Abstract
As a result of the experimental infection of rats with metacercariae of Paragonimus heterotremus Chen et Hsia, 1964 from crabs (Potamiscus tannanti) caught in Yen Bai province, Vietnam, it was found that worms migrated into the lungs, to the liver and less frequently to the tissue that lines body cavities of the hosts, where they reached the adult stage, but in the muscles, worms stayed at the larval stage. Studies have shown that for P. heterotremus, rats can simultaneously play the role of the final and paratenic host; herewith, an infection with the trematode of this species can lead to the development of three forms of paragonimiasis: pulmonary, hepatic and muscular. Eggs from the adult worms localised in the liver, unlike eggs from the adult worms localised in the lungs, were not excreted into the external environment, but accumulated inside the organ. Histology and description of changes, which take place on the external surface of organs affected with P. heterotremus, are given in this study. Based on the behavioural characteristics of worms during rat infection and molecular genetic data, we established that worms from Vietnam and India should be assigned to different species of Paragonimus. P. heterotremus distribution is limited to the territory of the Southeast China, Northern Vietnam, Laos and Thailand.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Flukes belonging to the genus Paragonimus Dollfus, 1939 are the causative agents of one of the most serious parasitic diseases, which lead to the loss of human work ability and, in some cases, death. Representatives of the genus are found in North, Centre and South America, Africa, the southeastern part of Eurasia and also the Pacific Islands (Blair et al. 1999a). The genus includes more than 50 species, the vast majority of which inhabits the territory of East, South and Southeast Asia (more than 40 species) (Singh et al. 2012, Kong et al. 2015). A sufficiently wide spectrum of prosobranch snails from five superfamilies (first intermediate host), as well as various crayfish and freshwater crabs (second intermediate host), participates in the life cycles of Paragonimus. Furthermore, it was indicated that the parasite’s circulation involves paratenic hosts mainly mammals (rodents, boars and others). The participation of paratenic hosts in the life cycles of species, i.e. Paragonimus westermani (Kerbert 1878), Paragonimus kellicotti Ward, 1908, Paragonimus skrjabini Chen, 1959, Paragonimus miyazakii Kamo, Nishida, Hatsushika et Tomimura, 1961, Paragonimus heterotremus Chen et Hsia, 1964 and Paragonimus mexicanus Miyazaki et Ishii, 1968, was experimentally confirmed (Blair et al. 1999a). In such hosts, fluke larvae are predominantly localised in the muscles and rarely in the other organs and tissues. The most complete information on the behaviour of paragonimus during mammalian infections was obtained for P. westermani ichunensis Chung, Hsu et Kao, 1978. A series of P. westermani ichunensis experiments showed that animals, such as rats and boars, can play the role of both paratenic and definitive hosts (Besprozvannykh 1994, 2002, 2004). Pulmonary (adult worms) and muscle (at the larval stage) localisation of the parasites was found in rats and boars, both when infected with P. westermani ichunensis metacercariae and muscle larvae from the paratenic hosts. In the first case of the P. westermani ichunensis metacercariae infection, only a few individuals of the parasite reached the lungs, and the rest were localised in the muscles. In the second case of the infection with muscle larvae from the paratenic hosts, during growth, the larvae increased in size by 1.5–2 times compared to metacercariae that penetrated the lungs, and the rest migrated again to the muscles (Besprozvannykh 1994, 2002, 2004). The definitive host spectrum of the Paragonimus species is wide and involves mammals whose nutrition includes malacostracans and potential paratenic hosts of the parasite (Kurochkin 1987; Blair et al. 1999a). According to various sources, from 7 to 12 species and subspecies of Paragonimus infect people with cases of both pulmonary and larval paragonimiasis (Miyazaki and Habe 1976; Miyazaki et al. 1978; Kurochkin 1987; Sykhanova and Kaminsky 1998; Singh et al. 2012; Kong et al. 2015).
Paragonimus heterotremus is known as one of the human paragonimiasis causative agents (Kurochkin 1987; Blair et al. 1999a; Narain et al. 2003; Devi et al. 2007; Doanh et al. 2011). According to results based either on the morphological characteristics of the worms in each developmental stage or on molecular genetic data, this species was registered in China, Thailand, Laos, Vietnam and India (Hu 1998; Yan et al. 1998; Blair et al. 1999a; Devi et al. 2010; Singh et al. 2012).
The life cycle of parasites was completed experimentally for the first time in China (Hu 1998). It was established that snails Tricula performs the role of the first intermediate host, and Somanniathelphusa crabs are utilised as the second intermediate host. Adult worms were grown in cats and rats infected with experimentally obtained metacercariae. In both cases, adult flukes were located in the lungs (Hu 1998). Other studies on worms designated as P. heterotremus demonstrated differences in their localisation in rats, which were infected by metacercariae from naturally infected crabs from China, India and Vietnam (Yan et al. 1998; Singh et al. 2011; Doanh et al. 2015a). Genetic data for the trematodes in these studies were not obtained.
About the first results of genetic studies for P. heterotremus from Thailand, using internal transcribed spacer 2 (ITS2) region of ribosomal DNA (AF159603) and the cox1 gene of mitochondrial DNA (AF159597) was reported by Blair et al. (1997, 1998, 1999b), but in any of those publications morphometric characterisation of the studied worms was not presented. Unfortunately, exactly these molecular data formed the basis for the subsequent systematic definitions of the species as P. heterotremus and phylogenetic reconstructions of the genus. Results of the molecular genetic analysis (simultaneously including ITS2 (DQ836248) and 28S rRNA gene (DQ836249)) for P. heterotremus were obtained using DNA extracted from eggs found in human saliva in India (Devi et al. 2007). The affiliation of the eggs to P. heterotremus species was determined based on the ITS2 sequence (DQ836248) (Devi et al. 2007). P. heterotremus formed a cluster on the phylogenetic tree with worms from Thailand (AF159603) (Blair et al. 1999b) and China (AY618758) (Chang et al. 2000). Subsequently, in 2010, Devi et al. published new partial 28S sequences of paragonimus species from China (HM172617) and India (HM172615) (no information concerning the morphology of worms is available), and these species where determined as P. heterotremus according to joint clustering with trematode from India, for which 28S data (DQ836249) were obtained earlier (Devi et al. 2007). Nevertheless, despite the clearly existing differences between the Chinese and Indian trematodes showed by both the ITS2 and 28S genetic markers, the affiliation of worms to one species P. heterotremus was suggested (Devi et al. 2007, 2010). All now available molecular data were obtained using DNA extracted from eggs and metacercariae, whose subsequent affiliation to P. heterotremus species was based only on the comparative analysis of ITS2 sequences (Doanh et al. 2013; Athokpam and Tandon 2015) with analogous data from GenBank (Blair et al. 1999b; Devi et al. 2007, 2010).
In order to establish the taxonomic status of trematodes from East Asia and India defined as P. heterotremus as well as identify the behaviour characteristics of P. heterotremus during infection, experimental infection of rats with metacercariae morphometrically similar to those of P. heterotremus from Vietnamese crabs was performed. For the experimentally grown worms, molecular genetic data were obtained using 28S and ITS2 rRNA gene sequences and additionally secondary structure models of the ITS2 transcripts were reconstructed.
Materials and methods
Parasite material
Metacercariae, morphologically similar to those of P. heterotremus (Fig. 1a), were obtained from freshwater crabs, Potamiscus tannanti (Rathbun 1904), collected in Luc Yen district, Yen Bai province, Vietnam (22°6′42″N 104°43′29″E). In total 149 crabs were dissected, the intensity of invasion in 4 individuals was more than 100 metacercariae per crab and, in 12 individuals, it varied between 30 and 40 metacercariae, in 4 crabs, 7–10 metacercariae. In another 20 crabs, the intensity of invasion was 1 to 2 metacercaria. The remaining 109 crabs were free from Paragonimus metacercariae. Metacercariae (85 in each bread ball) were given orally to 8 individuals of Rattus norvegicus (2 Wistar albino rats and 6 decorative ones). To establish the potential time when worms attain sexual maturity, 30 days after the beginning of the experiment, periodically every 5 days, the rat faeces were examined for the presence of paragonimid eggs. Rats were dissected at various intervals from 17 to 198 days from the moment they began infecting. Seven rats were dissected to identify the precise localisation of worms, their collection and fixation, and one rat was dissected to study the pathological changes in the organs inhabited by the Paragonimus. Worms were fixed in 70% and then stored in 96% ethanol. Whole-mounts for adult descriptions were made by staining the specimens with alum carmine, dehydrating the worms in a graded ethanol series and clearing in clove oil, followed by mounting in Canada balsam under a coverslip on a slide. For measurements, worms of the minimum and maximum sizes were used. All measurements are in millimetres (mm).
To perform histopathological studies, haematoxylin and eosin-stained histological sections of the lungs and liver affected with worms were made. All applicable international, national and/or institutional guidelines for the care and use of animals were followed. Euthanasia of laboratory animals was carried out in accordance with the Committee on the Ethics of Animal Experiments of the Federal Scientific Centre of the East Asia Terrestrial Biodiversity, Russia.
DNA extraction, amplification and sequencing
Genomic DNA was extracted, using the HotSHOT technique, from 35 worms: 18 muscle larvae, 11 adult worms infecting the liver, 6 individuals infecting lungs, obtained from rats during the period of 17–198 days since they had been infected (Truett et al. 2000). The classical PCR amplification of the full-length ITS2 sequences and 28S rDNA sequences was performed using the following primers: forward 3S (5′-CGCACATTAAGTCGTG-3′) together with reverse primer BD2 (5′-ATCTAGACCGGACTAGGCTGTG-3′) (Bowles and McManus 1993) and forward primer Dig12 (5′-AAGCATATCACTAAGCGG-3′), together with the reverse primer 1500R (5′-GCT ATC CTG AGG GAA ACT TCG-3′) (Tkach et al. 2003), respectively. The initial PCR reaction was carried out in a total volume of 20 μL. Each reaction contained 0.25 mM of each primer pair, combined with 4 μL of aqueous solution of DNA, DreamTaq buffer (including KCl and (NH4)2SO4 and 20 mМ MgCl2), 1.25 mM dNTP and 1 unit of DreamTaq DNA Polymerase. The amplification protocol for the ITS2 region was performed under the following conditions: initiation at 95 °C for 3 min, then 35 cycles including denaturation at 95 °C for 1 min, annealing at 55 °C for 1 min, extension at 72 °C for 1 min 30 s and a final cycle of 7 min at 72 °C, and for the 28S rDNA fragment the conditions were denaturation hold at 95 °C, 30 cycles of 30 s at 95 °C, 30 s at 54 °C, 2 min at 72 °C and 7 min extension hold at 72 °C. Each PCR reaction included negative and positive controls, using both primers. PCR products were enzymatically cleaned up with ExoSAP-IT PCR Product Cleanup Reagent from Thermo Scientific and then sequenced on an ABI 3130 Genetic Analyzer using the ABI BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems) (as instructed by the manufacturer) and internal sequencing primers: 300F (5′-AGGGTTCGATTCCGGAG-3′), 1200R (5′-GGGCATCACAGACCTG-3′), 900F (5′-CCGTCTTGAAACACGGACCAAG-3′), 1200F (5′-CCCGAAAGATGGTGAACTATGC-3′) described in Lockyer et al. (2003) for the 28S rDNA fragment. The sequencing primers were the same as those used for PCR of the ITS2 region. Contiguous sequences were assembled using MEGA 6.0 (Tamura et al. 2013) and submitted to GenBank under accession numbers (MK806548-MK806572 and MK828935-MK828940 for the ITS2 region; MK817531-MK817556 and MK828941-MK828944 for 28S rDNA).
Alignments and phylogenetic analysis
Sequences of worms obtained in this study (see above), and deposited in GenBank, and sequences of closely related taxa from GenBank as well (Supplementary Table 1), were aligned using the Clustal W program (Thompson et al. 1994). To clarify the phylogenetic relationships of the studied species with the other representatives of the genus Paragonimus, full-length sequences of the ITS2 region (including the portion of 5.8S rRNA gene (436 bp in length in total)) and partial sequences of the 28S rRNA gene of nuclear ribosomal DNA (1204 bp in length) were used.
Genetic divergence was estimated using genetic p-distance values, which were calculated by including all substitution types (Tajima 1983; Nei 1987), and phylogenetic relationships among taxa using the maximum likelihood (ML) method, with branch support values estimated by 1000 bootstrap replicates that were obtained using MEGA 6.0 software. Bayesian inference (BI) was obtained using MrBayes v. 3.1.2 software with the following nucleotide substitution parameters: nst = 6, rates = gamma (Huelsenbeck and Ronquist 2001). The MCMC algorithm was performed using 10.000.000 generations and two independent runs, where 25% of generations were discarded as burn-in. Modeltest 3.7 software (Posada and Crandall 1998) was used to select the nucleotide substitution model; TPM3+G and TPM3uf+G for ITS2 matrix and 28S, respectively (Huelsenbeck and Ronquist 2001). The resulting trees were rooted with the outgroup taxa (Haplorchis pumilio HM004191 and Fasciola hepatica AM709499).
Secondary structure prediction for ITS2 sequences
The individual secondary structures of the ITS2 were computed using RNA structure software package for “RNA secondary structure prediction and analysis” (Bellaousov et al. 2013). The web server offers RNA secondary structure prediction, including free energy minimization with maximum expected accuracy. The server creates an annotated group of secondary structures, starting with the maximum free energy (MFE) structure and including others with varied probabilities of correctness. Other structures are included because the lowest free energy structure may not be the correct one. RNA was folded at a fixed temperature of 37 °C, and the chosen structure had branching structures homologous to those previously published for digeneans (Michot et al. 1993; Morgan and Blair 1998; Voronova et al. 2017; Voronova and Chelomina 2018). Individual structure drawings were rendered as postscript, then generalised and processed in CorelDRAW Graphics Suite X8.
Results
Description
Paragonimus heterotremus Chen et Hsia, 1964
Host: Rattus norvegicus (Berkenhout, 1769) (experimentally).
Site: lungs, liver, rarely tissue adjacent to abdominal cavity.
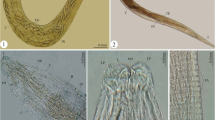
Adult worm (Table 1, Fig. 1b).
Body oval with singly arranged cuticular spines. Oral sucker terminal, round. Prepharynx absent, pharynx transversale oval, oesophagus absent or short. Caeca wide, curved, extending posterior end of body. Ventral sucker considerably less than oral sucker, round at middle third of body. Two testes, elongated, branched, symmetrical in middle of hindbody. Ovary branched, on right at level of ventral sucker. Uterus compact on left at level of ventral sucker. Eggs 0.073–0.089 × 0.039–0.050, oval, numerous, operculated, with aboral thickening and knob, unembryonated. Vitelline glands well developed, widely distribute on both sides of the body. Excretory vesicle I-shaped.
Remarks
The ovary and testes of different individuals do not have a constant form and number of branches. The shape of eggs varies as well, from oval to teardrop with narrowed anterior or posterior ends (Fig. 1c).
The results of the experiment
The experimental results showed that on day 17 after rats were infected (Table 2), worms at the larval stage were found only in the intercostal muscles of the thoracic spine region, in the muscles of the lumbar spine and in the muscles of the extremities.
Within 17 days, the worms significantly increased in size relative to metacercariae (Table 3). However, the growth rate of individual worms differed. Some of the worms, in comparison with metacercariae, increased in size by no more than 3 times, while others increased in size by 5–7 times (Table 3, Fig. 1d, e). In the largest individuals, the formation of the ovary and testes became visible. The further fate of different-sized individuals was different. The largest worms from muscles migrated to the tissue of their final localisation, mainly in the lungs and liver of definitive host, where their growth and development were continued; small worms stayed in the muscles (Table 3).
By 30 days from the beginning of the experiment, parasites with body sizes ranging from 1.86 to 3.53 mm (Fig. 1f) were found in the liver, and individuals whose body size did not exceed 0.80 mm stayed in the muscles. On day 30, in the dissected rat, most of the P. heterotremus worms were localised in the liver and muscles. Three individuals morphometrically similar to worms from the liver were located in the body cavity (Table 2). In the period from 30 to 60 days after infection, the formation of capsules occurred in the lungs and liver inhabited by the worms. On day 60, most of the parasites reached sexual maturity (eggs were found in the faeces of rats on 60–64 days from the moment of their infection) (Table 2). After the final localisation, the growth of flukes continued. At the same time, during the growth of individual trematodes, the initial differences (identified on day 17 in the worms obtained from the muscle tissue) were preserved. By day 198, the largest individuals had increased in body size by two times that of the small individuals (Table 1). Studies have shown that among the 60-day flukes, and flukes found in rats in the period up to 198 days, along with individuals producing eggs, there were worms without eggs in the uterus (Table 1). Such individuals, metrically, were similar with worms with the egg-filled uterus. In rats that were dissected in the period from 60–198 days, in addition to worms that penetrated the lungs or liver, the muscle larvae were detected (Table 2). On day 75, their sizes were similar with those of larger specimens of 17 daily worms from the rats’ muscles (Table 3). To sum up the results of the experiment, we stated that when feeding animals with metacercariae of paragonimus morphometrically similar to metacercariae of P. heterotremus, worms are localised at the sexually mature stage in the lungs, liver and body cavity, and at the larval stage, in the muscles. In Wistar rats, Paragonimus worms were equally distributed between the lungs and the liver. A total of 15 worms were found in both organs. In decorative rats, most of the worms were localised in the liver, and one rat had worms only in the liver. In one case, four adult worms were localised in the soft tissues surrounding the kidney (Table 2). Flukes usually reside in pairs. Moreover, all rats, including one dissected on the 198th day, had larvae in their muscles. Beginning from 60 to 64 days, all rats, except for one, had eggs in their faeces. Eggs in the faecal material were not detected only in rat with hepatic localisation of adult P. heterotremus; in other words, there was no egg excretion from the liver (Table 2).
Changes which take place on the external surface of organs affected with Paragonimus
Adult worms were localised in thick-walled, dense fibrous capsules in the liver and lungs. Capsules were filled with pressurised fluid; puncturing the capsule leads to a sharp ejection of its contents.
On day 30, traces of paragonimid movements left in the surface liver tissues and weakly formed capsules were observed (Fig. 2(a, b)). On day 60, and the next days, large nodes, often greyish and blackish shades, rising above the surface of the liver were found (Fig. 2(c)). Unions of the liver lobes, as well as stomach and diaphragm with the liver, were observed.
Organs and tissues of the rat infected with Paragonimus heterotremus. (a) Traces of worms’ movements left on the surface of the liver; (b) formed capsules in the liver; (c) capsules with worms in the liver; (d) capsules with worms in the lung; (e) tissue necrosis of the lung; (f) muscle larvae; (g) hyperaemia around the worms along their migration pathways; (h) heart
In the lungs, 60 days after rats were infected, the fibrous capsules presented a sacculated greyish appearance, and there were black spots detected on the surface of the lungs, the result of necrosis (Fig. 2(d, e)). The lung lobes united together with themselves and the oesophagus at the areas, affected by the worms, due to the proliferation of fibrous capsule tissue.
Muscle larvae were not encysted and capsulated, which ensured their ability to move through the soft host tissues (Fig. 2(f)). Hyperaemia was increased in the muscle tissue around the worms along their migration pathways (Fig. 2(g)). The intestines of all detected muscular larvae were filled with brown contents, which indicated their active feeding, probably myoglobin and the intercellular substance of the host.
Histology of organs affected by the parasites
Structural changes in the animals’ liver infected with P. heterotremus demonstrated dystrophy of the hepatocyte; progressive massive tissue necrosis, with sinusoidal expansion; and the presence of hyperplastic Kupffer cells, as well as hemosiderin (Fig. 3(a, b)). The intersegment connective tissue was oedematic, with focal leukocyte infiltration of the stroma and perioportal zone. Also in hepatosis, both microvesicular and macrovesicular fatty changes at different stages and enlarged hepatic portals with diapedetic microhaemorrhages were observed (Fig. 3(c, d)). There were diffuse swelling and proliferation of the endothelium of sinusoidal capillaries, with hepatic tissue autolysis. Along with the red blood corpuscles in the lumens of the capillaries, large histiocytes of round to oval shape were found. In the cytoplasm of individual Kupffer cells and histiocytes, small grains and hemosiderin clumps were detected. In the parenchyma of the organ in the area adjacent to the fibrous capsule containing the parasite’s eggs, there was fatty degeneration with pronounced cell death and disintegration of hepatocytes. Parasite eggs were observed in fibrous capsules delimited by connective tissue, where an inflammatory reaction was detected with extensive areas of necrosis and hepatocyte dystrophy. In the cytoplasm of histiocytes, small grains and clumps of hemosiderin were detected (Fig. 3(e, f)). Separately lying paragonimid eggs were not noticed in the hepatic tissue.
The histopathological changes in the lungs and liver tissues. (a) Progressive, massive tissue necrosis of the liver; (b) the presence of hemosiderin, hepatocyte dystrophy; (c) focal diffuse leukocyte infiltration of the stroma and perioportal zone, microvesicular and macrovesicular fatty changes of hepatocytes; (d) enlarged hepatic portals with diapedetic microhaemorrhages (× 100); (e) dense grains of hemosiderin, which delimited regions containing the parasite’s eggs in the liver; (f) fibrous capsule delimited by granulation bank consisting of numerous lymphocytic cells in the liver (× 80); (g) granulocyte macrophage infiltration around the damaged vessels in the lung; (h) plethora and capillary leak, capillary endothelial swelling, fibrinoid necrosis of the vascular wall of the lung (× 40); (i) destruction of endothelial and epithelial membranes, elastic fibres, cellular infiltration of the alveolar interstitium of the lung; (j) disruption of normal alveolar structure of the lung (× 80)
In the lung tissues, destructive pneumonia was observed, characterised by the presence of cavities, plethora in the large vessels, microvascular stasis with the formation of hyaline thrombi and diapedesis of red blood cells. The infiltration of neutrophils and macrophage was observed around the damaged vessels, as well as plethora and capillary leak, capillary endothelial swelling and fibrinoid necrosis of the vascular wall (Fig. 3(g, h)). Disruption of alveolar structures, endothelial and epithelial membranes and elastic fibres of the alveolar interstitium (Fig. 3(i, j)) were detected. Also, in the alveolar interstitium, there was a massive accumulation of collagen fibres with the development of diffuse pulmonary fibrosis. Inflammatory infiltrates were represented by eosinophils and destructive sacculated bronchiectases, with pronounced perifocal inflammation. Along with an acute inflammatory reaction in lung of the rats, the interstitial oedema and serofibrinous inflammation were observed, which was accompanied by persistent eosinophilia. Fibrous cysts 1–3 cm in diameter, or more, were formed around the worms which contained a caseous and purulent mass together with helminths eggs. In the area of some cysts, fragments of fibrous connective tissue were observed, and with their subpleural location the pleural empyema with a large number of eosinophils was observed.
Statistical and phylogenetic analyses of genetic data
28S rDNA
For Vietnamese P. heterotremus at different stages of development, localised in muscle tissue, the liver and lungs collected from rats, a total of 1204 alignable characters, obtained using 30 replicate sequences, were available for analysis in the 28S rDNA. All sequences were identical; GC content was 54 ± 0.6%.
When comparing newly obtained partial sequences of the 28S rDNA with sequences of specimens derived from adult worms of P. heterotremus from China (HM172617), no substitutions were observed, but when comparing them with the identical sequences of the worms derived from eggs (DQ836249; HM172615) of P. heterotremus from India (GenBank data), 6 variable and parsimony-informative sites were found: transitions T↔C and A↔G were 17% and 66%, respectively, and one transversion A↔T (17%). Pairwise distance (the p-distance) of sequences of the Vietnamese and Chinese worms in relation to the Indian worms (DQ836249; HM172615) was 0.5%. Three mutations were found between sequences of P. heterotremus from Vietnam and sequence of P. pseudoheterotremus (HM004189) from Thailand: insertion (C) at 1020 site, and two deletions (A and G) at 1088 and 1089 sites in P. pseudoheterotremus, which is close to 0.24% of divergence; 9 substitutions were found between sequences of P. pseudoheterotremus and those of Indian worms (DQ836249; HM172615) which is close to 0.7% of divergence (Table 4).
The observed substitutions in the nucleotide sequences of species mentioned above correlated with the resolution on the phylogenetic tree. Phylogenetic tree inferred from 28S rDNA sequences was well resolved; however, for bootstrap support values, there were relatively low values in some internodes, although posterior probabilities from BI analyses for each representative of Paragonimus were higher. Vietnamese, Chinese and Indian worms were grouped in a one separated clade which was divided into two major subclades (bootstrap support index 81% and a posterior probability of 0.99). First include P. heterotremus representatives from Vietnam, China, and P. pseudoheterotremus from Thailand (95%/1), and the second subclade encompasses Indian P. heterotremus (99%/0.99) (Fig. 4a).
Maximum Likelihood phylogeny of Paragonimus based on a partial 28S and b the full-length ITS2 rDNA sequences. Statistical branch support values are as follows: Maximum Likelihood bootstrap values/Bayesian inference posterior probabilities (ML/BI). Only bootstrap support values > 50% in ML and posterior probabilities > 0.7 in BI are shown. Specimens obtained from the nucleotide sequences in this study (in bold) are, together with others from DNA database, shown with accession number, species name, and country (locality)
ITS2 region
For Vietnamese P. heterotremus at different stages of development, localised in muscle tissue, the liver and lungs collected from rats, a total of 361 alignable characters, obtained using 31 replicate sequences, were available for analysis in the ITS2 region. All sequences were identical; GC content was 53 ± 0.4%.
When comparing newly obtained sequences of the ITS2 region with sequences of specimens derived from eggs, metacercariae and adult worms of P. heterotremus from Vietnam (AB827360-AB827369; LC025645) and Thailand (AB354221), substitutions were not observed, but when comparing them with sequences of worms derived from eggs (DQ836248; AB308378; AB456559) and metacercariae (AB308376; AB308377; AB456558) of P. heterotremus from India (GenBank data), 5 variable and parsimony-informative sites were found: transitions T↔C and A↔G were 40% and 20%, respectively, and transversions C↔G and A↔T were 20% each. Pairwise distance of sequences of the Vietnamese worms in relation to the Indian worms was 1.1% (Table 5).
Analogically to the 28S rRNA gene, the observed substitutions in the ITS2 nucleotide sequences of Paragonimus species correlated with the resolution on the resulted phylogenetic tree. All representatives of P. heterotremus were grouped in a big clade separated from other species of the genus Paragonimus with moderate statistical support (86%/0.88) (Fig. 4b). However, this clade was subdivided. All specimens of P. heterotremus from Vietnam and Thailand were grouped into one subclade (97%/0.96), and the second statistically well-supported (99%/1) subclade was formed by P. heterotremus from India.
Secondary structures of the ITS2
Reconstructed ITS2 secondary structures shared a model typical of digeneans, i.e. a palm with four fingers, first described in detail by Michot et al. (1993). Models for specimens of P. heterotremus from Vietnam and India differed, and accordingly to that, Gibbs free energy required for ab initio structure prediction was changed: − 100.3 kcal/mol for the Vietnamese specimens and − 96.2 kcal/mol for the Indian specimens (Fig. 5). While the folding patterns of domain A, the longest, hairpin-shaped domain C and domain D for P. heterotremus from Vietnam and India were identical, domain B showed variation. A single-point mutation at position 117 resulted in the loss of noncanonical hydrogen bonding in the apex of domain B and the formation of a large internal loop (marked with an arrow on Fig. 5) in the secondary structures for the Indian specimens. Other found substitutions did not lead to the folding changes.
Generalised secondary structure of the ITS2 region from the Vietnamese and Indian specimens of P. heterotremus with free energy − 100.3 kcal/mol and − 96.2 kcal/mol, respectively. There are two variants of domain B represented in comparison. Interspecific variable sites are in black circles; intraspecific—in squares. Substitutions in the sequences of the Indian worms are displayed on a grey background
Discussion
An investigation of susceptibility of various animals to infection caused by P. heterotremus metacercariae was conducted in China, India and Vietnam (Hu 1998; Yan et al. 1998; Singh et al. 2011; Doanh et al. 2015a). The results of these studies revealed significant differences in the localisation of flukes during infection in rats. In China, in one case, adult worms were only found in the lungs (Hu 1998). In another case, adult worms were in the lungs and liver, and larvae were detected in the muscles of the rats (Wistar rats) (Yan et al. 1998). Indian P. heterotremus in rats (Wistar rats) localised in the muscles at the juvenile stage (Singh et al. 2011). In Vietnam, after the infection of Rattus tanizumi rats with P. heterotremus metacercariae, adult worms localised in the lungs. At the same time, in mice, worms at the juvenile stage were detected in the muscles and liver (Doanh et al. 2015a). There are a number of studies showing the identity of the behaviour and localisation of individuals belonging to the same species of Paragonimus (P. westermani ichunensis) when rats were infected with metacercariae (Besprozvannykh 1994, 2002). The results of present studies are consistent with those obtained by Yan et al. (1998) for P. heterotremus from China. The similarity in the behaviour of flukes during infection of rats may indicate that the worms belong to the same species. In the rest of the above-mentioned cases, worms grown in rats infected with metacercariae (Hu 1998; Singh et al. 2011; Doanh et al. 2015a) may belong to different species. Unfortunately, as in Yan et al. (1998), as well as in studies by the other listed authors, the Paragonimus species identification was based only on morphometric data, which does not exclude the possibility of misidentification of the worms. Adult worms in our study were morphometrically identical to P. heterotremus described by Chen and Hsia (1964) from Guangxi Province China, which is located near Yen Bai province in northern Vietnam, where the material for these studies was collected.
Results of the genetic analysis of 28S rRNA gene and the ITS2 region sequenced for worms P. heterotremus from the lungs, liver and muscles of infected rats have shown relevant sequence identity and coincided with data for paragonimid individuals at various developmental stages from China, Vietnam and Thailand.
In both reconstructed trees based on the 28S rRNA gene and the ITS2 data sequences (Fig. 4), P. heterotremus specimens formed two well-supported separate subclades, in accordance with the geographic region and the observed level of genetic differentiation: first for the specimens from China, Vietnam and Thailand and the second for the Indian worms.
Ribosomal genes are generally considered to be highly conserved with a significantly slow mutation rate, but they are actually composed of a mixture of conserved and divergent regions (D) and are numbered in 5′ to 3′ direction of the mature rRNA. It was established that the D1–D3 (approximately 1000 bp) regions of LSU rDNA belong to the most variable parts and show a divergence rate, which is suitable to differentiate even closely related species for a wide variety of metazoan (Sonnenberg et al. 2007; Shylla et al. 2013). Detected variability in the 28S rRNA gene is very indicative (Olson et al. 2003; Blasco-Costa et al. 2016; Pérez-Ponce de León and Hernández-Mena 2019).
Pairwise distance between sequences of P. heterotremus from Southeast Asia and India was 0.5%. At the same time, when comparing partial sequences of the 28S rRNA gene, the divergence between species of Nanophyetus (early believed to belong to the same family as Paragonimus) varied from 0.1 to 0.6% (Voronova et al. 2017; Voronova and Chelomina 2018). Moreover, the genetic distances between sequences of P. heterotremus as well as of P. westermani from India and Southeast Asia were also compatible with published data and get into the range of interspecific differences of trematodes within the same genus (0.3–1.8%) (Zikmundova et al. 2014). Considering this, we may suggest that Indian and East Asian worms belong to different species. Unfortunately, due to the limited number of available 28S data, it is difficult to give an objective assessment of the interspecific level of differences for Paragonimus species.
At the same time, there is enough ITS2 data for Paragonimus which allows us to solve the problem of the taxonomic status of Indian and East Asian worms determined as P. heterotremus. The results of previous and our molecular analyses using sequences of ITS2 similarly showed no, or very limited, variation among specimens of P. heterotremus (metacercariae and adults), from Southeast Asia (Vietnam, Thailand) and Southern China, but P. heterotremus specimens from India differed markedly 1.1% differences in ITS2 from others, forming a separate group in a tree (Le et al. 2006; Devi et al. 2007; Doanh et al. 2015b). According to the literature data, interspecies divergence at the ITS2 is typically greater than 1% (Park et al. 2003; Vilas et al. 2005; Herrmann et al. 2014). For example, the genetic p-distance between Nanophyetus schikhobalowi and N. salmincola in ITS2 was 2% (Voronova et al. 2017). Latuterus tkachi and L. maldivensis differed from each other in 1.4% (Miller and Cribb 2007). Newly described species Alloglossidium floridense showed level of divergence from A. kenti and A. fonti (0.3 and 0.6%, respectively) (Kasl et al. 2014). Along with this, intraspecific variation in the ITS2 region was observed in a wide range of digeneans, and believed that it is typically less than 0.06% (Woodruff et al. 1987; Adlard et al. 1993; Agatsuma et al. 2000). The combination of such factors as differences in the interspecific and intraspecific variability among trematodes and the presence of higher differences between P. heterotremus from Vietnam and India than between the valid species P. harinasutai and P. bangkokensis (0.9%) indicates that worms from India registered as P. heterotremus belong to another species. The detected nucleotide diversity confirms the existing differences between Vietnamese and Indian specimens.
As for P. pseudoheterotremus from Thailand, despite it clustering together with P. heterotremus from China and Vietnam on the 28S tree, there were 0.24% differences between their gene sequences, and there is no data about biology, which currently indicates insufficient information to define the species status of P. pseudoheterotremus. ITS2 sequences obtained from GenBank (JQ796814; EF014340; KC894649-KC894659) have no variations with those obtained in this study, but indels observed in only one 28S rDNA sequence are needed to be revised.
Secondary structure data provided a final answer to the question about species individuality of P. heterotremus from Vietnam and India. Previously, it has been shown that the secondary structures of genes and spacers are an excellent tool for resolving disputes regarding the species affiliation of the organisms and the comparative sequences analysis is the most powerful tool for identifying the biologically relevant folding pattern of an RNA molecule, i.e. its native structure within the cellular context (Musters et al. 1990; van der Sande et al. 1992; Michot et al. 1993; Tacker and Schuster 1994; van Nues et al. 1995; Armache et al. 2010; Ghatani et al. 2012; Voronova et al. 2017). Secondary structures of ITS2 in Paragonimus showed the typical four-domain model thought for a number of different organisms from plants to worms (Mai and Coleman 1997; Schlotterer et al. 1994; Michot et al. 1993; Morgan and Blair 1998), but at the same time had a unique structure of domain B. Domain B is well suitable for distinguishing species from different trematode families due to accumulating a large percent of substitutions especially occurred between 80 and 130 bases from the 5′ end of ITS2 (Michot et al. 1993; Morgan and Blair 1998). The presence of one large internal loop in the stem of the apex of domain B in the secondary structures of the worms from India apparently was not energy-efficient compared to the model obtained for the Vietnamese representatives (Fig. 5). Similar data on the secondary structures of ITS2 for worms belonging to different species from the genus Nanophyetus have not got such excellent differences in the topology (Voronova et al. 2017), as shown for Paragonimus. Thus, the characteristics of the secondary structures of the P. heterotremus are consistent with the above data that Indian worms do not belong to this species.
Along with the obtained results, there is information that the first intermediate hosts of P. heterotremus are molluscs from different families Assimineidae and Pomatiopsidae, and the latter includes representatives of different genera Tricula and Oncomelania (Blair et al. 1999a). Given the narrowly specific relationships between the first intermediate hosts and trematodes, we can assume the presence in the East Asian region of morphologically similar worms region belonging to different species defined as P. heterotremus (Blair et al. 1999a). This can be indirectly confirmed by the results of rats’ infection. Experiments have shown that only 15 to 33% of paragonimid metacercariae infected animals. According to the literature, during the same experiment, the infection rate for P. heterotremus reached 40–100% (Doanh et al. 2015a), and for P. westermani ichunensis the infection rate was of 34–66% (Besprozvannykh 1994). Thus, it could be assumed that along with the loss of the number of metacercariae during animal feeding, and the presence of metacercariae that did not reach the infective stage, among the morphologically similar metacercariae, there could be individuals belonging to the species not capable of infecting rats.
Conclusion
The revealed differences in the behaviour of P. heterotremus worms from Vietnam and India during infection of rats, as well as high levels of genetic divergence in each rDNA region (nucleotide substitutions, alterations in the energies and secondary structures of the ITS2 transcript) and results of phylogenetic analysis generated from the 28S rDNA and ITS2 region datasets, provided strong support that P. heterotremus worms from Vietnam and India are different species. Southeast China is the type locality of P. heterotremus based on this, on the results of the current study and published data; the range of P. heterotremus is limited to the territory of the southeastern part of China, Northern Vietnam, Laos and Thailand.
References
Adlard RD, Barker SC, Blair D, Cribb TH (1993) Comparison of the second internal transcribed spacer (ribosomal DNA) from populations and species of Fasciolidae (Digenea). Int J Parasitol 23:423–425
Agatsuma T, Arakawa Y, Iwagami M, Honzako Y, Cahyaningsih U, Kang SY, Hong SJ (2000) Molecular evidence of natural hybridization between Fasciola hepatica and F. gigantica. Parasitol Int 49:231–238
Armache J, Jarascha A, Andreas M, Villab E, Beckera T, Bhushana S, Jossinetc F, Habeckd M, Dindara G, Franckenberga S, Marqueza V, Mielkef T, Thommh M, Berninghausena O, Beatrixa B, Södinga J, Westhofc E, Wilsona D, Beckmanna R (2010) Cryo-EM structure and rRNA model of a translating eukaryotic 80S ribosome at 5.5 – a resolution. PNAS 46:748–753
Athokpam VD, Tandon V (2015) A survey of metacercarial infections in commonly edible fish and crab hosts prevailing in Manipur, Northeast India. J Parasit Dis 39:429–440
Bellaousov S, Reuter JS, Seetin MG, Mathews DH (2013) RNAstructure: web servers for RNA secondary structure prediction and analysis. Nucleic Acids Res 41:W471–W474
Besprozvannykh VV (1994) The biology of Paragonimus westermani ichunensis in the Maritime Territory. Experimental research. Med Parazitol 4:28–31 (in Russian)
Besprozvannykh VV (2002) Development of Paragonimus westermani ichunensis in a reservoir host. Parazitologiia 36:427–429 (in Russian)
Besprozvannykh VV (2004) Age-related changes in trematodes Paragonimus westermani ichunensis (Chung, Hsu et Kao, 1978) during development in reservoir and atypical final hosts. In: Materials of the I and II international readings dedicated to the memory and 85th anniversary of the birth of S.S. Shulman. Kaliningrad: KSTU Editore, pp 201-209 (in Russian)
Blair D, Agatsuma T, Watanobe T, Okamoto M, Ito A (1997) Geographical genetic structure within the human lung fluke, Paragonimus westermani, detected from DNA sequences. Parasitology 115:411–417
Blair D, Waikagul J, Honzako Y, Agatsuma T (1998) Phylogenetic relationships among the Thai species of Paragonimus inferred from DNA sequences. In: Tada I, Kojima S, Tsuji M (eds) Proceedings of the 9th International Congress of Parasitology. Bologna: Monduzzi Editore, pp 643–647
Blair D, Xu ZB, Agatsuma T (1999a) Paragonimiasis and the genus Paragonimus. Adv Parasitol 42:113–222
Blair D, Wu B, Chang ZS, Gong X, Agatsuma T, Zhang YN, Chen SH, Lin JX, Chen MG, Waikagul J, Guevara AG, Feng Z, Davis GM (1999b) A molecular perspective on the genera Paragonimus Braun, Euparagonimus Chen and Pagumogonimus Chen. J Helminthol 73:295–299
Blasco-Costa I, Cutmore SC, Miller TL, Nolan MJ (2016) Molecular approaches to trematode systematics: «best practice» and implications for future study. Syst Parasitol 93:295–306
Bowles J, McManus DP (1993) Rapid discrimination of Echinococcus species and strains using a polymerase chain reaction-based RFLP method. Mol Biochem Parasitol 57:231–239
Chang ZS, Wu B, Blair D, Zhang YN, Hu L, Chen SH, Chen MG, Feng Z, Davis GM (2000) Gene sequencing for identification of Paragonimus eggs from a human case. Chin J Parasit Dis 18:213–215
Devi KR, Narain K, Bhattacharya S, Negmu K, Agatsuma T, Blair D, Wickramashinghe S, Mahanta J (2007) Pleuropulmonary paragonimiasis due to Paragonimus heterotremus: molecular diagnosis, prevalence of infection and clinicoradiological features in an endemic area of northeastern India. Trans R Soc Trop Med Hyg 101:786–792
Devi KR, Narain K, Agatsuma T, Blair D, Nagataki M, Wickramasinghe S, Yatawara L, Mahanta J (2010) Morphological and molecular characterization of Paragonimus westermani in northeastern India. Acta Trop 116:31–38
Doanh PN, Dung DT, Thach DTC, Horii Y, Shinohara A, Nawa Y (2011) Human paragonimiasis in Viet Nam: epidemiological survey and identification of the responsible species by DNA sequencing of eggs in patients’ sputum. Parasitol. Int 60:534–537
Doanh PN, Thaenkham U, An PT, Hien HV, Horii Y, Nawa Y (2013) Metacercarial polymorphism and genetic variation of Paragonimus heterotremus (Digenea: Paragonimidae), and a re-appraisal of the taxonomic status of Paragonimus pseudoheterotremus. J Helminthol 89:182–188
Doanh PN, Hien HV, An PT, Tu LA (2015a) Development of lung fluke, Paragonimus heterotremus, in rat and mice, and the role of paratenic host in its life cycle. Tap Chi Sinh Hoc 37:265–271
Doanh PN, Thaenkham U, An PT, Hien HV, Horii Y, Nawa Y (2015b) Metacercarial polymorphism and genetic variation of Paragonimus heterotremus (Digenea: Paragonimidae), and a re-appraisal of the taxonomic status of Paragonimus pseudoheterotremus. J Helminthol 89:182–188
Ghatani S, Shylla JA, Tandon V, Chatterjee A, Roy B (2012) Molecular characterization of pouched amphistome parasites (Trematoda: Gastrothylacidae) using ribosomal ITS2 sequence and secondary structures. J Helminthol 86:117–124
Herrmann KK, Poulin R, Keeney DB, Blasco-Costa I (2014) Genetic structure in a progenetic trematode: signs of cryptic species with contrasting reproductive strategies. Int J Parasitol 44:811–818
Hu W (1998) Studies on the life cycle of Paragonimus heterotremus. Chin J Parasitol Parasit Dis 16:347–352
Huelsenbeck JP, Ronquist F (2001) MrBayes: Bayesian inference of phylogeny. Biometrics 17:754–755
Kasl EL, Fayton TJ, Font WF, Criscione CD (2014) Alloglossidium floridense n. sp. (Digenea: Macroderoididae) from a spring run in North Central Florida. IJP 100:121–126
Kong Y, Doanh P, Nawa Y (2015) Paragonimiasis. In: Xiao L, Ryan U, Feng YY (eds) Biology of foodborne parasites. CRC Press, London, pp 445–462
Kurochkin YV (1987) Trematodes of the fauna of the USSR, Paragonimida. Science, Moscow (In Russian)
Le TH, Van De N, Blair D, McManus DP, Kino H, Agatsuma T (2006) Paragonimus heterotremus Chen and Hsia (1964), in Vietnam: a molecular identification and relationships of isolates from different hosts and geographical origins. Acta Trop 98:25–33
Lockyer AE, Olson PD, Littlewood DTJ (2003) Utility of complete large and small subunit rRNA genes in resolving the phylogeny of the Neodermata (Platyhelminthes): implications and a review of the cercomer theory. Biol J Linn Soc 78:155–171
Mai JC, Coleman AW (1997) The internal transcribed spacer 2 exhibits a common secondary structure in green algae and flowering plants. J Mol Evol 44:258–274
Michot B, Despres L, Bonhomme F, Bachellerie JP (1993) Conserved secondary structures in the ITS2 of trematode prerRNA. FEBS Lett 316:247–252
Miller T, Cribb T (2007) Two new cryptogonimid genera (Digenea, Cryptogonimidae) from Lutjanus bohar (Perciformes, Lutjanidae): analyses of ribosomal DNA reveals wide geographic distribution and presence of cryptic species. Acta Parasitol 52:104–113
Miyazaki I, Habe S (1976) A newly recognized mode of transmission of human infection with lung fluke, Paragonimus westermani (Kerbert, 1878). J Parasitol 62:646–648
Miyazaki I, Terasaki K, Iwata K (1978) Natural infection of muscle of wild boars in Japan by immature Paragonimus westermani (Kerbert, 1878). J Parasitol 64:559–560
Morgan JA, Blair D (1998) Trematode and monogenean rRNA ITS2 secondary structures support a four-domain model. J Mol Evol 47:406–419
Musters W, Boon K, Van der Sande CA, Van Heerikhuizen H, Planta RJ (1990) Functional analysis of transcribed spacers of yeast ribosomal DNA. EMBO J 9:3989–3996
Narain K, Devi KR, Mahanta J (2003) Paragonimus and paragonimiasis - a new focus in Arunachal Pradesh, India. Curr Sci 84:985–987
Nei M (1987) Molecular evolutionary genetics. Columbia University Press, USA
Olson PD, Cribb TH, Tkach VV, Bray RA, Littlewood DTJ (2003) Phylogeny and classification of the Digenea (Platyhelminthes: Trematoda). Int J Parasitol 33:733–755
Park GM, Im K, Yong TS (2003) Phylogenetic relationship of ribosomal ITS2 and mitochondrial CO1 among diploid and triploid Paragonimus westermani isolates. Korean J Parasitol 41:47–55
Pérez-Ponce de León G, Hernández-Mena D (2019) Testing the higher-level phylogenetic classification of Digenea (Platyhelminthes, Trematoda) based on nuclear rDNA sequences before entering the age of the ‘next-generation’ Tree of Life. J Helminthol 93:260–276
Posada D, Crandall KA (1998) Modeltest: testing the model of DNA substitution. Bioinformatics 14:817–818
Schlotterer C, Hauser MT, Von Haeseler A, Tautz D (1994) Comparative evolutionary analysis of rDNA ITS regions in Drosophila. Mol Biol Evol 11:513–522
Shylla JA, Ghatani S, Tandon V (2013) Utility of divergent domains of 28S ribosomal RNA in species discrimination of paramphistomes (Trematoda: Digenea: Paramphistomoidea). Parasitol Res 112:4239–4253
Singh S, Sugiyama H, Devi KR, Singh LD, Binchai S, Rangsiruji A (2011) Experimental infection with Paragonimus heterotremus metacercariae in laboratory animals in Manipur, India. SE Asian J Trop Med 42:34–38
Singh TS, Sugiyama H, Rangsiruji A (2012) Paragonimus & paragonimiasis in India. Indian J Med Res 136:192–204
Sonnenberg R, Nolte AW, Tautz D (2007) An evaluation of LSU rDNA D1-D2 sequences for their use in species identification. Front Zool 4:6
Sykhanova GI, Kaminsky YV (1998) Paragonimiasis: Typical and larval forms, clinical picture, pathological anatomy, diagnosis, treatment. Vladivostok, Russia
Tacker M, Schuster P (1994) Fast folding and comparison of RNA secondary structures. Monatshefte für Chemie 125:167–188
Tajima F (1983) Evolutionary relationships of DNA sequences in finite populations. Genetics 105:437–467
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: Molecular Evolutionary Genetics Analysis Version 6.0. Mol Biol Evol 30:2725–2729
Tkach VV, Littlewood DTJ, Olson PD, Kinsella JM, Swiderski Z (2003) Molecular phylogenetic analysis of the Microphalloidea Ward, 1901 (Trematoda: Digenea). Syst Parasitol 56:1–15
Truett GE, Heeger P, Mynatt RL, Truett AA, Walker JA, Warman ML (2000) Preparation of PCR - quality mouse genomic DNA with hot sodium hydroxide and tris (HotSHOT). Biotechniques 29:52–54
Van der Sande CAFM, Kwa M, Van Nues RW, Van Heerikhuizen H, Raue HA, Planta RJ (1992) Functional analysis of internal transcribed spacer 2 of Saccharomyces cerevisiae ribosomal DNA. J Mol Biol 223:899–910
Van Nues RW, Rientjes JMJ, Morre SA, Mollee E, Planta RJ, Venema J, Raue HA (1995) Evolutionarily conserved structural elements are critical for processing of internal transcribed spacer 2 from Saccharomyces cerevisiae precursor ribosomal RNA. J Mol Biol 250:24–36
Vilas R, Criscione CD, Blouin MS (2005) A comparison between mitochondrial DNA and the ribosomal internal transcribed regions in prospecting for cryptic species of platyhelminth parasites. Parasitology 131:839–846
Voronova AN, Chelomina GN (2018) Genetic diversity and phylogenetic relations of salmon trematode Nanophyetus japonensis. Parasitol Int 67:267–276
Voronova AN, Chelomina GN, Besprozvannykh VV, Tkach VV (2017) Genetic divergence of human pathogens Nanophyetus spp. (Trematoda: Troglotrematidae) on the opposite sides of the Pacific Rim. Parasitology 144:601–612
Woodruff DS, Merenlender AM, Upatham ES, Viyanant V (1987) Genetic variation and differentiation of three Schistosoma species from the Philippines, Laos, and peninsular Malaysia. Am J Trop Med Hyg 36:345–354
Yan T, Li G, Dong C (1998) Experimental studies on development of Paragonimus heterotremus in rats. Chin J Parasitol Parasit Dis 16:126–129
Zikmundova J, Georgieva S, Faltynkova A, Soldanova M, Kostadinova A (2014) Species diversity of Plagiorchis Luhe, 1899 (Digenea: Plagiorchiidae) in lymnaeid snails from freshwater ecosystems in central Europe revealed by molecules and morphology. Syst Parasitol 88:37–54
Funding
This work was partially supported by the FEB RAS (Far Eastern Branch of the Russian Academy of Sciences) project (18–4–039).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Section Editor: David Bruce Conn
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 18 kb)
Rights and permissions
About this article
Cite this article
Voronova, A.N., Besprozvannykh, V.V., Ngo, H.D. et al. Paragonimus heterotremus Chen et Hsia, 1964 (Digenea: Paragonimidae): species identification based on the biological and genetic criteria, and pathology of infection. Parasitol Res 119, 4073–4088 (2020). https://doi.org/10.1007/s00436-020-06929-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-020-06929-4