Abstract
The northern fur seal (NFS), Callorhinus ursinus (Mammalia: Otariidae), is a marine mammal species included into the IUCN Red List as the vulnerable species which population is dramatically declining. A significant amount of parasitological data collected previously and our recent data allowed us to clarify the list of NFS metazoan parasites and to perform a comprehensive analysis of the gastrointestinal helminth community. Gastrointestinal tracts from 756 NFSs (3- to 4-year-old males) were collected during the annual Aleut subsistence harvests in July–August of 2011–2014 from five separate rookeries on St. Paul Island, Alaska. Totally, 27,625 specimens of helminths and approximately 1000 nasal mites were collected and identified. Detailed analysis of the previously published and newly obtained data revealed 32 species of metazoan parasites, including trematodes (6 species), cestodes (4), nematodes (9), acanthocephalans (9) and arthropods (4). The gastrointestinal helminth community of newly studied NFSs comprised 19 species including trematodes (4), cestodes (3), nematodes (5) and acanthocephalans (7). Temporal changes in the helminth community structure were small but statistically significant. Gastrointestinal helminth infracommunities comprised from 1 to 10 species (average of 4). Small but significant correlation was found between the abundances of acanthocephalans (Corynosoma similis and C. strumosum), nematodes (Contracaecum osculatum, Pseudoterranova spp.) and cestode Diphyllobothrium tetrapterum.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The northern fur seal (NFS), Callorhinus ursinus Linnaeus, 1758 (Mammalia: Otariidae), is the only living species of the genus Callorhinus Gray, 1859. The distribution of NFS includes breeding areas in the North Pacific extending from the Kuril Islands of Russia, across the Bering Sea and south to the west coast of the USA with the southernmost rookery on San Miguel Island, the most western island in the Channel Islands of Southern California. The global NFS population was estimated to be approximately 1.29 million in 2014. This estimation reflects a decline of NFS population by approximately 658,000 from 1976, resulting in the NFS’s IUCN Red List categorization as a Vulnerable species under criterion A2b (Gelatt et al. 2015). The largest breeding rookeries of the NFS are now located on St. Paul Island, Pribilof Archipelago, Alaska (Gelatt et al. 2015). In the past, the Pribilof Archipelago represented approximately 75% of the world’s NFS population. Current metrics indicate that the Pribilof Archipelago’s population has declined by approximately 66% over the last three generations (1972–2014) with an annual 5.5–5.8% decline in pup production (Towell et al. 2006; Allen and Angliss 2015; Gelatt et al. 2015). Several hypotheses have been proposed to explain the causes for this decline that include predation, human interactions (e.g. by-catch, entanglement, ship disturbance, pollution) and food limitations. However, due to insufficient data on population vital rates, prey resources and potential causes of mortality, the exact reason behind the decline in NFS pup production is unknown (Trites 1992; NMFS 2007). Prior multi-year studies conducted on St. Paul Island have not shown specific bacterial, viral or parasitic diseases to be responsible for reducing the NFS population. Rather, emaciation and trauma were reported to be the primary causes of NFS mortality of pre-weaned pups (Spraker and Lander 2010).
Studies on NFS biology and ecology including their parasites and diseases have been performed in different localities for more than 100 years (Scott et al. 2006; Felix 2013). The historically first parasites reported from NFSs were anisakid nematodes discovered in pups on St. Paul Island in 1879 by Dr. White (see Scheffer et al. 1984). Later, Stiles and Hassall (1899) identified the nematodes Ascaris decipiens Krabbe, 1878 (now Pseudoterranova decipiens), Uncinaria sp. (now Uncinaria lucasi Stiles 1901) and the cestode Bothriocephalus sp. (now Adenocephalus pacificus Nybelin, 1931). The louse Echinophthirius callorhini Osborn 1899 (now Antarctophthirus callorhini) was described in the same year from NFS collected on Pribilof Archipelago. Later, Delyamure (1961) reported 21 helminth species from NFSs located on the Commander Archipelago. Since then, more than 40 species of metazoan parasites have been documented in NFSs in various parts of their range (Delyamure 1955, 1961; Dailey and Brownell 1972; Margolis and Dailey 1972; Dailey 1975; Yurakhno 1998; Felix 2013). Recent studies of anisakid nematodes based on molecular methods have revealed that the most common species, Anisakis simplex (Rudolphi, 1809), Pseudoterranova decipiens and Contracaecum osculatum (Rudolphi, 1802) are “species complexes” and include several morphologically indistinguishable cryptic species (Mattiucci and Nascetti 2008; Mattiucci et al. 2014, 2018). Therefore, at present, it is almost impossible to determine the precise number of species parasitizing NFSs. Moreover, it is extremely complicated to plan and perform a comprehensive parasitological investigation of NFSs due to the IUCN Red List of Threatened Species protections (Gelatt et al. 2015), which create an impasse in obtaining official permissions for the dissection of a significant number of NFSs for scientific purposes.
Most of the parasitological studies of NFSs on the Pribilof Archipelago were carried out between 1950 and 1970 and predominantly addressed Uncinaria lucasi (Olsen 1958; Olsen and Lyons 1962; Lyons 1963;) or Acanthocheilonema (Dipetalonema) odendhali (Perry, 1967) (Perry and Forrester 1971); however, these studies were based on occasional examinations of a small number of seals (Neiland 1961; Keyes 1965). Extensive studies of NFS parasites were carried out between 1960 and 1980 in the former USSR, mainly on the Commander and Kuril Archipelagos (Delyamure and Skrjabin 1960; Delyamure 1961; Chupakhina 1971; Timofeeva et al. 1972; Kovalenko 1975; Yurakhno and Taikov 1986; Yurakhno 1998). Recent studies have been carried out exclusively on St. Paul Island (Lyons et al. 2000, 2011, 2012, 2014; Ionita et al. 2008; Kuzmina et al. 2012, 2013, 2014, 2015, 2018).
The primary objective of this study was to review the published literature data addressing parasites of NFSs and to update the list of metazoan parasites with the inclusion of new data. Parasitological data collected during the last decade allowed us to perform a comprehensive analysis of the gastrointestinal helminth community of NFSs from St. Paul Island both at infracommunity and component community levels. Additionally, we hypothesized the existence of spatial and temporal differences in the gastrointestinal helminth community and tested the assumption using a linear model approach.
Material and methods
Collecting and identification of the material
This study was carried out on St. Paul Island (57° 09′ N, 170° 13′ W), Alaska, USA, in July–August of 2011–2014. Gastrointestinal tracts from 756 NFS males 3–4 years of age including additional organs were collected during the annual Aleut subsistence harvests from five separate haul-out areas adjacent to the five rookeries: Gorbatch (n = 125), Lukanin (n = 165), Morjovi (n = 120), Polovina (n = 164) and Zapadni (n = 182) (see map in our previous studies, Kuzmina et al. 2015, 2018).
Each gastrointestinal tract was separated into three parts (stomach, small intestine, large intestine), then dissected and examined according to Bowman and Lynn (1995). Intestinal content was collected separately from each segment of the digestive system and washed through 50 mesh sieves (0.297 mm) to collect small helminths. All parasites were collected manually under a dissecting microscope, washed in saline, and fixed in 70% non-denatured ethanol. A total of 27,625 specimens of helminths were collected and identified. Tapeworms were immediately killed through immersion in hot water, after which they were placed into 70% non-denatured ethanol. Before identification, all nematodes were cleared in lactophenol (25% phenol, 25% lactic acid, 25% glycerine and 25% distilled water) for approximately 3 h. Trematodes were clarified in lactophenol for 30 min before identification. Selected specimens of trematodes and cestodes were stained with acetocarmine, dehydrated in a graded ethanol series, cleared in clove oil, mounted permanently in Canada balsam and identified under a light microscope using morphological criteria (see Hernández-Orts et al. 2015; Kuzmina et al. 2018). All acanthocephalans were mounted in Berlese’s medium, examined and morphologically identified under light microscope Zeiss Axio Imager M1 (Kuzmina et al. 2012). Selected heads of 37 NFSs and additional internal organs of several NFSs were also examined for metazoan parasites. Mites were collected from NFS nasal cavities and nematodes were collected from the lungs and pelt subcutaneous tissues. All collected parasites were fixed in ethanol and identified by their morphology.
Morphological vouchers of studied metazoan parasites were deposited in the parasitological collection of the I. I. Schmalhausen Institute of Zoology NAS of Ukraine (Kyiv, Ukraine), the Helminthological Collection of the Institute of Parasitology, Czech Academy of Sciences (IPCAS) (České Budějovice, Czech Republic) and the National Museum of Natural History, Smithsonian Institution (USNM) (Washington, D.C., USA) (Kuzmina et al. 2012, 2014, 2015, 2018).
Data analysis
The prevalence (P), mean abundance (MA) and mean intensity (I) were calculated for each helminth species following the definitions of Bush et al. (1997). The relative abundance (RA) was calculated as the ratio (percentage) of the number of specimens of a particular species to the total number of helminth specimens in the sample. The analysis of the gastrointestinal helminth community was performed using only the complete gastrointestinal helminth collections from 651 of the 756 hosts examined in 2012–2014.
The calculations were performed using R software v. 3.6.1–3.6.3 (R Core Team 2020). Two packages were used in addition to the standard R packages: RRPP v. 0.6.0. (Collyer and Adams 2018, 2019) and corrplot v. 0.84 (Wei and Simko 2017). Effects of rookery and year were tested using linear model evaluation with a randomized residual permutation procedure realized in the lm.rrpp function of the R package RRPP. In total, 9999 permutations were used for testing.
Since our task was to evaluate the territorial and temporal variation in the levels of infection, we considered the set of annual estimates of the infection level in the studied rookeries as a sample from a great number of localities at different points of time. Therefore, the effects of rookery were treated as a random effect in the analysis, as well as the effect of the year. If the differences between the levels of infection between the rookeries occurred to be significant, one could make the pairwise comparisons of the levels of infection in different rookeries and describe the differences by multidimensional scaling or by an appropriate alternative technique. In our testing, significant differences between the rookeries did not occur (see the “Results” section below). Therefore, we did not conduct additional analyses through multidimensional scaling or other means.
To assess whether the gastrointestinal helminths of different taxa tend to occur together, the correlations between their numbers were analysed using the Spearman’s correlation coefficient (rS). The number of intestinal helminths of a certain taxon in the infracommunity of NFS was treated as a quantitative character, and the number of specimens of helminths of this taxon found in one individual of NFS was used as a value of this character for the individual. The p values of the obtained correlation coefficients were corrected for multiple comparisons by the Benjamini–Yekutieli method (Benjamini and Yekutieli 2001) conducted using the R function p.adjust. To find the sets of correlated characters, we applied the hierarchical cluster analysis realized in the R function hclust. We used (1 − rS) as the distance measure and the single linkage method as the clustering technique. To visualize the results, the R function hclust and the R package corrplot were used.
Results
Review of metazoan parasites of Callorhinus ursinus
Detailed analysis of the published data, including our previous studies, revealed 33 species documented as valid metazoan parasites of NFS, including 29 species of helminths (7 trematodes, 4 cestodes, 9 nematodes and 9 acanthocephalans) and 4 species of parasitic arthropods (Table 1; Fig. 1). Two species of trematodes, Apophallus (Pricetrema) callorhini (Yurakhno 1986) and Cryptocotyle (Ciureana) delamurei (Yurakhno 1987), were identified once in one NFS from the Commander Archipelago (Yurakhno 1986, 1987) (Fig. 1). Tatonova and Besprozvannykh (2019) listed the latter species as valid. A third trematode reported in NFS is the heterophyid Cryptocotyle jejuna (Nicholl, 1907) (Keyes 1965; Neiland 1961), a common parasite of European birds. The validity of these three species in NFS is questionable in the relation of other similar small trematodes reported from NFS. A recent extensive study by Kuzmina et al. (2018) molecularly confirmed two heterophyids (Phocitrema fusiforme Goto and Ozaki, 1930, Galactosomum ubelakeri (Dailey, 1969)) and one troglotrematid Nanophyetus salmincola (Chapin, 1926) infecting NFS on St. Paul Island. Seven species of tapeworms have been recognized in NFSs by Yurakhno (1998). However, only one immature tetrabothriid, two adult diphyllobothriids, and an accidental infection of cestode larvae (Scolex pleuronectis) that might belong to the larval type I of Jensen and Bullard (2010) and corresponds with members of the order Onchoproteocephalidea from elasmobranchs were confirmed as valid parasites of NFS (see Table 1). We found 7 of 8 nematode species confirmed as valid parasites of NFS (Table 2), but only 5 were included in the present analyses, due to their inhabitance of the gastrointestinal tract. Anisakis physeteris (Baylis, 1923) is a parasite of sperm whales, but its single report from an NFS in Japan was most probably a misidentification or an accidental finding (Nagasawa 1999). Finding microfilaria and identifying them as Dirofilaria immitis (Leidy, 1856) in NFSs also was reported by Nagasawa (1999). This was an unusual finding of a well-known dog parasite and most likely a misidentification with Acanthocheilonema odendhali (Perry, 1967). All 9 species of acanthocephalans reported from NFSs are considered as valid. We confirmed the presence of 7 species, including Corynosoma cameroni Van Cleave 1953, reported from NFSs for the first time (Kuzmina et al. 2012).
Microphotographs of the helminths from northern fur seals Callorhinus ursinus. a–e Trematoda. f, g Cestoda. h–o Nematoda. p–t Acanthocephala. a Apophallus (Pricetrema) callorhini. b Cryptocotyle (Ciureana) delamurei. c Galactosomum ubelakeri. d Nanophyetus salmincola. e Phocitrema fusiforme. f, g Anophryocephalus ochotensis. h, k, l Acanthocheilonema odendhali. i Pseudoterranova spp. j, o Uncinaria lucasi. m, n Contracaecum osculatum s. l.. p Bolbosoma nipponicum. q, r Corynosoma cameroni. s, t Corynosoma strumosum. a, b Material from type series collected by M. Yurakhno from Commander Archipelago. c–t Newly obtained material from St. Paul Island, Alaska, USA. a–f Light microscope photographs. k–o, r, t SEM photographs
From the 37 NFS heads examined, a total of approximately 1000 nasal mites were found. The greatest intensity of mite infection within an NFS head was 226 specimens, including larval stages. Specific studies of nasal mites were not performed. Additionally, the nematode Acanthocheilonema odendhali was found in subcutaneous tissues of 89 of 502 NFS examined (17.83%). Uncinaria lucasi was found in 9 NFS pups (see Lyons et al. 2012, 2014).
Orthohalarchne attenuata (Banks 1910) was documented in the nasal cavity of all 37 NFSs. Several individuals of O. diminuata Doetschman 1944, were found in the lower trachea of 6 seals. Two other dermal louse species previously reported from NFSs were not found, likely due to the absence of skin examination in this study. Yurakhno (1998) reported 2 species of ticks, Ceratixodes arcticus (Osborn 1899) (now Ixodes stignatus Birula, 1895) and Hyaloma puta (Pickard-Cambrige, 1876) (now I. uriae White, 1852) from NFSs, but these species are parasites of birds and their finding might represent accidental infections.
Based on this critical study, we confirmed only nematode (Uncinaria lucasi), anoplura (Antarctophthirius callorhini) and two trematodes (A. callorhini and C. delamurei) as specific parasites of NFS. All other metazoan parasites reported from NFSs are known from other pinnipeds, or even from terrestrial mammals and birds (Table 1).
Composition and structure of the component community of gastrointestinal helminths
In total, 19 species of gastrointestinal helminths collected from 651 NFSs were included in the community analysis. Cestodes were the most abundant taxonomic group and represented more than half of collected parasites (53%; 14,660 specimens; 2 species), followed by nematodes (29%; 7894; 5 species) and trematodes (14%; 3867; 4 species). The acanthocephalans were less abundant and composed only 4% (1204; 7 species) of all intestinal helminths (Table 2).
Acanthocephalans were represented by 7 species of the family Polymorphidae. Their total occurrence was comparatively low; 47.3% of the hosts harboured at least one acanthocephalan species, but none of the species had a mean abundance higher than 1.0. The highest intensity of infection was observed in Corynosoma strumosum (Rudolphi, 1802) with up to 30 specimens in one NFS, and C. similis Neiland 1962, up to 26 specimens (Table 2). One specimen of C. cameroni was found in each of six NFS individuals infected with this helminth.
Nematodes were found in 91.9% of the NFSs examined and included 5 species, all belonging to the family Anisakidae. Since it was not possible to distinguish the females of Pseudoterranova decipiens and those of P. azarazi (Yamaguti et Arima, 1942), we combined quantitative data for both species. They appeared to be the most prevalent group of nematodes (P = 84.2%) with the highest mean abundance of 5.5. The highest mean intensity among the nematodes in a single NFS was 265 specimens of Anisakis simplex (Table 2). However, the majority of A. simplex specimens (75.6%) were in larval stages. Phocascaris cystophorae (Goto and Ozaki, 1930) was the rarest nematode species (P = 5.5%) (Table 2).
Four recognized trematode species had a comparatively low total prevalence of 32.3%. Nanophyetus salmincola was found in one seal but with an impressive quantity of 1540 specimens. Phocitrema fusiforme was the most common trematode species with maximum infection intensity of 410 specimens in one seal followed by G. ubelakeri and A. zalophi Price, 1932 (Table 2).
With 3 species found, tapeworms were the least diverse group. While tapeworms lacked diversity, they were the most prevalent group of gastrointestinal helminths, found in 98.5% of examined seals. Comparatively high prevalence and abundance of tapeworms were observed in both diphyllobothriids, Adenocephalus pacificus and D. tetrapterum (Siebold, 1848), while the tetrabothriidean Anophryocephalus ochotensis Delyamure et Krotov, 1955, was found in 4 of the 651 seals (Table 2).
According to the prevalence values, 5 groups of parasite species were established (Fig. 2): dominant species (P > 70.1%), subdominant species (30.1% < P < 70%), common species (10.1% < P < 30%), rare species (1.1% < P < 10%) and occasional species (P < 1.0%). The tapeworm A. pacificus and the nematodes Pseudoterranova spp. were found in more than 80% of the seals; thus, they were dominant species. The group of subdominant species contained two anisakids (C. osculatum and A. simplex) and the diphyllobotriid D. tetrapterum. The group of common species had the trematode P. fusiforme and 4 acanthocephalans of the genus Corynosoma (C. strumosum, C. similis, C. semerme (Forssel, 1904) and C. villosum (Van Cleave 1953)). The group of rare species was comprised of 5 species: anisakid nematode Phocascaris cystophorae, trematodes G. ubelakeri, A. zalophi and acanthocephalans Bolbosoma nipponicum (Yamaguti, 1939) and C. validum (Van Cleave 1953). The acanthocephalans C. cameroni and C. alaskensis (Golvan, 1959), the tapeworm Anophryocephalus ochotensis and the trematode N. salmincola were found in less than 1% of hosts and were considered as occasional NFS parasites (Fig. 2).
Prevalence (%) and relative abundance (%) of gastrointestinal helminth species found in northern fur seals Callorhinus ursinus on St. Paul Island, Alaska. Black bars—dominant species; dark grey bars—subdominant species; grey bars—common species; light grey bars—rare species; white bars—occasional species
Analysis of relative abundance (RA) revealed that the most commonly occurring species A. pacificus and Pseudoterranova spp. were also the most abundant, composing almost 61% of the total number of helminths (Table 2, Fig. 2). The nematode A. simplex and the trematode P. fusiforme had the highest relative abundance other than A. pacificus and Pseudoterranova spp., at 9.2% and 7.3%, respectively (Fig. 2). Five dominant and subdominant species constituted more than 80% of the gastrointestinal helminth individuals found in this study. The total proportion of the other 14 species, including the trematode N. salmincola, was less than 20% (Fig. 2).
Temporal and spatial differences within the component community
We tested possible rookery/year effects on species composition and structure of NFS gastrointestinal helminth community using the Linear Model Evaluation with a Randomized Residual Permutation Procedure (Table 3; see “Material and methods” section for details).
We found significant differences between the three studied years (p = 0.0096) but not among the five rookeries (p = 0.84). No significant interaction was found between the effects of year and rookery (p = 0.15; i.e. year-to-year trends seem not to be different in different rookeries). Statistically significant differences in gastrointestinal helminth infections in NFS from the 5 rookeries for the entire period (2012–2014) were not found (p = 0.84). All of the studied effects explain a small piece of the total variation shown by R2 estimates (Table 3).
Characterization of gastrointestinal helminth infracommunities
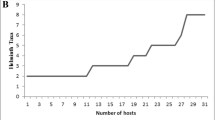
All examined NFSs were infected with helminths. The species richness ranged from 1 to 10 species (mean 4.3; median 4) and the abundance ranged from 1 to 452 helminth specimens (mean 36.2; median 29). The median value of the species richness in the infracommunities (4 species) was observed in the largest number of host individuals: 133 (20.4%), while the maximum number of helminth species (10) was observed only in three NFS individuals (0.5%) (Fig. 3).
Co-occurrence of gastrointestinal helminths in the infracommunities
The correlations between the numbers of the gastrointestinal helminths of different taxa and their p values are given in Table 4 and illustrated in Figs. 4 and 5. The matrix in Fig. 4 shows the correlations for each pair of selected helminths taxa. The areas of the filled circle sectors show the absolute value of corresponding correlation coefficients. The correlations of co-occurrence for most gastrointestinal helminth species found in NFSs are low (Table 2, Figs. 4 and 5). Most of the correlation coefficients are close to zero (− 0.12 < rS < 0.12; p > 0.05) indicating that there is no evidence for a relationship between the frequencies of gastrointestinal helminths. For some pairs of taxa (see Fig. 4), there is a slight positive correlation (i.e. two taxa of helminths were more often observed together than in the case of random infection: the heavier the parasite infection in an NFS with one species, the greater chance this seal will be infected with another species). Significant negative correlations between the abundance of any helminth species were not found. The maximum positive correlation in our data was found for acanthocephalans C. similis and C. strumosum (rS = 0.37) and for nematodes C. osculatum, Pseudoterranova spp. and the tapeworm D. tetrapterum (rS is about 0.3). The correlation was slightly lower between these two groups; correlation for A. simplex and B. nipponicum was also significant (Table 4, Figs. 4 and 5). Therefore, the higher the abundance of one of these 7 gastrointestinal helminth species (see Fig. 5), the higher the chance to find another species in the same individual seal.
The correlations between the numbers of gastrointestinal helminths of different taxa in the infracommunity of northern fur seals Callorhinus ursinus on St. Paul Island, Alaska. Aoc—Anophryocephalus ochotensis, Apa—Adenocephalus pacificus, Asi—Anisakis simplex, Aza—Apophallus zalophi, Bni—Bolbosoma nipponicum, Cca—Corynosoma cameroni, Cos—Contracaecum osculatum, Cse—Corynosoma semerme, Csi—Corynosoma similis, Cst—Corynosoma strumosum, Cva—Corynosoma validum, Cvi—Corynosoma villosum, Dte— Diphyllobothrium tetrapterum, Gub—Galactosomum ubelakeri, Pcy—Phocascaris cystophorae, Pfu—Phocitrema fusiforme, Pse—Pseudoterranova spp. See text for the details
Hierarchical cluster analysis for correlation between numbers of different gastrointestinal helminths infecting northern fur seal Callorhinus ursinus specimens. Aoc—Anophryocephalus ochotensis, Apa—Adenocephalus pacificus, Asi—Anisakis simplex, Aza—Apophallus zalophi, Bni—Bolbosoma nipponicum, Cca—Corynosoma cameroni, Cos—Contracaecum osculatum, Cse—Corynosoma semerme, Csi—Corynosoma similis, Cst—Corynosoma strumosum, Cva—Corynosoma validum, Cvi—Corynosoma villosum, Dte— Diphyllobothrium tetrapterum, Gub—Galactosomum ubelakeri, Pcy—Phocascaris cystophorae, Pfu—Phocitrema fusiforme, Pse—Pseudoterranova spp.
Analysis of the relations between infections by separate gastrointestinal helminth species and their co-occurrence revealed the absence of significant correlations for a number of pairs of helminth species (Fig. 4). Co-infection of NFSs with acanthocephalans C. strumosum, C. similis and nematodes of the genus Pseudoterranova were the most common. These species were found together in 58 NFSs (8.9%).
Discussion
Our study presents the first comprehensive analysis of the gastrointestinal helminth community in NFSs based on examination of a large number of seals within four consecutive years. Before our project, only fragmented studies of different groups of parasites were carried out on St. Paul Island (Neiland 1961; Keyes 1965; Perry and Forrester 1971). The diversity of helminth species found in NFSs on the Pribilof Archipelago was considered to be low and amounted to only 12 species (see Yurakhno 1998). According to our data, at least 23 species of helminths (Tables 1 and 2) and 2 species of nasal mites parasitize NFSs on St. Paul’s Island. Four species (trematodes G. ubelakeri and N. salmincola, and the acanthocephalans C. alaskensis and C. cameroni) were newly documented in NFSs in our previous and present surveys (Kuzmina et al. 2012, 2018; present study). Moreover, the examination of numerous freshly collected well-fixed material with molecular data allowed us to synonymise two species of tapeworms, Diplogonoporus violettae Yurakhno 1986 and Diphyllobothrium (Diplogonoporus) tetrapterum (Kuzmina et al. 2015). Additionally, the collected material and molecular data allowed for the invalidation of all other diphyllobothriids reported in NFSs, with exception of A. pacificus (Kuzmina et al. 2015; Hernandez-Orts et al. 2018).
Ionita et al. (2008) misidentified acanthocephalan Corynosoma semerme collected from NFSs from St. Paul Island as C. obtuscens (now synonymised with C. australe). This was recently confirmed also by molecular methods (Lisitsyna et al. 2019). The validity is unclear of two trematode species, Apophallus callorhini and Cryptocotyle delamurei, described from a single seal from Bering Island, the Commander Archipelago, by Yurakhno (1986, 1987), as these parasites have not been reported since they were initially described. We hypothesize that the heterophyid A. callorhini could be synonymous with A. zalophi. The original description of A. callorhini was based on 13 exemplars. This species was distinguished from the congeners due to the larger size of its mouth, abdominal suckers, pharynx and spines which are not suitable discriminating characters for species of this group. Moreover, several metrical characters of A. zalophi reported in the original description of A. callorhini were incorrect (see Yurakhno 1986). The original description of opisthorchid C. delamurei was based on 25 specimens and was distinguished from other species of the genus Ciuraena (= Cryptocotyle) by a smaller length and width of the body, in addition to smaller oral and genital suckers, testes, ovary and eggs (Yurakhno 1987). The differentiation of C. delamurei and C. jejuna, both rarely reported from NFSs (see Table 1), was not performed. We examined several specimens of both species from the type series (Fig. 1), but their low quality did not allow us to confirm or synonymise these species. New material from the type locality is necessary for the final decision.
Ecological studies of NFS parasites have been carried out only on the Commander and Kuril Archipelagoes of the Russian North Pacific, where several hundred NFSs were examined and 14 helminth species were reported (Delyamure 1961; Yurakhno and Taikov 1986; Yurakhno 1998). However, our study allowed us to compare our data from the Pribilof Archipelago only with the data from the Commander Archipelago, in the Bering Sea (see fig. 1 in Kuzmina et al. 2015). The NFS populations from the Pribilof and Commander Archipelagos may partially overlap in the Bering Sea during feeding migrations (Sokolov 1998; Gentry and Kooyman 2014). Therefore, similar parasite fauna could be expected in both populations. A comparison of the parasite community structure of these two Archipelagos allowed the following conclusions.
The tapeworm A. pacificus was the most dominant helminth species in both populations ( P = 92.5% in Commander population and P = 97.2% in Pribilof population); D. tetrapterum was confirmed only in the Pribilof population. Onchoproteocephalidean larvae of Scolex pleuronectis were found in only 9.6% of the NFSs from the Commander Islands (Yurakhno 1998). Pseudoterranova spp. were the most common nematodes in both populations ( P = 70.12%, intensity 2.7 in the Commander population; P = 84.2% in the Pribilof population). Phocascaris cystophorae was more common in the Commander population ( P = 50.3%, intensity 2.6) than in the Pribilof population (Table 2). Moreover, C. osculatum was an extremely rare species in the Commander population ( P = 1.07%) in contrast to its prevalence in the Pribilof population ( P = 45.5%) (Table 2). Uncinaria lucasi was the most pathogenic parasite of NFS pups on the Pribilof Archipelago causing high pup mortality (Olsen 1958; Lyons 1963). However, its prevalence has decreased in recent decades from around 90% in 1978, to 6–10% in 1999 and 3–5% in 2007–2011 (Lyons et al. 2011, 2012, 2014). This species was found to be quite rare on the Commander Archipelago (prevalence < 1%) (Yurakhno 1998). We also documented the dramatic decline in the intensity of anisakid nematodes compared with the data collected on St. Paul Island 30–40 years ago (Keyes 1964; Spraker et al. 2003), which may be associated with changes in NFS diet as well as a decline in NFS populations in the North Pacific during last decades (Kuzmina et al. 2014; Gelatt et al. 2015).
Acanthocephalans were the most diverse group of helminths in this study with 7 species found. Half of NFSs were infected with acanthocephalans, mainly with C. strumosum (Table 2). In the Commander population, 5 species of acanthocephalans were found. Corynosoma villosum was the most common species (prevalence 58%), while C. strumosum was found only in 10.7% of seals (Yurakhno 1998). According to Delyamure (1955) and Delyamure et al. (1976), the main hosts of C. strumosum are spotted seals (Phoca largha) and bearded seals (Erignathus barbatus). During the last several decades, global climatic change and decreasing ice habitats in the North Pacific has led to a dramatic decline of spotted and bearded seal populations, especially near Alaskan shores (Boveng et al. 2009; Cameron et al. 2010). The decline in the population of spotted and bearded seals could lead to significant changes in the helminth community in NFSs, including acanthocephalans.
Trematodes also were a diverse group of helminths including four species in the Pribilof population; almost one third of NFSs were infected with Phocitrema fusiforme (Table 2). The Commander population harboured three species, P. fusiforme, A. callorhini and Ciureana delamurei (Table 1), with a low prevalence of 1.07% (Yurakhno 1998). Our present study was performed more than 30 years after the observations on the Commander population (Yurakhno and Taikov 1986; Yurakhno 1998) and several climatic and environmental factors including a decline of fish stock or decrease of NFS population mainly influenced the noted differences (Trites 1992; Towell et al. 2006; Sinclair et al. 2008; Allen and Angliss 2015; Gelatt et al. 2015).
In our study, tapeworms were the most prevalent group found in almost all NFSs examined (P = 98.5%), followed by nematodes (91.9%), acanthocephalans (47.3%) and trematodes (32.3%).
The two dominant taxa, tapeworm A. pacificus and nematodes Pseudoterranova spp., composed almost 61% of the total number of helminths, while the total proportion of the 10 rare and occasional species was less than 5%. It could be that these dominant gastrointestinal helminth species have a pathogenic influence on NFS health (Keyes 1965; Yurakhno and Taikov 1986), while the effects of rare and occasional species are less impactful. Still, the results of multi-year studies on NFS mortality did not reveal any significant effects of parasites and other pathogens compared with effects of starvation, emaciation and trauma (Spraker and Lander 2010).
We suggest that there is a common pattern for infections in NFSs with helminths, which varies from rookery to rookery and from year to year due to environmental factors. However, the effect of year and rookery on the distribution of helminth taxa was very small (Table 3). Absence of significant differences among different rookeries indicated that the gastrointestinal helminth community on St. Paul Island may be considered as a whole, even though the feeding grounds of NFSs from different rookeries are separated (Robson et al. 2004; Sterling and Ream 2004; Zeppelin and Ream 2006). The positive correlation (rS = 0.37) between the abundance of the acanthocephalans C. similis and C. strumosum may be explained by similarities in their life cycles and, consequently, simultaneous infection of NFSs with both species. Similarly, a positive correlation for the nematodes C. osculatum and Pseudoterranova spp. may be due to their similar transmission methods (Delyamure 1955; Yurakhno 1998). Interestingly, the occurrence and abundance of the cestode D. tetrapterum positively correlated with those of C. osculatum and Pseudoterranova spp. However, information on the life cycles and transmission of these parasites in the Bering Sea ecosystems is insufficient; several parasites may use the same fish species as their intermediate host. Complex parasitological studies of marine mammals and fish, as well as different groups of invertebrate intermediate hosts, are needed to explain these correlations.
References
Afanasev VP (1941) Parasite fauna of commercial mammals of the Commander Islands. Uchenye Zapiski LGU, Ser Biol 74:93–117 [In Russian]
Allen BM, Angliss RP (2015) Alaska marine mammal stock assessments, 2014. U.S. Dep. Commer., NOAA Tech. Memo. NMFSAFSC-301, 1–304. https://doi.org/10.7289/V5NS0RTS
Banks N (1910) New American mites (Arachnoidea; Acarina). Proc Ent Soc Wish 12:2–12
Benjamini Y, Yekutieli D (2001) The control of the false discovery rate in multiple testing under dependency. Ann Stat 29:1165–1188. https://doi.org/10.1214/aos/1013699998
Blagoveschenskij DI (1966) New forms of lice (Siphunculata) parasites of pinnipeds and hares. Entomol Obozr 45:806–813 [In Russian]
Boveng PL, Bengtson JL, Buckley TW, Cameron MF, Dahle SP, Kelly BP, Megrey BA, Overland JE, Williamson N J (2009) Status review of the spotted seal (Phoca largha). US Dep Commer, NOAA Tech Memo NMFS-AFSC-200:1–153
Bowman DD, Lynn RC (1995) Parasitology for veterinarians, sixth edn. W.B. Saunders Company, Philadelphia
Bush AO, Lafferty KD, Lotz JM, Shostak AW (1997) Parasitology meets ecology on its own terms: Margolis et al. revisited. J Parasitol 83:575–583. https://doi.org/10.2307/3284227
Cameron MF, Bengtson JL, Boveng PL, Jansen JK, Kelly BP, Dahle SP, Logerwell EA, Overland JE, Sabine CL, Waring GT, Wilder JM (2010) Status review of the bearded seal (Erignathus barbatus). US Dep Commer, NOAA Tech Memo NMFS-AFSC-211: 1–247
Chupakhina TI (1971) Helminth fauna of the fur seals from Tulenij island. Trudy Atlant NIRO 39:166–170 [In Russian]
Collyer ML, Adams DC (2018) RRPP: an R package for fitting linear models to high-dimensional data using residual randomization. Methods Ecol Evol 9:1772–1779 https://doi.org/10.1111/2041-210X.13029
Collyer ML, Adams DC (2019) RRPP: Linear Model Evaluation with Randomized Residuals in a Permutation Procedure. https://CRAN.R-project.org/package=RRPP
Dailey MD (1975) The distribution and intraspecific variation of helminth parasites in pinnipeds. Rapp P-V Reun Cons Int Explor Mer 169:338–352
Dailey MD, Brownell RL Jr (1972) A checklist of marine mammal parasites. In: Ridgway SH (ed) Mammals of the sea, biology and medicine, Charles C. Thomas, Springfield, IL
Delyamure SL (1955) Helminthofauna of marine mammals (ecology and phylogeny). Izdatel’stvo Akademii Nauk USSR, Moscow [In Russian]
Delyamure SL (1961) Ecological and geographical survey of helminth fauna of the northern fur seal. Helmintologia 3:73–79 [In Russian]
Delyamure SL, Skrjabin AS (1960) Helminthofauna of Commander northern fur seals. Nauchnye Doklady Vysshej Shkoly. Biol Nauk 1960:11–14 [in Russian]
Delyamure SL, Yurakhno MV, Popov VN (1976) On the helminth fauna of Bering Sea pinnipeds from the Karaginsk gulf. Parazitologiya (Leningrad) 10:325–332 [in Russian]
Doetschman WH (1944) A new species of endoparasitic mite of the family Halarachnidae (Acarina). Trans Am Microsc Soc 63:68–72
Ewing HE (1923) New genera and species of sucking lice. J Wash Acad Sci 13:146–149
Felix JR (2013) Reported Incidences of Parasitic Infections in Marine Mammals from 1892 to 1978. Zea E-Books Book, p 20
Ferris GF (1934) Contributions toward a monograph of the sucking lice. Vol. 2. Part VII. Stanford Univ Publ Univ Ser BioI Sci 2:471–526
Ferris GF (1951) The sucking lice. Mem Pac Coast Ent Soc 1:1–320
Fisher WK (1952) The status of the harbour seal in British Columbia, with particular reference to the Skeena River. Fish Res Bd Can Bull 93:1–58
Gelatt T, Ream R, Johnson D (2015) Callorhinus ursinus. The IUCN red list of threatened species 2015: e.T3590A45224953. https://doi.org/10.2305/IUCN.UK.2015-4.RLTS.T3590A45224953.en
Gentry RL, Kooyman GL (2014) Fur seals: maternal strategies on land and at sea, Course book edn. Princeton University Press, Princeton
Gerber JA, Roletto J, Morgan LE, Smith DM, Gage LJ (1993) Findings in pinnipeds stranded along the central and northern California coast, 1984–1990. J Wildl Dis 29:423–433. https://doi.org/10.7589/0090-3558-29.3.423
Hernández-Orts JS, Scholz T, Brabec J, Kuzmina T, Kuchta R (2015) High morphological plasticity and global geographical distribution of the Pacific broad tapeworm Adenocephalus pacificus (syn. Diphyllobothrium pacificum): molecular and morphological survey. Acta Trop 149:168–178. https://doi.org/10.1016/j.actatropica.2015.05.017
Hernandez-Orts J, Scholz T, Brabec J, Kuzmina T, Kuchta R (2018) Does the number of genital organs matter? Case of the seal tapeworm Diphyllobothrium (syn. Diplogonoporus) tetrapterum (Cestoda: Diphyllobothriidea). Can J Zool 96:193–204. https://doi.org/10.1139/cjz-2017-0013
Ionita M, Varela MG, Lyons ET, Spraker TR, Tolliver SC (2008) Hookworms (Uncinaria lucasi) and acanthocephalans (Corynosoma spp. and Bolbosoma spp.) found in dead northern fur seals (Callorhinus ursinus) on St. Paul Island, Alaska in 2007. Parasitol Res 103:1025–1029. https://doi.org/10.1007/s00436-008-1087-0
Jellison WL (1952) Anoplura from mammals of the Pribilof Islands. J Parasitol 38:274–275
Jensen K, Bullard SA (2010) Characterization of a diversity of tetraphyllidean and rhinebothriidean cestode larval types, with comments on host associations and life-cycles. Int J Parasitol 40(8):889–910. https://doi.org/10.1016/j.ijpara.2009.11.015
Kagei N, Oda T (1975) Dipetalonema odendhali Perry, 1967 found from Fur seal, Callorhinus ursinus (Linnaeus, 1758). Bull Inst Publ Health 24:203–205 [in Japanese]
Keyes MC (1964) Research in fur seal mortality, St. Paul Island, Alaska. 8 July to 24 September 1963. US Dep inter, US FishWildl Serv, Mar Mamm Biol Lab, Seattle, Washington
Keyes MC (1965) Pathology of the northern fur seal. J Amer Vet Med Assoc 147:1090–1095
Kim, Chung K (1972) Louse populations of the northern fur seal (Callorhinus ursinus). Am J Vet Res 33:2027–2036
Kovalenko LM (1975) Helminth fauna of eared seals inhabiting the Kurile Islands. In: marine mammals, part I. Kiev; Naukova Dumka. [In Russian]
Krotov AI, Delyamure SL (1952) Some aspects of the parasitic worm fauna of mammals and birds of the URSS. Tr Gelmintol Lab 6:278–292 [In Russian]
Kuzmina TA, Lisitsyna OI, Lyons ET, Spraker TR, Tolliver SC (2012) Acanthocephalans in northern fur seals (Callorhinus ursinus) and a harbor seal (Phoca vitulina) on St. Paul Island, Alaska: species, prevalence, and biodiversity in four fur seal subpopulations. Parasitol Res 111:1049–1058. https://doi.org/10.1007/s00436-012-2930-x
Kuzmina TA, Kuzmin YI, Tkach VV, Spraker TR, Lyons ET (2013) Ecological, morphological, and molecular studies of Acanthocheilonema odendhali (Nematoda: Filarioidea) in northern fur seals (Callorhinus ursinus) on St. Paul Island, Alaska. Parasitol Res 112:3091–3100. https://doi.org/10.1007/s00436-013-3483-3
Kuzmina TA, Lyons ET, Spraker TR (2014) Anisakids (Nematoda: Anisakidae) from stomachs of northern fur seals (Callorhinus ursinus) on St. Paul Island, Alaska: parasitological and pathological analysis. Parasitol Res 113:4463–4470. https://doi.org/10.1007/s00436-014-4131-2
Kuzmina TA, Hernández-Orts JS, Lyons ET, Spraker TR, Kornyushyn VV, Kuchta R (2015) The cestode community in northern fur seals (Callorhinus ursinus) on St. Paul Island, Alaska. Int J Parasitol Parasites Wildl 4(2):256–263. https://doi.org/10.1016/j.ijppaw.2015.04.007
Kuzmina TA, Tkach VV, Spraker TR, Lyons ET, Kudlai O (2018) Digeneans of northern fur seals Callorhinus ursinus (Pinnipedia: Otariidae) from five subpopulations on St. Paul Island, Alaska. Parasitol Res 117:1079–1086. https://doi.org/10.1007/s00436-018-5784-z
Lisitsyna OI, Kudlai O, Spraker TR, Tkach VV, Smales LR, Kuzmina TA (2019) Morphological and molecular evidence for synonymy of Corynosoma obtuscens Lincicome, 1943 with Corynosoma australe Johnston, 1937 (Acanthocephala: Polymorphidae). Syst Parasitol 96:95–110. https://doi.org/10.1007/s11230-018-9830-0
Lyons ET (1963) Biology of the hookworm Uncinaria lucasi stiles, 1901, in the northern fur seal Callorhinus ursinus Linn. On the Pribilof Islands, Alaska: PhD dissertation. Colorado State University
Lyons ET, Spraker TR, Olson KD, Tolliver SC, Bair HD (2000) Prevalence of hookworms (Uncinaria lucasi Stiles) in northern fur seal (Callorhinus ursinus Linnaeus) pups on St. Paul Island, Alaska, USA: 1986–1999. Comp Parasitol 67:218–223
Lyons ET, Melin SR, Nadler SA, Tolliver SC (2011) Review of research on hookworms (Uncinaria lucasi Stiles, 1901) in northern fur seals (Callorhinus ursinus Linnaeus, 1758). Parasitol Res 109:257–265. https://doi.org/10.1007/s00436-011-2420-6
Lyons ET, Kuzmina TA, Tolliver SC, Spraker TR (2012) Update on the prevalence of the hookworm, Uncinaria lucasi, in northern fur seals (Callorhinus ursinus) on St. Paul Island, Alaska, 2011. Parasitol Res 111:1397–1400. https://doi.org/10.1007/s00436-012-2881-2
Lyons ET, Kuzmina TA, Carie JL, Tolliver SC, Spraker TR (2014) Prevalence of hookworms, Uncinaria lucasi (Ancylostomatidae) in northern fur seals (Callorhinus ursinus) on St. Paul Island, Alaska. Vest Zool 48:221–230. https://doi.org/10.2478/vzoo-2014-0025
Machida M (1969) Parasites of the northern fur seal and their relationships to the breeding islands. Proc Jap Soc Syst Zool 5:16–17 [In Japanese]
Machida M (1977) Two species of Dipetalonema from pinnipeds caught off northern Japan. Bull Nat Sci Mus Tokyo Ser A Zool 3:67–71
Margolis L (1956) Parasitic helminths and arthropods from Pinnipedia of the Canadian Pacific coast. J Fish Res Bd Canada 13:489–505
Margolis L, Dailey MD (1972) Revised annotated list of parasites from sea mammals caught off the west coast of North America. NOAA technical report NMFS, SSRF-647:1–23. https://doi.org/10.5962/bhl.part.23513
Mattiucci S, Nascetti G (2008) Advances and trends in the molecular systematics of anisakid nematodes, with implications for their evolutionary ecology and host–parasite co-evolutionary processes. Adv Parasitol 66:47–148. https://doi.org/10.1016/S0065-308X(08)00202-9
Mattiucci S, Cipriani P, Webb SC, Paoletti M, Marcer F, Bellisario B, Gibson DI, Nascetti G (2014) Genetic and morphological approaches distinguish the three sibling species of the Anisakis simplex species complex, with a species designation as Anisakis berlandi n. sp. for A. simplex sp. C (Nematoda: Anisakidae). J Parasitol 100:199–214. https://doi.org/10.1645/12-120.1
Mattiucci S, Cipriani P, Levsen A, Paoletti M, Nascetti G (2018) Molecular epidemiology of Anisakis and anisakiasis: an ecological and evolutionary road map. Adv Parasitol 99:93–263. https://doi.org/10.1016/bs.apar.2017.12.001
Miller FH Jr (1971) Scanning electron microscopy of Echinophthirius horridus (von Olfers), Antarctophthirus callorhini (Osborn), and Proechinophthirius fluctus (Ferris) with emphasis on the antennal structures (Anoplura: Echinophthiriidae). J Parasitol 57:668–674. https://doi.org/10.2307/3277937
Nagasawa K (1999) Parasites of pinniped (Mammalia: Carnivora) in Japan: checklist and bibliography. Bull Nat Res Inst Far Seas Fish 36:27–32 [In Japanese]
National Marine Fisheries Service (2007) Conservation plan for the eastern Pacific stock of northern fur seal (Callorhinus ursinus). NMFS, Juneau, AK
Neiland KA (1961) Suspected role of parasites in non-rookery mortality of fur seals (Callorhinus ursinus). J Parasitol 47:5. https://doi.org/10.2307/3275461
Neiland KA (1962) Alaskan species of acanthocephalan genus Corynosoma Lühe, 1904. J Parasitol 48:69–75. https://doi.org/10.2307/3275415
Nikolskij OR (1969) On the parasite fauna of fur seals in a pelagic period of their life. In: Parazitologii P (ed) Proceedings of the 6th Scientific Conference of Parasitologists of the USSR, part 1. Naukova Dumka, Kiev, pp 179–184 [In Russian]
Olsen OW (1958) Hookworm Uncinaria lucasi Stiles, 1901, in fur seals Callorhinus ursinus (Linn.), on the Pribilof Islands. Trans 23rd N am Wildl Conf (3–5 march 1958). Wildlife management institute, Washington DC, pp 152–175
Olsen OW, Lyons ET (1962) Life cycle of the hookworm, Uncinaria lucasi Stiles, of northern fur seals, Callorhinus ursinus on the Pribilof Islands in the Bering Sea. J Parasitol 48(Suppl):42–43
Osborn H (1899) Acarina. In: The fur seals and the Fur-Seal Islands of the North Pacific Ocean. Part 3. U.S. Treasury Department, Washington, 553–554
Oudemans AC (1926) Halarachne-Studien. Arch Naturgesch Abt A 91:48–108
Perry ML, Forrester DJ (1971) Dipetalonema odendhali (Nematoda: Filarioidea) from the northern fur seal, with a description of the microfilaria. J Parasitol 57:469–472
Petrochenko VI (1958) Acanthocephala of domestic and wild animals, vol II. Akademiya Nauk SSSR, Moscow [In Russian]
R Core Team (2020) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria URL https://www.R-project.org/
Robson BR, Goebel ME, Baker JD, Ream RR, Loughlin TR, Francis RC, Antonelis GA, Costa DP (2004) Separation of foraging habitat among breeding sites of a colonial marine predator, the northern fur seal (Callorhinus ursinus). Can J Zool 82:20–29. https://doi.org/10.1139/z03-208
Scheffer VB, Fiscus CH, Todd EI (1984) History of scientific study and Management of the Alaskan Fur Seal, Callorhinus ursinus, 1786–1964. NOAA Tech Rep NMFS SSRF-780
Scott TL, Yano KM, Baker JD, Rickey MH, Eames M, Fowler CW (2006) The northern fur seal (Callorhinus ursinus): A bibliography. AFSC Processed Rep. 2006–05; 1–246
Sinclair EH, Vlietstra LS, Johnson DS, Zeppelin TK, Byrd GV, Springer AM, Ream RR, Jr GL H (2008) Patterns in prey use among fur seals and seabirds in the Pribilof Islands. Deep-Sea Res II 55:1897–1918
Skrjabin AS, Yurakhno MV (1972) Study of the nematoda Terranova azarazi (Yamaguti et Arima, 1942) A. Skrjabin, 1958. Parasites, parasitoses and ways of their eradication. Kyiv. Naukova Dumka 1:164–167 [In Russian]
Sokolov VE, Aristov AA, Lisitzina TU (1998) The northern fur seal. Systematic, Morphology, Ecology, Behaviour, Nauka, Moscow [In Russian]
Spraker TR, Lander ME (2010) Causes of mortality in northern fur seals (Callorhinus ursinus), St. Paul Island, Pribilof Islands, Alaska, 1986–2006. J Wildl Dis 46:450–473. https://doi.org/10.7589/0090-3558-46.2.450
Spraker TR, Lyons ET, Tolliver SC, Bair HD (2003) Ascaridoid nematodes and associated lesions in stomachs of subadult male northern fur seals (Callorhinus ursinus) on St. Paul Island, Alaska: (1987–1999). J Vet Diagn Investig 15:432–437. https://doi.org/10.1177/104063870301500505
Sterling JT, Ream RR (2004) At-sea behavior of juvenile male northern fur seals (Callorhinus ursinus). Can J Zool 82:1621–1637. https://doi.org/10.1139/z04-136
Stiles CW (1901) Uncinariasis (Ancylostomiasis) in man and animals in the United States. Tex Med News 10:523–532
Stiles CW, Hassall A (1899) Internal parasites of the fur seal. In: the fur seals and fur seal islands of the North Pacific Ocean (David Starr Jordan Report 1899), part 3. U.S. Treasury Department, Washington, DC, pp 99–177
Tatonova YV, Besprozvannykh VV (2020) Description of a new species, Cryptocotyle lata sp. nov., and discussion of the phylogenetic relationships in Opisthorchioidea. Parasitol Int 73: [in press]. https://doi.org/10.1016/j.parint.2019.101939
Timofeeva AA, Lvov DK, Gromov AI, Baturova RA, Evseeva TI, Chupakhina TI, Scherbina RD, Pogrebenko AG (1972) Complex foci of infection on the Tulenij Island in the Okhotsk Sea. Zool Zh 51:932–935 [In Russian]
Towell RG, Ream RR, York AE (2006) Decline in northern fur seal (Callorhinus ursinus) pup production on the Pribilof Islands. Mar Mam Sci 22:486–491. https://doi.org/10.1111/j.1748-7692.2006.00026.x
Trites AW (1992) Northern fur seals: why have they declined? Aquat Mamm 18:3–18
Van Cleave HJ (1953) Apreliminary analysis of the acanthocephalan genus Corynosoma in mammals of North America. J Parasitol 39:1–13
Wei T, Simko W (2017) R package "corrplot": Visualization of a Correlation Matrix (Version 0.84). Available from https://github.com/taiyun/corrplot
Yamaguti S (1951) Studies on the helminth fauna of Japan: part 47. Cestodes of marine mammals and birds. Acta Med Okayama 7:307–316
Yurakhno MV (1986) Pricetrema callorhini sp. n. (Trematoda, Heterophyidae) a parasite of fur seal. Parazitologia 20:317–320 [In Russian]
Yurakhno MV (1987) Ciureana delamurei sp. n. (Trematoda, Heterophyidae) a parasite of northern fur seal. Parazitologia 21:752–754 [In Russian]
Yurakhno MV (1998) Diseases and parasites. In: Sokolov VE, Aristov AA, Lisitzina TU (eds) The northern fur seal. Systematic, morphology, ecology, behaviour, pp 810–899 Nauka, Moscow, [In Russian]
Yurakhno MV, Taikov IM (1986) Some preliminary results of parasitological autopsies of commander seals in 1984–1985. In: study, protection and management of marine mammals. Proceedings of the 9th All-Union Conference, Arkhangelsk, pp 435–436. [In Russian]
Zeppelin TK, Ream RR (2006) Foraging habitats based on the diet of female northern fur seals (Callorhinus ursinus) on the Pribilof Islands, Alaska. J Zool 270:565–576. https://doi.org/10.1111/j.1469-7998.2006.00122.x
Acknowledgements
We thank the people of the Aleut community, who allowed us to collect the stomachs and intestines from the harvested NFS males. We thank the National Marine Mammal Laboratory scientists for allowing us the use of their laboratory for collection of the parasites from the digestive system of the seals. We thank the anonymous reviewers for criticisms and valuable suggestions. We thank Sam McGuffin for reviewing this manuscript. This work was performed under the authority of the Marine Mammal Protection Act Permit Number 14327 issued to the National Marine Mammal Laboratory.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Section Editor: Elizabeth Marie Warburton
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kuzmina, T.A., Kuzmin, Y., Dzeverin, I. et al. Review of metazoan parasites of the northern fur seal (Callorhinus ursinus) and the analysis of the gastrointestinal helminth community of the population on St. Paul Island, Alaska. Parasitol Res 120, 117–132 (2021). https://doi.org/10.1007/s00436-020-06935-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-020-06935-6