean intensity (average number of a parasite considering only the infected animals of the host species) of A. alata mesocercariae were calculated (Bush et al. 1997; Reiczigel et al. 2019). Confidence intervals (95%) of prevalence were calculated using the Clopper-Pearson method (Clopper and Pearson 1934). Confidence level of median was reported as the shortest interval that reaches the desired confidence level (Bush et al. 1997; Carpenter and Bithell 2000). The differences of mesocercariae prevalence and medians between species, age groups, sex, and predilection sites were analyzed with the Fisher’s exact test and Mood’s median test (Sen 2005; Reiczigel et al. 2008). Statistical analyses were performed using R (R Core Team 2019).
Results
In total, A. alata mesocercariae were detected in 28 of 86 (32.6%; CI 95% 23.6–43.1) randomly selected wetlands (Fig. 1). Mesocercariae of A. alata were found in all analyzed frog species, while none of the analyzed common toads were found to be infected (Table 1). There were no significant differences observed between the prevalence in different host species. Nonetheless, 108 of the 390 amphibians examined for A. alata, including tadpoles, were infected with an overall prevalence of 27.7% (CI 95% 23.5–32.3). From all collected specimens, including tadpoles (n = 80), the highest (85.9%; n = 335) was from the water frog group. Overall, from 2120 observed mesocercariae, 7.0% (95% CI 6.0–8.2%) were encysted (Fig. 2).
Notably, only tadpoles from the water frog group were analyzed, and the A. alata mesocercariae prevalence in tadpoles was significantly higher (p < 0.0001) than in adults from the same water frog group. Additionally, no significant differences between median intensity in different amphibian development stages were observed.
Sex was determined for 210 of 255 adult water frogs and 18 of 19 common frog adults (Table 2). Still, no significant differences (p > 0.05) were observed between the prevalence and median mesocercariae intensity and different sexes, although a tendency of higher mesocercariae median intensity in males of the water frog group was observed.
Overall weight (0.3–80.0 g) and size (1.1–10.5 cm) were determined for 240 of 255 adult water frogs. When the abundance of A. alata mesocercariae was analyzed, a slight linear tendency was detected with weight (r = 0.19, p = 0.0034) and size (r = 0.22, p = 0.0004).
Our study has shown that A. alata mesocercariae predilection sites in adult frogs were the head, internal organs, and visceral membranes (Table 3). Moreover, mesocercariae in tadpoles were found predominantly in the head and torso regions. Significantly higher prevalence was observed in the tadpole head (p < 0.0001) and torso (p < 0.0001) compared with the adults of the same species and sites. Most often, mesocercariae in adult frogs were observed in both the head and visceral membrane (64.7%, CI 95% 41.2–82.8), while mesocercariae in tadpoles were mostly detected in both the head and torso (55.0%, CI 95% 34.2–74.19). Internal organs were analyzed separately, and significantly higher (p = 0.005) median intensity was observed in the livers of water frogs. Yet, due to the size of the tadpole, it was not possible to distinguish the internal organs. Furthermore, in one specimen of an adult common frog, mesocercariae were found in the intestinal wall. Overall, 1487 of 2120 mesocercariae (70.1%, CI 95% 68.2–72.1) were detected in the body cavity—925 (43.6%; CI 95% 41.5–45.8) in the internal organs and 562 (26.7%; CI 95% 24.7–28.4) in the visceral membranes.
Discussion
This study shows a high prevalence of A. alata mesocercariae in the water frog group. Common frogs and moor frogs indicated that these amphibians were suitable intermediate hosts for A. alata, while none of the analyzed common toads was found to be infected in Latvia. In the previous studies, common toads had been found to be suitable intermediate hosts for A. alata as well. For instance, mesocercariae were observed in 2 out of 25 (0.8%) common toads from Belorussian Polesie (Shimalov and Shimalov 2001). Also, in the Volga Basin, in Russia, the common toad was found to be a suitable second intermediate host for A. alata (Chikhlyaev et al. 2016). In the present study, the analyzed number of common toads was limited (n = 31), and likely the prevalence and median intensity of A. alata mesocercariae could have been underestimated.
In the present study, prevalence of A. alata mesocercariae in common frogs and moor frogs was 15.8% (n = 19) and 33.3% (n = 3), respectively. The prevalence of A. alata mesocercariae in common frogs can reach 27.0% (n = 37) (Patrelle et al. 2015), though there are no previously published data on prevalence in moor frogs. Nevertheless, the adult water frog group analysis showed similar results compared with our study, 17.3% (n = 29) and 22.4% (n = 225), respectively (Patrelle et al. 2015). All investigated frog species spawn from April to May and only water frogs inhabit the aquatic environment all the time, while common and moor frogs are present in water only when spawning. These frogs mostly prefer stagnant waters where only a few snails have the capacity to infect the frogs in a waterbody (Portier et al. 2012; Patrelle et al. 2015).
The presence of A. alata mesocercariae in the intermediate and paratenic hosts as a facultative part of a parasite’s life cycle increased transmission opportunities. In this parasite development stage, A. alata could survive several host transitions unharmed (Möhl et al. 2009). Experimental studies with A. arisimoides and A. canis cercariae showed that amphibians could get infected in two ways: via cercariae penetration through the skin and/or via cannibalism of other amphibians infected with mesocercariae (Pearson 1956). According to the previous studies, depending on infection route, amphibians could act as second intermediate hosts if the infection occurred with cercariae penetrations or paratenic hosts if infection occurred via consumption of other infected amphibians (Pearson 1956; Patrelle et al. 2015). Cannibalism has been reported for Ranidae frogs, and it could be observed in several other frog species, especially in cases where there were dense populations of tadpoles (Ruchin and Ryzho 2002; Covaciu-Marcov et al. 2005; Mollov et al. 2010). The changes in the ecological conditions in the habitat and the increased growth of population could force specimens toward cannibalism as cannibalism emerges as a mechanism to increase the survival rate of amphibians (Crump 1992; Stebbins and Cohen 1995).
In the present study, 7.0% of detected mesocercariae in frogs were encapsulated and located mostly in the visceral membrane. Several previous studies had also mentioned the presence of similar pseudocysts (Tǎbǎran et al. 2013; Patrelle et al. 2015; Uhrig et al. 2015). Similar to other cysts, they are circular and encapsulated, but are not lined by a functional epithelium. The mucus around the mesocercariae was most likely produced by the host; inflammation was the result of direct tissue damage rather than an immune reaction targeted toward the parasitic antigens (Tǎbǎran et al. 2013; Uhrig et al. 2015).
After being ingested, A. alata mesocercariae could migrate through the intestinal wall and circulate to the muscle tissues and different organs (Möhl et al. 2009), while Pearson (1956) observed that regardless the route of infection, mesocercariae in frog tissues distribute equally, in the muscle tissues ventrally in the distal part of the thighs or between the sternum and the hyoid. In the current study, the following predilection sites were determined for A. alata mesocercariae in amphibians: the head, especially periorbital muscles, torso, especially under skin, internal organs, including the lungs, liver, kidneys, intestinal wall, and organ visceral membranes. Although A. alata mesocercariae were mostly found in the head, including periorbital region, these results correspond with the previous studies, which also had reported head, hindlimbs, and torso as a predilection sites for A. alata mesocercariae in amphibians (Patrelle et al. 2015; Voelkel et al. 2019). The highest mesocercariae intensity in the tissues around the eyes could possibly lead to impaired vision of frogs and tadpoles and, thus, make them more susceptible to predators assuring A. alata transmission (Patrelle et al. 2015).
The present study is the first one that shows no differences in A. alata mesocercariae prevalence in either the water frog group or in the common frogs between different sexes. An earlier study comparing the pattern of the other parasite infection and the amphibian host sex also did not show any differences (Hamann et al. 2006). It should be noted, however, that there is no notable difference in behavior or biology in tadpoles of different sex of these two species because these differences only become apparent once the amphibians sexually mature into adults (Lannoo 2005).
Significant difference was observed between A. alata mesocercariae prevalence in tadpoles and adult frogs. Tadpoles probably have higher risk of infection due to their activity and easy access through the thin skin; the epidermis is much thinner, compared with adult frogs which have a thicker, harder outer skin (Pearson 1956). Still, in a previous study where 23 tadpoles and 29 adults of water frog group were analyzed, no significant differences between the frogs’ developmental stages were observed (Patrelle et al. 2015).
To our knowledge, this is the most comprehensive study on A. alata mesocercariae occurrence and their predilection sites in amphibians not only in the Baltic region but also in all of Europe. Present study shows that A. alata is common in various species of amphibians in Latvia, and it indicates the gaps of knowledge regarding potential A. alata 2nd intermediate hosts in different regions in Europe.
References
Bannikov AG, Darevsky IS, Ishchenko VG, Rustamov AK, Szczerbak NN (1977) Opredelitel zemnovodnyh i presmykayushchihsya fauny SSSR. USSR, Moscow (in Russian)
Berger EM, Paulsen P (2014) Findings of Alaria alata mesocercariae in wild boars (Sus scrofa, Linnaeus, 1758) in West Hungary (Transdanubia regions). Wien Tierärztl Monat-Vet Med Austria 101:120–123
Borgsteede FHM (1984) Helminth parasites of wild foxes (Vulpes vulpes L.) in the Netherlands. Z Parasitenkd 70:281–285
Bush AO, Lafferty KD, Lotz JM, Shostak AW (1997) Parasitology meets ecology on its own terms: Margolis et al. Revisited J Parasitol 83:575–583
Carpenter J, Bithell J (2000) Bootstrap confidence intervals: when, which, what? A practical guide for medical statisticians. Stat Med 19:1141–1164
Castro O, Venzal JM, Félix ML (2009) Two new records of helminth parasites of domestic cat from Uruguay: Alaria alata (Goeze, 1782) (Digenea, Diplostomidae) and Lagochilascaris major Leiper, 1910 (Nematoda, Ascarididae). Vet Parasitol 160:344–347
Chikhlyaev I, Ruchin A (2014) The helminth fauna study of European common brown frog (Rana temporaria Linnaeus, 1758) in the Volga basin. Acta Parasitol 59:459–471
Chikhlyaev IV, Ruchin AB, Fayzulin AF (2016) The helminth fauna study of European common toad in the Volga Basin. Nat Environ Pollut Technol 15:1103–1109
Clopper CJ, Pearson ES (1934) The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika 26:404–413
Core Team R (2019) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Covaciu-Marcov SD, Sas I, Cupşa D, Bogdan H, Lukacs J (2005) The seasonal variation of the food of a non-hibernated Rana ridibunda Pallas 1771 population from the thermal lake 1 Mai spa, Romania. Analele Univ Oradea, Fasc Biologie 12:77–85
Craig HL, Craig PS (2005) Helminth parasites of wolves (Canis lupus): a species list and analysis of published prevalence studies in Nearctic and Palearctic populations. J Helminthol 79:95–103
Crump ML (1992) Cannibalism in amphibians. In: Elgar MA, Crespi BJ (eds) Cannibalism: ecology and evolution among diverse taxa. Oxford University Press, New York, pp 256–276
ESRI (2011) ArcGIS desktop: release 10.3. Environmental Systems Research Institute, Redlands
Galaktionov KV, Dobrovolskij AA (2003) The biology and evolution of Trematodes. Kluwer Academic Publishers, Dordrecht
Guillen J (2012) FELASA guidelines and recommendations. J Am Assoc Lab Anim Sci 51(3):311–321
Hamann MI, González CE, Kehr AI (2006) Helminth community structure of the oven frog Leptodactylus latinasus (Anura, Leptodactylidae) from Corrientes, Argentina. Acta Parasitol 51:294–299
Huguenin A, Depaquit J, Villena I, Ferté H (2019) MALDI-TOF mass spectrometry: a new tool for rapid identification of cercariae (Trematoda, Digenea). Parasite 26:11
Justine JL, Briand MJ, Bray RA (2012) A quick and simple method, usable in the field, for collecting parasites in suitable condition for both morphological and molecular studies. Parasitol Res 111:341–351
Khalil MI, El-Shahawy IS, Abdelkader HS (2014) Studies on some fish parasites of public health importance in the southern area of Saudi Arabia. Rev Bras Vet Parasitol 23:435–442
Lannoo M (ed) (2005) Amphibian declines: the conservation status of United States species, 1st edn. University of California Press, Berkeley
Malešević M, Smulders FJM, Petrović J, Eta J, Paulsen P (2016) Alaria alata mesocercariae in wild boars (Sus scrofa) in northern Serbia after the flood disaster of 2014. Wiener Tierärztliche Monatsschrift 103:345–349
Martinković F, Sindičić M, Lučinger S, Šimac I, Bujanić M, Živičnjak T, Stojčevićjan D, Šprem N, Popović R, Konjević D (2017) Endoparasites of wildcats in Croatia. Vet Arhiv 87:713–729
Möhl K, Große K, Hamedy A, Wüste T, Kabelitz P, Lücker E (2009) Biology of Alaria spp. and human exposition risk to Alaria mesocercariae-a review. Parasitol Res 105:1–15
Mollov I, Boyadzhiev P, Donev A (2010) Trophic role of the marsh frog Pelophylax ridibundus (Pallas, 1771) (Amphibia, Anura) in the aquatic ecosystems. Bulg J Agric Sci 16:298–306
Olsen OW (1974) Animal parasites. Their life cycles and ecology. University Park Press, Baltimore, pp 237–240
Ozoliņa Z, Bagrade G, Deksne G (2018) The host age related occurrence of Alaria alata in wild canids in Latvia. Parasitol Res 117:3743–3751
Ozoliņa Z, Bagrade G, Deksne G (2019) First confirmed case of Alaria alata mesocercaria in Eurasian lynx (Lynx lynx) hunted in Latvia. Parasitol Res 119:759–762
Patrelle C, Portier J, Jouet D, Delorme D, Ferté H (2015) Prevalence and intensity of Alaria alata (Goeze, 1792) in water frogs and brown frogs in natural conditions. Parasitol Res 114:4405–4412
Paulsen P, Ehebruster J, Irschik I, Lücker E, Riehn K, Winkelmayer R, Smulders FJM (2012) Findings of Alaria alata mesocercariae in wild boars (Sus scrofa) in eastern Austria. Eur J Wildl Res 58:991–995
Paulsen P, Forejtek P, Hutarova Z, Vodnansky M (2013) Alaria alata mesocercariae in wild boar (Sus scrofa, Linnaeus, 1758) in south regions of the Czech Republic. Vet Parasitol 197:384–387
Pearson JC (1956) Studies of the life cycles and morphology of the larval stages of Alaria arisaemoides (Augustine and Uribe, 1927) and Alaria canis (LaRue and Fallis, 1936) (Trematoda: Diplostomatidae). Can J Zool 34:295–387
Portier J, Jouet D, Vallée I, Ferté H (2012) Detection of Planorbis planorbis and Anisus vortex as first intermediate hosts of Alaria alata (Goeze, 1792) in natural conditions in France: molecular evidence. Vet Parasitol 190(1–2):151–158
Reiczigel J, Abonyi-Tóth Z, Singer J (2008) An exact confidence set for two binomial proportions and exact unconditional confidence intervals for the difference and ratio of proportions. Comput Stat Data An 52:5046–5053
Reiczigel J, Marozzi M, Fábián I, Rózsa L (2019) Biostatistics for parasitologists - a primer to quantitative parasitology. Trends Parasitol 35:277–281
Rentería-Solis Z, Hamedy A, Michler FU, Michler BA, Lücker E, Stier N, Wibbelt G, Riehn K (2013) Alaria alata mesocercariae in raccoons (Procyon lotor) in Germany. Parasitol Res 112:3595–3600
Riehn K, Hamedy A, Große K, Wüste T, Lücker E (2012) Alaria alata in wild boars (Sus scrofa, Linnaeus, 1758) in the eastern parts of Germany. Parasitol Res 111:1857–1861
Rodríguez-Ponce E, González JF, Conde de Felipe M, Hernández JN, Raduan Jaber J (2016) Epidemiological survey of zoonotic helminths in feral cats in gran Canaria island (Macaronesian archipelago-Spain). Acta Parasitol 61:443–450
Ruchin AB, Ryzho MK (2002) On diet of the marsh frog (Rana ridibunda) in the diet Sura and moksha watershed, Mordovia. Adv Amphib Res Former Soviet Union 7:197–205
Segovia JM, Guerrero R, Torres J, Miquel J, Feliu C (2003) Ecological analyses of the intestinal helminth communities of the wolf, Canis lupus, in Spain. Folia Parasitol 50:231–236
Sen PK (2005) Multivariate median and rank sum tests. In: Armitage P, Colton T (eds) Encyclopedia of Biostatistics. Wiley, Hoboken
Shimalov VV, Shimalov VT (2000) Helminth fauna of snakes (Reptilia, Serpentes) in Belorussian Polesye. Parasitol Res 86(4):340–341
Shimalov VV, Shimalov VT (2001) Helminth fauna of toads in Belorussian Polesie. Parasitol Res 87(10):84
Shimalov VV, Shimalov VT (2003) Helminth fauna of the red fox (Vulpes vulpes Linnaeus, 1758) in southern Belarus. Parasitol Res 89:77–78
Shimalov VV, Shimalov VT, Shimalov AV (2000) Helminth fauna of lizards (Reptilia, Sauria) in the southern part of Belarus. Parasitol Res 86(4):343
Shimalov VV, Shimalov VT, Shimalov AV (2001) Helminth fauna of newts in Belorussian Polesie. Parasitol Res 87(4):356
Skrjabin KI (1960) Trematodes of animals and man; essentials of trematodology, vol XVIII. Ketter Press, Jerusalem, pp 327–343
Stebbins RC, Cohen NW (1995) A natural history of amphibians. Princeton University, New Jersey
Strokowska N, Nowicki M, Klich D, Bełkot Z, Wiśniewski J, Didkowska A, Chyla P, Anusz K (2020) The occurrence of Alaria alata mesocercariae in wild boars (Sus scrofa) in North-Eastern Poland. Int J Parasitol Parasites Wildl 12:25–28
Szczęsna J, Popiołek M, Schmidt K, Kowalczyk R (2008) Coprological study on helminth fauna in Eurasian Lynx (Lynx lynx) from the Białowieża Primeval Forest in eastern Poland. J Parasitol 94:981–984
Tǎbǎran F, Sandor AD, Marinov M, Cătoi C, Mihalca AD (2013) Alaria alata infection in European mink. Emerg Infect Dis 19:1547–1549
Takeuchi-Storm N, Mejer H, Al-Sabi MN, Olsen CS, Thamsborg SM, Enemark HL (2015) Gastrointestinal parasites of cats in Denmark assessed by necropsy and concentration McMaster technique. Vet Parasitol 214:327–332
Uhrig EJ, Spagnoli ST, Tkach VV, Kent ML, Mason RT (2015) Alaria mesocercariae in the tails of red-sided garter snakes: evidence for parasite-mediated caudectomy. Parasitol Res 114:4451–4461
Voelkel AC, Dolle S, Koethe M, Haas J, Makrutzki G, Birka S, Lücker E, Hamedy A (2019) Distribution of Alaria spp. mesocercariae in waterfrogs. Parasitol Res 118:673–676
Acknowledgments
We thank Daina Pūle for her valuable work in preparing maps for this study. This study was conducted under the auspices of the project: “The biological and ecological triggers causing the expansion of the invasive fish: the Chinese Sleeper (Perccottus glenii), in Eastern Europe” (Latvian – Ukrainian project Nr. LV-UA/2018/6).
Author information
Authors and Affiliations
Corresponding author
Additional information
Section Editor: David Bruce Conn
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ozoliņa, Z., Deksne, G., Pupins, M. et al. Alaria alata mesocercariae prevalence and predilection sites in amphibians in Latvia. Parasitol Res 120, 145–152 (2021). https://doi.org/10.1007/s00436-020-06951-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-020-06951-6