Abstract
The freshwater snail Bithynia siamensis goniomphalos serves as the first intermediate host of liver fluke Opisthorchis viverrini, a foodborne parasite, of which human infection has persisted in Southeast Asia for decades. The snail Filopaludina martensi martensi has been proposed as a biological control agent against B.s. goniomphalos, but knowledge on the snail ecology and population dynamics between the two species remains rudimentary. This study investigated selected abiotic and biotic factors influencing the distribution and abundance of B.s. goniomphalos and F.m. martensi. Water quality, soils, and snails were collected from 34 localities in Northeast Thailand. Soil properties and snail soft tissue elemental concentrations were analyzed. Experiments were performed to examine interspecific competition. Statistical analysis was conducted to explore the associations between water and soil properties and soft tissue elemental concentrations. The results showed that B.s. goniomphalos had the highest mean dominance in streams and red-yellow podzolic soils, while F.m. martensi snails preferred ponds and latosol soils. Negative correlation in species abundances was found between the two species. Interspecific competition was detected, with B.s. goniomphalos growth rates hampered by the presence of F.m. martensi. Despite the possibility of using F.m. martensi to control B.s. goniomphalos, B.s. goniomphalos exhibited a greater adaptability to different water and soil properties, suggesting that the species could colonize a wide range of environmental conditions. This study provides further insights into the ecology of the two snail species, underscoring the importance of considering abiotic factors when assessing the possible biological control agent to control O. viverrini transmission.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The liver fluke, Opisthorchis viverrini, is a fish-borne trematode which causes a major public health problem in continental Southeast Asia, affecting countries of Thailand, Lao People’s Democratic Republic (PDR), Cambodia, Vietnam, and Myanmar (Sithithaworn et al. 2012; Aung et al. 2017). The life cycle of O. viverrini requires freshwater snails of the genus Bithynia as the first intermediate host, cyprinid fish as the second intermediate host, and humans and other fish-eating mammals such as cats and dogs as the definitive host (Kaewkes 2003). Humans are infected by eating raw or undercooked fish contaminated with metacercariae, the infective stage of the parasite. An estimated 10 million people in Thailand and Lao PDR alone are infected with O. viverrini (Sripa et al. 2011) because of the fishing-dependent livelihood and the long-standing tradition of raw fish consumption (Wang 2012). Moreover, O. viverrini has been classified as a group-1 carcinogen for cholangiocarcinoma (CCA), a bile duct cancer (IARC 2012), and the highest CCA incidence has been reported in Northeast Thailand (Sripa and Pairojkul 2008; Alsaleh et al. 2019).
Considerable efforts have been devoted to investigating O. viverrini prevalence in the two intermediate hosts. The infection in fish varies greatly, ranging from 2.1 to 100% (Wang 2012), but is generally high as some places have reported about 90% of infection in several cyprinid fish species (Sithithaworn and Haswell-Elkins 2003; Rim et al. 2008). On the contrary, the prevalence of O. viverrini in Bithynia snails is low, varying from 0.03 to 1.73% (Brockelman et al. 1986; Namsanor et al. 2015; Kiatsopit et al. 2016). However, the number of cercariae released from infected snails have been found high, for example in Northeast Thailand, 284–789 cercariae/snail/day (Laoprom et al. 2016). Bithynia snails thus play a key amplification role in the O. viverrini life cycle, which undergo asexual reproduction through the stages of cercariae. Three Bithynia subspecies from the Mekong region with mostly allopatric distributions act as the first intermediate host of O. viverrini: Bithynia siamensis goniomphalos in Northeast Thailand, Lao PDR and Southern Vietnam, B.s. siamensis in Central Thailand and the southern Myanmar, and B. funiculata in North Thailand (Brandt 1974; Petney et al. 2012; Piratae 2015).
Snail control strategies have been considered a priority for reducing parasitic disease transmission (WHO 2017). Niclosamide has been widely used for its molluscicidal activity in the schistosomiasis control in endemic areas (Perrett and Whitfield 1996; Coelho and Caldeira 2016). Field trials of niclosamide on B.s. goniomphalos revealed low mortality rates in the snails, but adversely increased mortality rates in other aquatic animals, such as silver barb (Puntius gonionotus), shrimp (Macrobrachium lanchesteri) and ricefish (Oryzias mekongensis) (Tesana et al. 2012). Due to the toxic effects on no-target animals and environmental pollution, biological control agents have been considered as an alternative approach to chemical molluscicides (Pointier et al. 1989; Slootweg et al. 1994).
Biological controls, however, should be approached with caution as they could adversely alter ecological equilibrium. Introduction of putative biological control agents without explicit regard for the native species can cause irreversible ecosystem alterations (Pointier and David 2004). For example, the introduction of the common carp as a biological control agent for the invasive apple snails has an unexpected diminishing of snail diversity in ponds (Wong et al. 2009). The possibility of utilizing biological control agents to impede the completion of the O. viverrini life cycle has been suggested, with Filopaludina martensi martensi (F.m. martensi), one of the three subspecies of F. martensi commonly found in the Mekong region, being identified as a potential agent (Haruay et al. 2008). Different from most instances where the introduction of a biological control agent constitutes as a foreign invasive species, F.m. martensi is native to Northeast Thailand (Brandt 1974). Additionally, F.m. martensi coexists in similar habitats alongside B.s. goniomphalos (Haruay and Piratae 2019), although F.m. martensi has been observed as the most abundant species in river habitats (Nahok et al. 2017), while B.s. goniomphalos dominates rice paddies (Wang et al. 2015), probably because of the shallow, slow water flowing environments of the rice paddies (Brockelman et al. 1986; Petney et al. 2012). It is thus imperative to understand the snail ecology of F.m. martensi and B.s. goniomphalos, particularly the abiotic and biotic factors influencing the two snail species’ distribution and abundance.
Freshwater gastropods are inherently sensitive to alterations in the environment and often exist under specific environmental conditions (Moran and Emlet 2001). Abiotic factors, especially water quality and soil properties, function as the primary determinants in delineating their suitable habitat ranges. Water quality variables of temperature, dissolved oxygen, salinity, and acidity have been studied in relation to B.s. goniomphalos snail abundance (Wang et al. 2015; Kim et al. 2016), while soil properties of the snail habitats and chemical compositions of snail soft tissues have not been investigated. Exchanges between soils and snails occur via both soil ingestion and absorption through the foot (Gomot et al. 1989). Consequently, different soil properties, such as soil types, pH, moisture levels, and chemical elements, can affect snail ecology (Mourier et al. 2011). For example, Calcium (Ca) supports the shell growth of snails and the development of their eggs and embryos (Tunholi et al. 2011). This element is hence fundamental to snail survival and colonization in the environment. Regarding biotic factors, interactions among different species for resources influence species abundance. Field samplings by Haruay et al. (2008) and Wang et al. (2015) have shown negative correlations between B.s. goniomphalos and F.m. martensi snail abundances, but it remains unclear how these two species engage in interspecific competition while coexisting within similar habitats. Experimental work is desirable to examine the interactions between the two species, thereby assessing the possible utilization of F.m. martensi as a biological control agent for B.s. goniomphalos, the first intermediate host of O. viverrini.
The objective of this study is to scrutinize the effects of selected abiotic and biotic factors on the abundance and distribution of B.s. goniomphalos and F.m. martensi snails in Northeast Thailand where infections of O. viverrini still persist. Specifically, this study aims to (1) investigate snail species diversity across different water habitats and soil types, (2) examine abiotic factors of water quality and soil properties and their association with snail dominances, (3) analyze the associations between water and soil properties and snails’ physical sizes and soft tissue elemental concentrations, and (4) assess the biotic effects of interspecific competition on the growth of B.s. goniomphalos and F.m. martensi snails in laboratory experiments. The study will provide insights into the factors influencing the abundance and distribution of B.s. goniomphalos and F.m. martensi. It will contribute to a better understanding of snail ecology and population dynamics between B.s. goniomphalos and F.m. martensi snails, which is currently lacking in the public health–focused literature on O. viverrini. Such understanding will bridge the literature gap to inform disease and ecosystem health management strategies of considering F.m. martensi as a possible biological control agent for limiting O. viverrini transmission.
Materials and methods
Study area and sampling sites
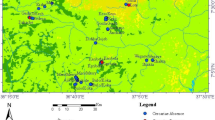
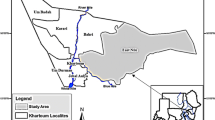
The study area covers the provinces of Khon Kaen, Nong Bua Lamphun, and Kalasin in Northeast Thailand, where high human O. viverrini prevalence, high intensity of fish infection, and O. viverrini infected B.s. goniomphalos snails have been reported (Aunpromma et al. 2012; Sithithaworn et al. 2012; b; Ong et al. 2016; Wang et al. 2017). Northeast Thailand experiences pronounced wet and dry seasons. The wet season is from May to October, and the dry season occurs between November and March. Sampling was conducted in three main water bodies of snail habitats, including rice paddies, ponds, and streams. In addition to these water habitats, sampling sites were chosen based on soil types and O. viverrini prevalence to present a matrix of areas of intersecting environmental conditions with areas of known parasitic transmissions. A total of 34 sites feasible for sampling were identified, covering three different soil types (Table 1; Fig. 1). Snails, water quality measurements, and soil properties were collected from all 34 sites during the wet season, from June to September in 2016.
Snail sampling and species dominance and diversity
To compare snail abundance, species diversity and dominant species across different sites, sampling methods of Lohachit (2004–2005) and Wang et al. (2015) were adopted. Snails were collected along the shallow edges of water bodies using quadrat sampling. A 0.25-m2 square quadrat was positioned four times along a transect line with a meter interval between each quadrat to create a 1-m2 sampling area for each site. Each quadrat was sampled for 10 min. Snails of all species were collected by scoop net or handpicking. The collected snails were brought back to the laboratory for species identification based on shell morphology in Brandt (1974). Snail numbers were counted by species and sites.
To compare species diversity and dominance across different habitats and soil types, the following indices from Magurran (2004) and Wang et al. (2015) were used. First, dominance of individual snail species was computed as the relative proportion of the number of an individual snail species among the total number of all snail species sampled at each site, and it was expressed as a percentage from 0 to 100%. The association between the dominances of B.s. goniomphalos and F.m. martensi in different sites were further examined using an x–y plot and Pearson’s correlation coefficient (SPSS, version 17.0), so as to understand the potential interspecies competition between the two snail species.
Second, species richness (S) was calculated as the total number of species present at each habitat or soil type. Third, Shannon diversity index (H) was derived using the following formula:
where pi was the proportion of species i relative to the total number of species. A higher H indicated higher diversity.
Fourth, species evenness (E) was derived as follows:
The index E ranged between 0 and 1, with values closer to 1 denoting even populations that consisted of several species with similar abundances.
Water quality and soil property analysis
Water quality, including temperature (°C), salinity (ppt), conductivity (mS/cm), total dissolved solids (TDS) (g/L), dissolved oxygen (mg/L), percentage dissolved oxygen (%), and pH, was measured at each sampling site using the YSI 556 Handheld Multi-Probe System. These variables were suggested as possible factors affecting the distribution of snails (Chitramvong et al. 1981; Wang et al. 2015).
Soil samples were collected from the shallow edges of water bodies where the greatest density of snails was found. Using a shovel, approximately 250 g of soil were dug out up to a maximum depth of 15 cm. Soil samples were brought back to the laboratory, heated, and dried overnight in the oven at 40 °C, and then left to cool at room temperature. Organic constituents such as grass, leaves, and roots were sieved out before the subsequent analyses on soil pH, organic matter, soil base cations, and texture.
Soil pH determines the chemical processes that occur and the subsequent nutrient availability (Bloom and Skyllberg 2012). To measure soil pH, samples were prepared according to Brady and Weil (2002) using a soil:water ratio of 1:1 (w/v, weight per volume). Dry soil (20 g) and 20 ml of deionized water were shaken together at 240 rpm for 2 h to ensure a well mix, and then left to equilibrate for an hour to reach ambient temperature. A pH meter (Orion 3 Star pH Benchtop) was used to take the measurements.
Soil organic matter offers information on nutrient availability for organisms. The loss-on-ignition (LOI) method based on percent weight (Santisteban et al. 2004) was employed. Samples were placed in the oven for 2 h at 105 °C to remove all moisture. Subsamples of 5 g (± 0.01 g) were obtained and placed in a ceramic crucible where it was combusted at 550 °C for 8 h in a muffle furnace (Carbolite OAF 1000, Fisons, UK). The samples were then cooled to room temperature in a dessicator to prevent moisture from accumulating before being weighed again to obtain the final weight. The organic matter content (%) was then calculated as follows:
where A represented weight (5 ± 0.01 g) of soil and crucible before combustion and B was weight of soil and crucible after combustion.
Soil base cations represent the available exchangeable ions and are used as indicators of soil fertility and nutrient availability (Zarabi and Jalali 2012). The ammonium acetate extraction method by Lavkulich (1981) was employed to determine the exchangeable contents of sodium (hereafter Exch. Na for brevity), magnesium (Exch. Mg), potassium (Exch. K), and calcium (Exch. Ca) cations in each soil sample. The soil-extractant mixture was subsequently placed on the orbiter shaker at 180 rpm for 2 h (Stuart orbiter shaker, mini, SSM1) before being centrifuged (Centurion Scientific K3 Series) for 10 min at 6000 min−1. A 0.45-μl syringe filter was utilized to extract the supernatant from the mixture to form a resultant 10-ml solution, which was analyzed using the Inductive Coupled Plasma Optical Emission Spectroscopy (ICP-OES) machine (Perkin Elmer Optima 8300). A 6-point calibration regression method was prepared at concentrations of 0.1, 0.2, 0.5, 1, 5, and 10 ppm using 100 ppm standards and ammonium acetate. A reagent blank sample was also prepared and analyzed with its value being subtracted to give the final concentration. All sample, standard, and blank solutions were analyzed using 15-s integrations. The exchangeable bases (cmolc/kg) were calculated with the following equations (Robertson et al. 1999):
where C was the concentration of ions (Ca, K, Mg, Na) in extract (mg/L) with blank subtracted, V denoted the volume of extract used, and W represented the mass of dry weight.
where Cg represented the element mass (in μg element/g soil); N denoted valence of ion; A was the atomic mass of ion (Ca = 20.04, K = 39.10, Mg = 12.15, Na = 23.00).
Soil texture was determined by the international pipette method (Beretta et al. 2014). Dried soil (2.5 g ± 0.005 g) was weighed and removed of any organic materials. Next, 6.25 ml of deionized water along with the respective proportions of dispersing agents—6.25 ml of 2(N) HCL, 1.25 ml of hydrogen peroxide, 2.5 ml of sodium hexametaphosphate and 0.6 ml of sodium carbonate—were added to the dry soil. The mixture was wet sieved using a 0.5-mm sieve where soil particles greater than 0.5 mm in size were dried and weighed. Particles that were lesser than 0.5 mm were analyzed using the Malvern Mastersizer 2000 particle size analyzer with laser obscuration values of between 12 and 13%. The proportion of clay, silt, and sand were determined according to the UDSA particle size classification (Blott and Pye 2012).
All data obtained from the analyses of 15 water quality and soil properties were summarized using descriptive statistics to show their means and ranges of values across the habitats and soil types. Canonical Correspondence Analysis (CCA) with Type 1 scaling was then performed using the Palaeontological Statistics (PAST) Software Version 4.0 (Hammer et al. 2001) to relate B.s. goniomphalos and F.m. martensi snail species dominances to water and soil properties.
Snail soft tissue elemental analysis
B.s. goniomphalos and F.m. martensi snails were retained and their shell heights and widths were measured using a digital vernier caliper. Next, their soft tissues were extracted and separated from their shells for elemental analysis according to Mostafa (2007). Snail soft tissues were placed in the oven at 40 °C for 2 h to remove moisture before being grinded into fine powder using a mortar and pestle. The soft tissue was weighed to attain a weight of 0.05 g. Using 10 ml of 69% nitric acid (HNO3), the soft tissue was digested on a hot plate by boiling to dryness. The sample volume was made up to 20 ml with 2% HNO3. The sample tubes are analyzed using the ICP-OES (Perkin Elmer Optima 8300) for the elemental concentrations of nine metallic elements namely calcium (Ca), copper (Cu), iron (Fe), potassium (K), lead (Pb), manganese (Mn), nickel (Ni), sodium (Na), and zinc (Zn) because prior studies have reported the accumulation of these elements in snail tissues (Evans et al. 2001; Kaufer et al. 2002; Mostafa 2008). The final elemental concentration (mg/g) was calculated as follows:
where C denoted the concentration value in ppm with blank subtracted.
Elemental concentrations of B.s. goniomphalos and F.m. martensi snail tissues were first tabulated by habitat and soil types for comparison. Principal component analysis (PCA) was conducted using PAST software, to explore the associations between soft tissue elemental concentrations and respective habitat and soil types. Finally, Pearson’s correlation coefficients were employed to assess the associations between individual water and soil properties and each of the snail characteristics (i.e., snail heights, widths, and elemental concentrations in soft tissues).
Laboratory experiment
Interspecific competition can be manifested in the reduction in growth, survivorship, or fecundity of a species, as a result of resource exploitation or interference by another species (Begon et al. 1996; Eccard and Ylönen 2003). To assess the interspecific competition between B.s. goniomphalos and F.m. martensi snails, their shell growth rate, an indicator of snail’s health and performance, was measured over a 30-day period. Only snails without shell damage were used in the experiments to observe the competitions between the two species. B.s. goniomphalos snails over 8 mm in height were classified as adults (Tesana et al. 2012), but quite a few of them had shell damage in their shell apex and aperture. It has also been suggested that small-sized B.s. goniomphalos snails are more susceptible to parasitic infection (Chanawong and Waikagul 1991; Kiatsopit et al. 2014; Prasopdee et al. 2015). Consequently, the B.s. goniomphalos snails used in the experiments were all juveniles, and their shell heights ranged from 5 to 6.5 mm. F.m. martensi snails within 18 mm in height were classified as juveniles while those over 18 mm were classified as adults (Palasun and Intanai 2015).
Four types of treatments were conducted (Table 2), each of which was replicated five times. Treatments #1 and #2 were single-species treatments, with 1 B.s. goniomphalos juvenile and 1 F.m. martensi juvenile, respectively. Treatments #3 and #4 were mixed-species treatments, with 1 B.s. goniomphalos juvenile and 1 F.m. martensi adult in #4, and 1 B.s. goniomphalos and 5 F.m. martensi adults in #4. Snails for individual treatments were kept in the same plastic cups with 200 ml of deionized water, and 5 g of fresh ivy gourd leaves were supplied every 2 days. The heights and widths of the snail shells were measured using a digital vernier caliper at the onset of the experiment, every 10 days. At the end of the experiment, two measures were employed to assess the performance of B.s. goniomphalos and F.m. martensi snails during the competition. First, percentage of growth (%) was calculated using the percentage of length increase. Second, specific growth rate (G) of the snails was computed with the following equation (Kaufmann 1981; Seppälä et al. 2013):
where S1 and S2 represented the sizes at the beginning and at the end of the time period Δt, respectively, and S was their geometric mean. Mann–Whitney U test was used to determine if the percentage of growth and the specific growth rate were statistically significantly different between the treatments.
Results
Snail diversity and dominance across habitats and soil types
A total of 11,294 snails were collected, consisting of eight taxa: B.s. goniomphalos (84.41%), Pomacea canaliculata (7.40%), F.m. martensi (5.59%), Melanoides tuberculata (1.33%), Tarebia granifera (0.50%), Brotia sp. (0.33%), Indoplanorbis exustus (0.32%), and Lymnaea auricularia rubiginosa (0.12%). B.s. goniomphalos snails were found in all 34 sites, while F.m. martensi snails were found in 24 sites (about 70% of the study sites). The average number of snails collected per site was the highest at the stream habitats, followed by the paddy and the pond habitats (Table 3). The ponds had a higher species richness (7 species) and the highest species diversity (H = 0.469). Conversely, the streams had a lower species richness (5 species), the lowest diversity (H = 0.396), and the lowest species evenness (E = 0.297) among the three types of habitats. Mean B.s. goniomphalos dominance was the highest in streams at 88.3%, while mean F.m. martensi dominance was the highest in ponds at 24.1%. Pearson’s correlation coefficient showed a significant negative relationship between the dominance of B.s. goniomphalos snails and that of F.m. martensi snails in the sampling sites (r = − 0.8, p < 0.05) (Fig. 2), underscoring the possible interspecies competition between the two snail species.
Quantification of snail diversity and dominance across three soil types showed that the average number of snails was the highest in soil type Lato followed by soil types LHG and RYP (Table 4). Soil type Lato also exhibited the greatest values in both species richness (8 species) and diversity (H = 0.500). The species evenness was the highest in soil type LHG (0.46) and the lowest in soil type RYP. B.s. goniomphalos dominated all three soil types, with the highest mean dominance at 88.6% in soil type RYP. Alternatively, F.m. martensi had the lowest mean dominance at 3.8% in soil type RYP and its highest mean dominance at 21.0% in soil type Lato.
Water quality and soil properties across different habitat types and soil types
Water quality and soil properties varied differently across different habitat and soil types (Table 5). Among the three habitat types, the stream was characterized by the lowest soil organic contents and the highest water pH, soil pH, and Exch. Ca, while paddy was marked by the highest water temperature, conductivity, TDS, and salinity. Both habitat types observed higher mean B.s. goniomphalos snail dominances. The pond habitat recorded low DO and Exch. Ca and was preferred by F.m. martensi snails. Among the soil types, RYP was characterized by the lowest conductivity, TDS, salinity, and silt composition, as well as the highest sand composition. The RYP soil type also recorded the highest mean B.s. goniomphalos snail dominance and the lowest mean F.m. martensi snail dominance. Alternatively, F.m. martensi snails had the highest mean snail dominance in the soil type Lato where conductivity, TDS, and salinity were the highest, but clay composition was the lowest. The Lato soil type, similar to the pond habitat, also reported the lowest Exch. Ca. CCA ordination separated F.m. martensi and B.s. goniomphalos snail dominances based on water and soil properties (Fig. 3). The first axis was positively correlated with F.m. martensi, soil pH, sand, DO, and water pH environment. B.s. goniomphalos was likely to be associated with Exch. K, although the concentrations detected from soils in the study sites were low.
Elemental concentrations in snail soft tissues in different habitat types and soil types
The nine metallic elements extracted from the snail soft tissues showed that overall, Ca had the highest mean concentrations in both B.s. goniomphalos and F.m. martensi snails, followed by K and Na. Nevertheless, the elemental concentrations varied between B.s. goniomphalos and F.m. martensi snail species, and across different habitat and soil types (Table 6). In terms of habitats, the highest mean Ca concentration in B.s. goniomphalos soft tissues was found in the paddies, whereas that in F.m. martensi soft tissues was high in both streams and ponds. The highest mean K concentrations for both snail species soft tissues were detected in the paddies. The highest mean Ca concentration in B.s. goniomphalos soft tissues was found in soil type Lato, while that in F.m. martensi soft tissues was detected in soil type RYP. The highest mean K concentrations for both species were detected in soil type LHG.
PCA of snail soft tissue elemental concentrations and soil properties showed that nine elements were clustered into three groups (Fig. 4). First, Ca from B.s. goniomphalos soft tissues was positively correlated with ponds and paddies as well as soil types Lato and RYP. Second, Mn, Fe, and Pb from F.m. martensi soft tissues were correlated with ponds and soil type RYP. Third, Ni, Cu, Na, Zn, and K from B.s. goniomphalos soft tissues were correlated with streams and soil type LHG. While there were no distinct separations across snails from different habitat and soil types, variabilities of elemental concentrations in F.m. martensi snails were generally less than the concentrations in B.s. goniomphalos snails, when individual habitat and soil types were compared.
PCA based on soil properties and elemental concentrations of B.s. goniomphalos (Bsg) and F.m. martensi (Fmm) snail soft tissues by (a) habitat types and (b) soil types (cf. Table 1 for soil descriptions)
Pearson’s correlation coefficients showed that water pH was the most important environmental variable affecting both the physical (e.g., snail size) and chemical characteristics (i.e., most of the elemental concentrations) of the two snail species (Tables 7 and 8). The strongest positive correlation for B.s. goniomphalos snails was between water pH and soft tissue K concentration (r = 0.653, p < 0.01), while the strongest negative correlation was between water pH and soft tissue Ca concentration (r = − 0.856, p < 0.01) (Table 7). For F.m. martensi snails, the strongest positive correlation was between soil Exch. Na and soft tissue Na concentration (r = 0.499, p < 0.01), while the strongest negative correlation was between water pH and soft tissue Mg concentration (r = − 0.473, p < 0.05) (Table 8). Overall, there were more significant correlations between snail characteristics and water and soil properties for B.s. goniomphalos compared with F.m. martensi snails. In addition to water pH, water temperature, conductivity (including its relevant indices TDS and salinity), soil sand composition and silt composition, soil Exch. Na, and soil pH exhibited statistically significant correlations with six or more characteristics for B.s. goniomphalos. Likewise, water pH, conductivity, TDS, and salinity affected six or more characteristics of F.m. martensi, while water pH was the only variable significantly associated with F.m. martensi snail size.
Interspecific competition between B.s. goniomphalos and F.m. martensi snails
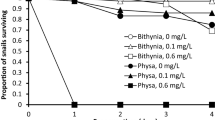
A total of 50 snails were used in the four treatments. At the end of the experiment, 43 snails survived so they were used in growth measurements. Of the seven dead snails, five occurred in Treatment #4, while Treatments #1 and #2 had one casualty each.
Comparison of Treatments #1 and #2 showed that when there was no species competition, the percentage of growth of B.s. goniomphalos juveniles increased much more at 10.6% than that of F.m. martensi juveniles at 3.5% (Table 9); their respective specific growth rates were also significantly different (p = 0.009) (Fig. 5). Analysis of the strength of interspecific competition indicated that B.s. goniomphalos snails grew at a much slower rate in the high level of competition in Treatment #4 than in the low level of competition in Treatment #3 (Table 9; Fig.5, p = 0.016). The findings suggested that the growth rates of B.s. goniomphalos were influenced by the densities of F.m. martensi. In addition, the growth inhibition of B.s. goniomphalos snails might be attributed to the acts of aggression from F.m. martensi snails. It was observed during the experiment that F.m. martensi snails attacked and gnawed on the shells of juvenile B.s. goniomphalos snails, causing shell erosion and discoloration (Fig. 6).
Discussion
B.s. goniomphalos and F.m. martensi snail dominances across habitat and soil types
B.s. goniomphalos was the most abundant snail species in the study area, accounting for more than 80% of the snails collected, while F.m. martensi ranked third in abundance at 5.59%. The findings of snail habitat and soil preferences of this study corroborated prior work in two aspects. First, similar to Papasarathorn et al. (1990), the abundance of B.s. goniomphalos was the highest in soil type RYP. Second, echoing Haruay and Piratae (2019), F.m. martensi snails preferred the pond habitat.
Conversely, the stream habitat of this study recorded the highest B.s. goniomphalos snail dominance at 88.3%. This was different from previous work that observed the highest dominance of B.s. goniomphalos snails in the paddies among the three water habitat types (Wang et al. 2015). The difference could be due to the lower number of stream habitats sampled in this study. Nevertheless, the abundance of B.s. goniomphalos in the paddy habitat was still high, at 83.4%, emphasizing the favorable habitat conditions provided by rice paddies. As Tesana et al. (2012) noted, the availability of plant and shallow water in the rice paddy environment might have accounted for the common presence of B.s. goniomphalos.
B.s. goniomphalos snails were reported in most, if not all, of the sampling sites in studies of different spatial extents in Northeast Thailand. Within a province, Haruay et al. (2008) found B.s. goniomphalos in all 20 localities sampled, whereas F.m. martensi was found in 9 out of 20 localities. Across three provinces, this study reported the presence of B.s. goniomphalos snails in all 34 sampling sites. At a larger spatial extent of the whole Northeast Thailand, Suwannatrai et al. (2011) observed B.s. goniomphalos in 50 of 56 localities. Studies also reported that B.s. goniomphalos survived at high temperature of 41.14 °C (Wang et al. 2015) and extreme water salinity of 22.11 ppt (Suwannatrai et al. 2011). All in all, B.s. goniomphalos seemed to be more tolerant in a wider range of environmental conditions than F.m. martensi.
Water quality, soil properties, and snail elemental concentrations
Water quality and soil properties varied across the habitat and soil types. Similar to prior work (e.g., Wang et al. 2015), the rice paddy generally had higher water temperature and lower water pH than the other two habitat types. Conversely, lower conductivity was detected in this study, marked by conductivity of 0.03 mS/cm, salinity of 0.01 ppt, and TDS of 0.02 g/l in the pond habitat in Khon Kaen Province. This was possibly due to dilution during the rainy season at the time of sampling in September (Thongwat and Terakulsatit 2019).
B.s. goniomphalos snails are typically found in areas with red-yellow podzolic soils (Papasarathorn et al. 1990) and mixed sand–mud substrates (Chitramvong 1992). The findings of this study echo these previous studies that higher dominance of B.s. goniomphalos snails are found in the LHG and RYP soil types with red-yellow podzolic soils. High dominance of B.s. goniomphalos snails have also been observed in rice paddies, of which soils are mainly alluvial and low humic gley soils (Kyuma 2004), with higher clay content and lower pH (Kyuma and Kawaguchi 1966). Likewise, this study illustrates that the paddy habitat has the lowest water and soil pH among the three water habitats. Furthermore, water pH has been found to be significantly negatively associated with B.s. goniomphalos snail heights, underscoring the species’ tolerance of lower pH environments (Table 7).
For F.m. martensi, it has been suggested that they prefer muddy and sandy substrates (Haruay and Piratae 2019). The result of this study refines this understanding. On the one hand, it strengthens the association between F.m. martensi snails and sandy substrates, evident in the highest abundance of F.m. martensi snail in soil type Lato (Table 5), which is characterized by a sandy texture (Topark-Ngarm and Gutteridge 1986). Pearson’s correlation coefficients also show positive correlations between soil sand composition and F.m. martensi snail sizes, although the relationship is not significant (Table 8). On the other hand, analysis of soil composition of this study does not show clear associations between F.m. martensi snails and muddy substrates, which mainly consist of silt and clay.
Findings of this study provide further insights into the association between DO and the two snail species. CCA biplot and Pearson’s correlation coefficients suggest stronger associations between DO and F.m. martensi snail characteristics than between DO and B.s. goniomphalos (Fig. 3, Tables 7 and 8). Higher DO has been observed at soil types LHG and Lato where F.m. martensi snail dominates. Alternatively, lower DO has been found at the pond habitat, with the lowest value recorded at 0.06 mg/l (Table 5). Indeed, DO concentrations are naturally low at the bottom of water bodies (Culberson and Piedrahita 1996), and F.m. martensi snails tend to reside at the bottom of ponds where they bury themselves into (Haruay and Piratae 2019). These results indicate that F.m. martensi can be adapted to survive in a wider range of oxygen levels. It should, however, be noted that the concentration of DO in surface water is affected by temperature (Bachand and Horne 1999).
The mean concentrations of the metallic elements are generally higher in B.s. goniomphalos than in F.m. martensi, except for Na and Zn (Table 6). This is likely attributed to the different substrates that the two species feed on. B.s. goniomphalos snails tend to ingest substrates on the surface and in the upper layers of the soil, where most of the elements and organic matter are accumulated (Hartmann 2008). F.m. martensi are commonly found at the ponds where soil is low in concentrations of organic matter and nutrients (Boyd 1995). Additionally, the soil type Lato in which a F.m. martensi dominates is of low organic matter and low cation exchange capacity, marked by the low readings of organic content, Exch. Ca, Exch. K, and Exch. Mg (Table 5).
Compared to Kawaguchi and Kyuma (1974) that reported Exch. Ca at 7.2 cmolc/kg, Exch. Mg at 4.3 cmolc/kg, and Exch. K at 0.3 cmolc/kg in Thai paddy soils, this study recorded higher levels of Exch. Ca (10 cmolc/kg) and lower levels of Exch. Mg (2.08 cmolc/kg) and Exch. K (0.17 cmolc/kg) from the paddy habitat. The element Ca in soil has been considered as more important than Mg, K, and Na in snail distribution (Ondina et al. 1998) because it is critical to a variety of functions in metabolism and reproduction (Porcel et al. 1996). This is seen in both B.s. goniomphalos and F.m. martensi soft tissues that the Ca has the highest elemental concentration among the nine elements analyzed. Besides, the mean Ca concentrations in B.s. goniomphalos were almost twice the amount than those in F.m. martensi in all soil types and water habitats, except for the pond habitat. Other essential elements of K, Na, Fe, and Zn in soft tissues also play a vital role in the nerve–muscle transmission, protein synthesis, and enzyme activation (Zelck et al. 1995; Zsombok et al. 2000; Mostafa et al. 2014), and the concentrations of K and Fe are generally higher in B.s. goniomphalos than in F.m. martensi. The concentrations of heavy metal Ni and Pb are low compared to other elements, suggesting no major toxic contamination in the study area, but both snail species in the pond habitat observe the highest Pb concentrations among the three water habitats. It has been suggested that snails can potentially serve as bioindicators for water pollution, particularly F.m. martensi because of its high tolerance to waste water and metal accumulations (Aroonsrimorakot et al. 2018, Neeratanaphan and Phalaraksh 2017). This finding of this study, however, shows that Pb concentrations are mostly higher in B.s. goniomphalos than in F.m. martensi.
Interspecific competition
The laboratory experiment provided evidences of interspecific competition between B.s. goniomphalos and F.m. martensi snails. In the single-species treatments, the juvenile growth of the two snail species differed, with B.s. goniomphalos exhibiting a much faster growth rate than F.m. martensi. The differences suggested that F.m. martensi snails took a longer duration to attain maturity and adulthood. In the mixed-species treatments, interactions between B.s. goniomphalos and F.m. martensi slowed down their growth rates. Indeed, interspecific competition shapes population dynamics primarily through the reduction of growth rates in competitor species (Baur 1988). Moreover, the interspecific competition between the two snail species were asymmetric as growth rates of B.s. goniomphalos snails were severely reduced by the presence of F.m. martensi snails, but not vice versa (Fig. 5).
The growth inhibition of B.s. goniomphalos might be further explained by interference competition, in which the competition between organisms is direct, often through the aggression display between the competing organisms (Krebs 2009). This is different from exploitation competition where species interact indirectly as they compete for common resources, such as food. Since food was non-limiting in this experimental design, interference competition occurred. The shell erosion and discoloration of B.s. goniomphalos snail juveniles (Fig. 6) revealed a direct act of aggression by F.m. martensi snails. Although it was unknown if the act of aggression was from an individual F.m. martensi or a few of them, or possibly due to the confined space in the experiments, such antagonistic behavior indicated an interference competition between both snail species. Likewise, it has been noted by Kimura and Chiba (2010) that the aggressive behavior by the competitor snail Euhadra peliomphala has led to shell erosion of a subordinate snail species. Alternatively, it was postulated that acts of aggression by F.m. martensi could be in the form of chemical stimuli produced in their mucus trails and feces. The release of such chemical compounds could either directly or indirectly inhibit snail growth through the alteration of behavior interactions between the two snail species (Pearce 1997). Freshwater snails often interact through the detection of chemicals in the trail (Turner and Chislock 2010). As such, chemicals released could lead to behavioral alterations and affect feeding patterns and activities, subsequently inhibiting nutrient intake and ultimately impeding growth (Cross and Benke 2002). Therefore, antagonistic behavior either through direct or indirect means suggested that both B.s. goniomphalos and F.m. martensi engaged in interspecific competition.
This study reported the first laboratory experiment that observed interspecific competition between B.s. goniomphalos and F.m. martensi snails. Although the study has focused on B.s. goniomphalos juveniles for their susceptibility to O. viverrini infection (cf. Kiatsopit et al. 2014; Prasopdee et al. 2015), more analyses from laboratory experiments are needed to reinforce the findings of this study. For example, B.s. goniomphalos adults can be included, and the number of B.s. goniomphalos snails in the treatments can be increased to investigate if the degree of interspecific competition remains the same. It is possible that aggression will still occur because F.m. martensi snails are generally three times larger than B.s. goniomphalos snails. A systematic approach to measure aggression will thus be desirable. Other aspects of competitions, particularly relating to fecundity, should be considered. Given that B.s. goniomphalos growth reduction has been observed in this study with the presence of F.m. martensi, it is important to know how growth reduction may affect snail reproduction rate, for its potential consequences on O. viverrini transmission. More combinations of treatments over a longer duration of observation to incorporate how growth reduction may affect reproduction rate will further substantiate the interspecific competition between B.s. goniomphalos and F.m. martensi.
Implications for disease control
Interspecific competition has been considered as the determining force shaping snail population dynamics among different types of interaction between snail species (Bloch and Willig 2012). In addition, the “decoy effect” in the context of snail-borne infections has suggested an encounter or transmission reduction by non-host snails as a sponge intercepting snail-infective stages as well as a reduction of susceptible hosts by adding competing or predating non-host snail species to the system (Stensgaard et al. 2016). F.m. martensi has therefore been proposed to be a biological control agent against B.s. goniomphalos, the first intermediate hosts of O. viverrini because of the potential competition between the two snail species for feeding on similar types of plankton (Haruay et al. 2008). It has also been noted that F.m. martensi snails may feed on feces contaminated with O. viverrini eggs in sediments (Haruay et al. 2008). This study, similar to Haruay et al. (2008) and Wang et al. (2015), shows a negative correlation in species abundances between B.s. goniomphalos and F.m. martensi. Moreover, this study provides evidences in interspecific competition between the two snail species from lab experiments that the growth rates of B.s. goniomphalos are limited in the presence of F.m. martensi, and the rates are further hampered in treatments with more F.m. martensi snails (Fig. 5).
Nevertheless, the use of F.m. martensi snails to control B.s. goniomphalos snails requires continuous research efforts. First, experiments can be expanded to investigate the reproductive rates of both snail species under competition and the encountering rate between B.s. goniomphalos snails and O. viverrini. On the one hand, growth reduction in B.s. goniomphalos, as observed in this study, may enable snail energy allocation to reproduction due to life history trait evolution (Stearns 2000), thereby contributing to more B.s. goniomphalos individuals. On the other hand, if the snails are infected by trematodes, snail growth rate can be accelerated to exhibit gigantism (Mostafa 2007; Kiatsopit et al. 2012), but their fecundity may be affected as shown in the egg capsule defects (Serbina 2015). An earlier study has suggested that young snails appeared to be more susceptible to O. viverrini infection, and medium exposure to O. viverrini eggs (50 eggs per snail, as opposed to 30 and 90 eggs per snail) yielded the highest percentage of living positive Bithynia snails (Chanawong and Waikagul 1991). Follow-up work is needed to examine the host–parasite relationship, under the competition with F.m. martensi.
Second, sufficient risk assessments need to be conducted before the introduction of any biological control agent. In this study area and the larger Mekong region context, F.m. martensi snails also serve as both first and second intermediate hosts of several trematodes. In particular, the intestinal flukes, Echinostoma, uses F.m. martensi as the second intermediate host for the development of their infective metacercaria stage. F.m. martensi is consumed by some villagers in Thailand as a food source, and humans can get infected with Echinostomiasis if the snails are undercooked. Biological control using F.m. martensi snail, albeit may bring down B.s. goniomphalos snail abundance, should hence be carefully assessed to avoid the cascade effects of other parasitic infections. Another possible snail-control option of using Anentome (Clea) helana snails has been proposed, as Haruay and Piratae (2019) have reported that the species consumed both B.s. goniomphalos and F.m. martensi. Follow-up research on this is desirable because A. helana snail abundance has been low in water habitats—it had a low relative abundance of 2.5% in Wang et al. (2015) and was not come across in the sampling sites of this study.
Third, the dynamics of the ecosystems should be considered for ecosystem-based disease control. The higher mean dominances of B.s. goniomphalos snails than F.m. martensi snails across all habitat and soil types as well as the broader coverages of B.s. goniomphalos snails in the PCA biplots, suggest a greater adaptability of B.s. goniomphalos snails to the water quality and soil properties examined in this study. Nonetheless, it is crucial to note that species dominance can vary across locality and seasonality; species interactions in the field occur beyond just B.s. goniomphalos and F.m. martensi, and species diversity may buffer population expansion of some species in the ecosystem. While F.m. martensi seems to exhibit a limited environmental tolerance, other studies have found the dominance of F.m. martensi snails in rivers (Nahok et al. 2017). Furthermore, the possible transmission pathways between snails and cyprinid fish, the second intermediate host of O. viverrini, need to be integrated to prioritize field experiments in areas where snails and cyprinid fish have closer interactions, such as rivers and ponds, for O. viverrini transmission reduction. An ecosystem-based, biodiversity-oriented approach to investigate multiple species communities across different sites and seasons is desirable, so as to inform disease control strategies and better align with integrated ecosystem health management.
References
Alsaleh M, Sithithaworn P, Khuntikeo N, Loilome W, Yongvanit P, Chamadol N, Hughes T, O'Connor T, Andrews RH, Holmes E, Taylor-Robinson SD (2019) Characterisation of the urinary metabolic profile of liver fluke-associated cholangiocarcinoma. J Clin Exp Hepatol 9(6):657–675
Aroonsrimorakot S, Sakulkiatpanya T, Muangkun S (2018) Heavy metal concentration in the components of Golden apple snail (Pomacea canaliculata) and pond snail (Filopaludina martensi). Interdiscip Res Rev 12(5):5–10
Aung WP, Htoon TT, Tin HH, Thinn KK, Sanpool O, Jongthawin J, Sadaow L, Phosuk I, Rodpai R, Intapan PM, Maleewong W (2017) First report and molecular identification of Opisthorchis viverrini infection in human communities from Lower Myanmar. PLoS One 12:e0177130
Aunpromma S, Tangkawattana P, Papirom P, Kanjampa P, Tesena S, Sripa B, Tangkawattana S (2012) High prevalence of Opisthorchis viverrini infection in reservoir hosts in four districts of Khon Kaen Province, an opisthorchiasis endemic area of Thailand. Parasitol Int 61:60–64
Bachand PAM, Horne AJ (1999) Denitrification in constructed free-water surface wetlands: II. Effects of vegetation and temperature. Ecol Eng 14:17–32
Baur B (1988) Microgeographical variation in shell size of the land snail Chondrina clienta. Biol J Linn Soc Lond 35:247–259
Begon M, Harper JL, Townsend CR (1996) Ecology: individuals, populations, communities. Blackwell Science, Oxford
Beretta AN, Silbermann AV, Paladino L, Torres D, Bassahun D, Musselli R, Garćia-Lamohte A (2014) Soil texture analyses using a hydrometer: modification of the Bouyoucos method. Cienc Investi Agrar 41(2):263–271
Bloch CP, Willig MR (2012) Density compensation suggests interspecific competition is weak among terrestrial snails in tabonuco forest of Puerto Rico. Caribb J Sci 46(2–3):159–168
Bloom PR, Skyllberg U (2012) Soil pH and pH buffering. In: Huang PM, Li Y, Sumner ME (eds) Handbook of soil sciences, 2nd edn. CRC Press, Boca Raton, Florida, pp 1–14
Blott SJ, Pye K (2012) Particle size scales and classification of sediment types based on particle size distributions: review and recommended procedures. Sedimentology 59(7):2071–2096
Boyd CE (1995) Bottom soils, sediment, and pond aquaculture. Springer, Boston, Massachusetts
Brady NC, Weil RR (2002) The nature and properties of soils. Prentice Hall, Upper saddle River
Brandt RAM (1974) The non-marine aquatic Mollusca of Thailand. Arch Mollusken 105:1–423
Brockelman WY, Upatham ES, Viyanant V, Ardsungnoen S, Chantanawat R (1986) Field studies on the transmission of the human liver fluke, Opisthorchis viverrini, in northeast Thailand: population changes of the snail intermediate host. Int J Parasitol 16(5):545–552
Chanawong A, Waikagul J (1991) Laboratory studies on host-parasite relationship of Bithynia snails and the liver fluke, Opisthorchis viverrini. Southeast Asian J Trop Med Public Health 22:235–239
Chitramvong YP (1992) The Bithniidae (Gastropoda: Prosobranchia) of Thailand: comparative external morphology. Malacol Rev 25:21–38
Chitramvong YP, Upatham ES, Sukhapanth N (1981) Effects of some physico-chemical factors on the survival of Bithynia siamensis siamensis, Radix rubiginosa and Indoplanorbis exustus. Malacol Rev 14:43–48
Coelho P, Caldeira R (2016) Critical analysis of molluscicide application in schistosomiasis control programs in Brazil. Infect Dis Poverty 5:57
Cross WF, Benke AC (2002) Intra- and interspecific competition among coexisting lotic snails. Oiko 96:251–264
Culberson SD, Piedrahita RH (1996) Aquaculture pond ecosystem model: temperature and dissolved oxygen prediction-mechanism and application. Ecol Model 89:231–258
Eccard JA, Ylönen H (2003) Interspecific competition in small rodents: from populations to individuals. Evol Eco 17:423–440
Evans TW, Irwin SWB, Fitzpatrick S (2001) The effect of digenean (Platyhelminthes) infections on heavy metal concentration in Littorina littorea. J Mar Biol Assoc UK 81:349–350
Gomot A, Gomot L, Boukraa S, Bruckert S (1989) Influence of soil on the growth of the land snail Helix aspersa. An experimental study of the route for the stimulating factors. J Molluscan Stud 55:1–7
Hammer Ø, Harper DAT, Ryan, PD (2001) PAST: paleontological statistics software package for education and data analysis. Palaeontol Electron 4(1):1–9.http://palaeo-electronica.org/2001_1/past/issue1_01.htm. Accessed 26 Feb 2020
Hartmann R (2008) Aggregation. In: Chesworth W (ed) Encyclopedia of soil science. Springer, Dordrecht, pp 30–32
Haruay S, Piratae S (2019) Situation and cercarial infection of freshwater mollusk from Sirindhorn Reservoir, Ubon Ratchathani Province, Thailand. Iran J Parasitol 14(3):421–429
Haruay S, Tesana S, Suwannatrai A, Piratae S, Kampoosa P, Thammasiri C, Duangprompo W, Kaewkes S, Tarbsripair P, Paiboonsak S (2008) Effects of surface salt on distribution on density of snails in Family Viviparidae in Khon Kaen province, Thailand, analyses by using geographic information system. KKU Res J 8:40–50
IARC (2012) A review of human carcinogens, biological agents. IARC Monogr Eval Carcinog Risks Hum 100B. International Agency Research On Cancer, Lyon
Kaewkes S (2003) Taxonomy and biology of liver flukes. Acta Trop 88(3):177–186
Kaufer SW, Chejlava M, Fried B, Sherma J (2002) Effects of Euhaplorchis californiensis (Trematoda) infection on metallic ions in the host snail Cerithidea californica (Gastropoda). Parasitol Res 88:1080–1082
Kaufmann KW (1981) Fitting and using growth curves. Oecologia 49:293–299
Kawaguchi K, Kyuma K (1974) Paddy soils in Tropical Asia part 1. Description of fertility characteristics. Southeast Asian Stud 12(1):3–24
Kiatsopit N, Sithithaworn P, Saijuntha W, Boonmars T, Tesana S, Sithithaworn J, Petney TN, Andrews RH (2012) Exceptionally high prevalence of infection of Bithynia siamensis goniomphalos with Opisthorchis viverrini cercariae in different wetlands in Thailand and Lao PDR. Am J Trop Med Hyg 86:464–469
Kiatsopit N, Sithithaworn P, Kopolrat K, Andrews RH, Petney TN (2014) Seasonal cercarial emergence patterns of Opisthorchis viverrini infecting Bithynia siamensis goniomphalos from Vientiane Province, Lao PDR. Parasit Vectors 7:551
Kiatsopit N, Sithithaworn P, Kopolrat K, Namsanor J, Andrews RH, Petney TN (2016) Trematode diversity in the freshwater snail Bithynia siamensis goniomphalos sensu lato from Thailand and Lao PDR. J Helminthol 90(3):312–320
Kim CS, Echaubard P, Suwannatrai A, Kaewkes S, Wilcox BA, Sripa B (2016) Seasonal and spatial environmental influence on Opisthorchis viverrini intermediate hosts, abundance, and distribution: insights on transmission dynamics and sustainable control. PLoS Negl Trop Dis 10(11):e0005121
Kimura K, Chiba S (2010) Interspecific interference competition alters habitat use patterns in two species of land snails. Evol Ecol 24:815–825
Krebs CJ (2009) Ecology: the experimental analysis of distribution and abundance, six edn. Pearson Benjamin Cummings, San Francisco
Kyuma K (2004) Paddy soil science. Kyoto University Press, Kyoto & Trans Pacific Press, Melbourne
Kyuma K, Kawaguchi K (1966) Major soils of Southeast Asia and the classification of soils under rice cultivation (Paddy soils). Southeast Asian Stud 4:290–312
Laoprom N, Kiatsopit N, Sithithaworn P, Kopolrat K, Namsanor J, Andrews RH, Petney TN (2016) Cercarial emergence patterns for Opisthorchis viverrini sensu lato infecting Bithynia siamensis goniomphalos from Sakon Nakhon Province, Thailand. Parasitol Res 115(9):3313–3321
Lavkulich LM (1981) Methods manual, pedology laboratory. Department of Soil Science, University of British Columbia, Vancouver, British Columbia
Lohachit C (2004-2005). Ecological studies of Bithynia siamensis goniomphalos, a snail intermediate host of Opisthorchis viverrini, in Khon Kaen Province, Northeast Thailand. Malacol Rev 37/38:1–26
Magurran AE (2004) Measuring biological diversity. Blackwell, Malden, Massachusetts
Moormann FR, Rojanasoonthon S. (Map makers) (1967) Kingdom of Thailand general soil map. Bangkok: Soil Survey Division, Land Development Department. http://eusoils.jrc.ec.europa.eu/ESDB_Archive/EuDASM/Asia/images/maps/download/TH2004_1SO.jpg. Accessed 1 December 2019
Moran AL, Emlet RB (2001) Offspring size and performance in variable environments: field studies on a marine snail. Ecology 82(6):1597–1612
Mostafa OM (2007) Effects of Schistosoma mansoni and Schistosoma haematobium infections on calcium content in their intermediate hosts. Parasitol Res 101:963–966
Mostafa OM (2008) Effects of Fasciola gigantica experimental infection on some inorganic elements in the snail host Lymnaea natalensis. Exp Parasitol 118:468–471
Mostafa OM, Mossa AT, El Einin HM (2014) Heavy metal concentrations in the freshwater snail Biomphalaria alexandrina uninfected or infected with cercariae of Schistosoma mansoni and/or Echinostoma liei in Egypt: the potential use of this snail as a bioindicator of pollution. J Helminthol 88(4):411–416
Mourier B, Fritsch C, Dhivert E, Gimbert F, Cœurdassier M, Pauget B, Vaufleury A, Scheifler R (2011) Chemical extractions and predicted free ion activities fail to estimate metal transfer from soil to field land snails. Chemosphere 85:1057–1065
Nahok B, Srifa A, Tumpeesuwan C, Tumpeesuwan S (2017) Freshwater molluscan assemblages in upper part of Choen River Basin, northeastern Thailand. Trop Nat Hist 17(1):11–24
Namsanor J, Sithithaworn P, Kopolrat K, Kiatsopit N, Pitaksakulrat O, Tesana S, Andrews RH, Petney TN (2015) Seasonal transmission of Opisthorchis viverrini sensu lato and a lecithodendriid trematode species in Bithynia siamensis goniomphalos snails in northeast Thailand. Am J Trop Med Hyg 93(1):87–93
Neeratanaphan L, Phalaraksh C (2017) Contamination of heavy metals in sediment and mollusks collected form Bueng Jode reservoir, Khon Kaen province. Asia Pac J Sci Technol 13:1058–1064
Ondina P, Mato S, Hermida J, Outeiro A (1998) Importance of soil exchangeable cations and aluminium content on land snail distribution. Appl Soil Ecol 9(1–3):229–232
Ong X, Wang YC, Sithithaworn P, Grundy-Warr C, Pitaksakulrat O (2016) Dam influences on liver fluke transmission: fish infection and human fish consumption behavior. Ann Am Assoc Geogr 106(4):755–772
Palasun S, Intanai I (2015) Effect of copper on hatching, egg development and metabolism of freshwater pond snail Filopaludina martensi. Proc the 6th Hatyai Nat. Int Conference, Thailand, 26 Jun 2015. pp 879–890.
Papasarathorn T, Sumethanurugkul P, Viboolyavatana J, Temcharoen P, Tongkoom B (1990) Studies of life history and ecological requirements of Bithynia (Digoniostoma) siamensis goniomphalos, the snail intermediate host of Opisthorchis viverrini. J Parasitol Trop Med Assoc Thailand 3:21–27
Pearce TA (1997) Interference and resource competition in two land snails: adult inhibit conspecific juvenile growth in field and laboratory. J Molluscan Stud 63:389–399
Perrett S, Whitfield PJ (1996) Currently available molluscicides. Parasitol Today 12:156–159
Petney T, Sithithaworn P, Andrews R, Kiatsopit N, Tesana S, Grundy-Warr C, Ziegler A (2012) The ecology of the Bithynia first intermediate hosts of Opisthorchis viverrini. Parasitol Int 61:38–45
Piratae S (2015) Bithynia siamensis goniomphalos, the first intermediate host of Opisthorchis viverrini in Thailand. Asian Pac J Trop Med 8(10):779–783
Pointier JP, David P (2004) Biological control of Biomphalaria glabrata, the intermediate host of schistosomes, by Marisa cornuarietis in ponds of Guadeloupe: long-term impact on the local snail fauna and aquatic flora. Biol Control 29:81–89
Pointier JP, Guyard A, Mosser A (1989) Biological control of Biomphalaria glabrata and B. straminea by the competitor snail Thiara tuberculata in a transmission site of schistosomiasis in Martinique, French West Indies. Ann Trop Med Parasitol 83(3):363–369
Porcel D, Bueno JD, Almendros A (1996) Alterations in the digestive gland and shell of the snail Helix aspersa Müller (Gastropoda, Pulmonata) after prolonged starvation. Comp Biochem Physiol 115A:11–17
Prasopdee S, Kulsantiwong J, Piratae S, Khampoosa P, Thammasiri C, Suwannatrai A, Laha T, Grams R, Loukas A, Tesana S (2015) Temperature dependence of Opisthorchis viverrini infection in first intermediate host snail, Bithynia siamensis goniomphalos. Acta Trop 141(Pt a):112–117
Rim HJ, Sohn WM, Yong TS, Eom KS, Chai JY, Min DY, Lee SH, Hoang EH, Phommasack B, Insisengmay S (2008) Fishborne trematode metacercariae detected in freshwater fish from Vientiane Municipality and Savannakhet Province, Lao PDR. Korean J Parasitol 46(4):253–260
Robertson GP, Coleman DC, Bledsoe CS, Sollins P (1999) Standard soil methods for long-term ecological research. Oxford University Press, New York
Santisteban JI, Mediavilla R, López-Pamo E, Dabrio CJ, Zapata MR, García MG, Castaño S, Martínez-Alfaro PE (2004) Loss on ignition: a qualitative or quantitative method for organic matter and carbonate mineral content in sediments? J Paleolimnol 32(3):287–299
Seppälä O, Karvonen A, Kuosa M, Haataja M, Jokela J (2013) Are sick individuals weak competitors? Competitive ability of snails parasitized by a gigantism-inducing trematode. PLoS One 8(10):e79366
Serbina EA (2015) The effect of trematode parthenites on the individual fecundity of Bithynia troscheli (Prosobranchia: Bithyniidae). Acta Parasit 60:40–49
Sithithaworn P, Haswell-Elkins M (2003) Epidemiology of Opisthorchis viverrini. Acta Trop 88(3):187–194
Sithithaworn P, Andrews RH, Nguyen VD, Wongsaroj T, Sinuon M, Odermatt P, Nawa Y, Liang S, Brindley PJ, Sripa B (2012) The current status of opisthorchiasis and clonorchiasis in the Mekong Basin. Parasitol Int 61:10–16
Slootweg R, Malek EA, McCullough FS (1994) The biological control of snail intermediate hosts of schistosomiasis by fish. Rev Fish Biol Fisher 4:67–90
Sripa B, Pairojkul C (2008) Cholangiocarcinoma: lessons from Thailand. Curr Opin Gastroenterol 24(3):349–356
Sripa B, Bethony JM, Sithithaworn P, Kaewkes S, Mairiang E, Loukas A, Mulvenna J, Laha T, Hotez PJ, Brindley P (2011) Opisthorchiasis and Opisthorchis-associated cholangiocarcinoma in Thailand and Laos. Acta Trop 120(Suppl 1):S158–S168
Stearns SC (2000) Life history evolution: successes, limitations, and prospects. Naturwissenschaften 87:476–486
Stensgaard A, Kristensen TK, Jørgensen A, Kabatereine NB, Rahbek C (2016) Associations between patterns of human intestinal schistosomiasis and snail and mammal species richness in Uganda: Can we detect a decoy effect? Front Biogeogr 8(3):e21748
Suwannatrai A, Suwannatrai K, Haruay S, Piratae S, Thammasiri C, Khampoosa P, Kulsantiwong J, Prasopdee S, Tarbsripair P, Suwanwerakamtorn R, Sukchan S, Boonmars T, Malone JB, Kearney MT, Tesana S (2011) Effect of soil surface salt on the density and distribution of the snail Bithynia siamensis goniomphalos in northeast Thailand. Geospat Health 5(2):183–190
Tesana S, Thapsripair P, Thammasiri C, Prasopdee S, Suwannatrai A, Harauy S, Piratae S, Khampoosa P, Kulsantiwong J, Donthaisong C, Chalokepanrat P, Viyanant V, Malone JB (2012) Effects of Bayluscide on Bithynia siamensis goniomphalos, the first intermediate host of the human liver fluke, Opisthorchis viverrini, in laboratory and field trials. Parasitol Int 61(1):52–55
Thongwat W, Terakulsatit B (2019) Using GIS and map data for the analysis of the relationship between soil and groundwater quality at saline soil area of Kham Sakaesaeng District, Nakhon Ratchasima, Thailand. Int J Mater Metall Eng 13(1):32–39
Topark-Ngarm A, Gutteridge RC (1986) Forages in Thailand. In: Blair GJ, Ivory DA, Evans TR (eds.), Forages in South East Asia and South Pacific Agriculture. Proc Int Workshop CisarruaIndonesia19–23 Aug 1985, ACIAR Proc 12:96–103
Tunholi VM, Lustrino D, Tunholi-Alves VM, Garcia JS, Mello-Silva CC, Maldonado A Jr, Rodrigues Mde L, Pinheiro J (2011) Influence of Echinostoma paraensei (Lie and Basch, 1967) infection on the calcium content in Biomphalaria glabrata (Say, 1818). Exp Parasitol 129(3):266–269
Turner AM, Chislock MF (2010) Blinded by the stink: nutrient enrichment impairs the perception of predation risk by freshwater snails. Ecol Appl 20(8):2089–2095
Wang YC (2012) Examining landscape determinants of Opisthorchis viverrini transmission. EcoHealth 9(3):328–341
Wang YC, Ho R, Feng CC, Namsanor J, Sithithaworn P (2015) An ecological study of Bithynia snails, the first intermediate host of Opisthorchis viverrini in Northeast Thailand. Acta Trop 141:244–252
Wang YC, Yuen R, Feng CC, Sithithaworn P, Kim IH (2017) Assessing the role of landscape connectivity on Opisthorchis viverrini transmission dynamics. Parasitol Int 66(4):402–412
WHO (2017) Field use of molluscicides in schistosomiasis control programmes: an operational manual for programme managers. World Health Organization, Geneva
Wong PK, Kwong KL, Qiu JW (2009) Complex interactions among fish, snails and macrophytes: implications for biological control of an invasive snail. Biol Invasions 11:2223–2232
Zarabi M, Jalali M (2012) Leaching of nitrogen and base cations from calcareous soil amended with organic residues. Environ Technol 33(14):1577–1588
Zelck UE, Becker W, Bayne CJ (1995) The plasma proteins of Biomphalaria glabrata in the presence and absence of Schistosoma mansoni. Dev Comp Immunol 19(3):181–194
Zsombok A, Schrofner S, Hermann A, Kerschbaum HH (2000) Nitric oxide increase excitability by depressing calcium activated potassium current in snail neurons. Neurosci Lett 295:85–88
Acknowledgements
The authors are thankful to Liren Ling, Huixian Tan, Shalom Tan, and Siewping Yeo for their assistance in field work, and to the National University of Singapore for funding support through the Academic Research Fund (Grant Number: FY2017-FRC3-008, WBS: R-109-000-235-115).
Availability of data and material
The authors confirm that all data underlying the findings are fully available without restriction. Data will be deposited at the National University of Singapore digital institutional repository ScholarBank@NUS.
Code availability
Not applicable
Funding
This research was supported by the National University of Singapore through the Academic Research Fund (Grant Number: FY2017-FRC3-008, WBS: R-109-000-235-115). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design and commented on previous versions of the manuscript. Manuscript writing was done by Yi-Chen Wang and Jutamas Namsanor. Methodology was done by Yi-Chen Wang and Timothy Zherui Liew. Investigation and analysis were done by Timothy Zherui Liew, Jutamas Namsanor, and Yi-Chen Wang. Funding acquisition was done by Yi-Chen Wang. Resources were handled by Yi-Chen Wang and Paiboon Sithithaworn. Manuscript revision was done by all the authors.
Corresponding authors
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that they have no conflicts of interest.
Ethics approval
Not applicable
Consent to participate
Not applicable
Consent for publication
Not applicable
Additional information
Handling Editor: Una Ryan
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, YC., Liew, T.Z., Namsanor, J. et al. Assessing the role of Filopaludina martensi martensi as a biocontrol agent of Bithynia siamensis goniomphalos, the first intermediate host of Opisthorchis viverrini. Parasitol Res 119, 3415–3431 (2020). https://doi.org/10.1007/s00436-020-06837-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-020-06837-7