Abstract
Ultrastructural and phylogenetic description of a fish-infecting myxosporean found infecting the gallbladder wall of the teleostean Eugerres brasilianus Cuvier, 1830, collected from the Atlantic coast near the city of Maceió (Alagoas State), Brazil. Groups of mature pseudo-conical myxospores, agglutinated forming pseudocyst structures, occurring in the mucosa of gallbladder were 5.2 ± 0.8 μm (4.5–6.0) (n = 30) long, 4.3 ± 0.6 μm (3.8–4.7) (n = 25) thick, and 2.9 ± 0.2 μm (2.7–3.2) (n = 25) wide. The two ellipsoidal polar capsules, 1.8 ± 0.4 × 1.2 ± 0.4 μm (n = 25), opened close to the sutural line, each containing an isofilar polar tubule. The latter consisted of a single coil with five to six turns, arranged obliquely to the axis of the polar capsule. This myxosporean parasite, while being morphologically similar to Sphaerospora spp., displays tissue tropism and phylogenetic relationships distinct from the latter. Bayesian inference (BI) and maximum likelihood (ML) analyses showed the parasite and two other related species clustering within the marine clade, more specifically within a subclade of the larger Kudoa (Multivalvulida) clade. Consequently, this atypical new myxozoan species was classified as Kudoa eugerres n. sp. and two other histozoic Sphaerospora spp. sensu lato were transferred to the genus Kudoa.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Myxosporeans are metazoan parasites belonging to the phylum Cnidaria Hatschek, 1888, characterized by a complex life cycle, in which there is an alternation of stages between an invertebrate definitive host (Polychaeta or Oligochaeta), and a poikilothermic vertebrate as intermediate host (usually freshwater, brackish, or marine fishes, but also amphibians or reptiles) (Lom and Dyková 2006). Presently, it is widely recognized that the main evolutionary trends are the definitive and intermediate hosts and the tissue tropism (Carriero et al. 2013; Holzer et al. 2018).
Numerous myxosporeans have been reported from freshwater and marine habitats in different geographic regions. The genus Sphaerospora Thélohan, 1892 accounts for at least 102 species described from Eurasia and North America, being most of them coelozoic parasites of the urinary system (Lom and Dyková 2006; Jirků et al. 2007; Patra et al. 2018) and, more rarely, of the gallbladder (Gb) (Su and White 1994; Zhao et al. 2015). Some occurrences in other organs which involve histozoic development have also been reported (Lom and Dyková 1992). Sphaerospora molnari infects the gill filaments and skin of the common carp Cyprinus carpio (Lom et al. 1983; Eszterbauer et al. 2013), S. testicularis infects testicular tissue and intestine of wild and cultured of Dicentrarchus labrax (Sitjà-Bobadilla and Alvarez-Pellitero 1990; Fioravanti et al. 2004), S. ovophila was found in Canada in the ovary of the teleostean Lepomis gibbosus (Xiao and Desser 1997), and S. dicentrarchi is a systemic parasite of European seabass, D. labrax (Sitjà-Bobadilla and Alvarez-Pellitero 1992; Fioravanti et al. 2004) having been described from the tissues of several organs, predominantly the Gb and intestine, with a prevalence of 100% and 70.5% in wild and cultured fishes, respectively (Sitjà-Bobadilla and Alvarez-Pellitero 1993). The latter species was also reported from spotted seabass, D. punctatus, caught in the estuary of the Alvor River (southern Portugal) (Xavier et al. 2013).
Phylogenetic analyses show the genus Sphaerospora as one the most polyphyletic groups among myxozoans. Sphaerospora (sensu stricto) (s.s.), also known as “true Sphaerosporids,” form a basal clade in which most species are included, while Sphaerospora (sensu lato) (s.l.) appeared dispersed among several subclades of marine and freshwater clades (Bartošová et al. 2013; Holzer et al. 2013). Previous phylogenetic studies have shown that Sphaerospora s.l. are not true Sphaerosporids and, therefore, should be taxonomically reclassified (Diamant et al. 2005; Bartošová et al. 2011; Jones et al. 2011).
This study describes the occurrence of a Sphaerospora-like myxosporean from a South American fish of the genus Eugerres (Family Gerreidae). This genus accounts for 8 species, from which no myxozoan parasitosis was ever reported. The Brazilian fish E. brasilianus is distributed along the Western Central Atlantic: from South Carolina (USA) to the South of Brazil, being broadly caught along the northeastern region of Brazil and in the lower São Francisco River. In the latter region, this fish represents one of the most important marine species, much appreciated and of great commercial value, namely as a potential species to be introduced in aquaculture (Soares et al. 2016). In the present study, a new species, Kudoa eugerres n. sp., is described from the gallbladder wall (GbW) of E. brasilianus. The morphological characteristics and phylogenetic positioning of marine histozoic bivalvulids are discussed, resulting in the emendation of the diagnosis of the order Multivalvulida, Family Kudoidae and genus Kudoa in order to accommodate the parasite in the study and two other known species.
Materials and methods
Fish sampling
Nineteen specimens of the teleost fish Eugerres brasilianus Cuvier, 1830 (Teleostei, Gerreidae) (Brazilian common name “carapeba” or “mojarra”) (about 20 to 30 cm long, and about 150 to 250 g), were collected between July and December 2017, from the Atlantic coast (09° 44′ S/35° 49′ W), near the city of Maceió (Alagoas State), Brazil. Fishes were transported alive to the laboratory and maintained for 3–6 days in an aquarium with aerated seawater, following the abiotic parameters calculated at the site of fish collection: salinity 3.0–3.5‰, pH 6.0–6.5, temperature ~ 25 °C. All specimens were periodically observed and later anesthetized with MS 222 before being dissected.
Light microscopy and transmission electron microscopy
For LM, small fragments of tissues of different organs were checked under a microscope for a parasitological survey. Small fragments of infected GbW were removed and photographed using a light microscope.
For TEM, small fragments of infected GbW were fixed in 4–5% glutaraldehyde in 0.2 M sodium cacodylate buffer (pH 7.2–7.4) for 20–24 h at 4 °C and then washed overnight in the same buffer at 4 °C, and post-fixed with 2% osmium tetroxide in the same buffer for 3 h at the same temperature, dehydrated through an ascending ethanol and propylene oxide series, and embedded in Epon. The semi-thin sections were stained with methylene blue-Azur II for LM observations. Ultrathin sections were double stained with uranyl acetate and lead citrate before observation in a JEOL 100CXII TEM (JEOL Optical, Tokyo, Japan), operated at 60 kV.
DNA extraction, PCR amplification, and DNA sequencing
Small fragments of infected GbW were collected from three specimens and preserved in 80% ethanol at 4 °C before genomic DNA extraction, which was performed using a GenElute™ Mammalian Genomic DNA Miniprep Kit for each sample, following the manufacturer’s instructions. The DNA was stored in 50 μL of TE buffer at − 20 °C until further use. Amplification of the SSU rDNA gene was achieved using both universal and specific myxosporean primers: the 5′-end with the primers 18e (5′CTG GTT GAT CCT GCCAGT3′) (Hillis and Dixon 1991)/MYX4r (5′CTGACAGATCACTCCACGAAC3 ′) (Hallett and Diamant 2001), and Kud6r (5′CGTTCATTCCGATAGTGA3′) (Whipps et al. 2003); and the 3′-end with the primers MyxospecF (5′TTCTGCCCTATCAACTTGTTG3′) (Fiala 2006), and MYX4f (5′GTTCGTGGAGTGATCTGTCAG3 ′) (Rocha et al. 2015)/18r (5′CTACGGAAACCTTGTTACG3′) (Whipps et al. 2003). PCR was carried out in 50-μL reactions using 10 pmol of each primer, 10 nmol of each dNTP, 2.5 mM MgCl2, 5 μL 10 × Taq polymerase buffer, 1.5 units Taq DNA polymerase (Nzytech, Lisbon, Portugal), and approximately 50–100 ng of genomic DNA. The reactions were run on a Hybaid P × E Thermocycler, with initial denaturation at 95 °C for 3 mins, followed by 35 cycles of 94 °C for 45 s, 53 °C for 45 s, and 72 °C for 90 s. The final elongation step was performed at 72 °C for 7 mins. Five-microliter aliquots of the PCR products were electrophoresed through a 1% agarose 1 × tris–acetate–EDTA buffer (TAE) gel stained with ethidium bromide. The purification of PCR products was achieved using a single-step enzymatic cleanup that eliminates unincorporated primers and dNTPs by means of the ExoFast method. Purified PCR products were directly sequenced using a Big Dye Terminator v.1.1 from the Applied Biosystems Kit and were run on an ABI3700 DNA analyzer (Perkin-Elmer, Applied Biosystems, Stabvida, Caparica, Portugal).
Distance and phylogenetic analysis
The obtained forward and reverse sequence segments were manually aligned with ClustalW (Thompson et al. 1994) in MEGA 7.0.9 software (Kumar et al. 2016), and ambiguous bases were clarified using corresponding ABI chromatograms. To evaluate the relationship of this species to other myxozoans, a homology search was performed using BLAST software, resulting in the selection of 78 SSU rDNA sequences from GenBank namely several Kudoa species, Sphaerospora species (excluding replicate sequences), as well as some representatives of coelozoic myxozoans genera (Chloromyxum, Gadimyxa, Myxidium, Myxobilatus, Sphaeromyxa, Parvicapsula, and Zschokkella), and histozoic myxozoans of the genera Enteromyxum, Gastromyxum, Monomyxum, and Unicapsula. Tetracapsuloides bryosalmonae (U70623) and Buddenbrockia plumatellae (AY074915) were selected as the outgroup. The sequences were aligned using the software multiple alignments using fast Fourier transform version 7 (MAFFT v.7) available online, and subsequent phylogenetic and molecular evolutionary analyses were conducted in MEGA 7.0.9.
For inferring phylogenetic relationships, Bayesian inference (BI) and maximum likelihood (ML) methods were used. BI analyses were performed using MrBayes v.3.2.6 (Ronquist and Huelsenbeck 2003). The general time reversible model with gamma-shaped rate variations across sites (Invgamma) (GTR + I + Γ) was used, in accordance with the model test algorithm of the software. Posterior probability distributions were generated using the Markov chain Monte Carlo (MCMC) method, with four chains running simultaneously for 1,000,000 generations, and every 100th tree sampled. ML analyses were conducted in MEGA 7.0.9, using the general time reversible substitution model with four gamma-distributed rate variation among sites, with bootstrap confidence values calculated from 500 replicates.
Distance estimation was carried out for some sequences of the main marine clade (Kudoa subclade, 2 Sphaerospora spp., and the myxosporean described here), which was first aligned with the software MAFFT version 7, and then, the distance calculated using a p distance model distance matrix for transitions and transversions in MEGA 7.0.9. All positions containing gaps and missing data were eliminated; all ambiguous positions removed for each sequence pair.
Results
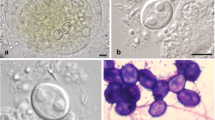
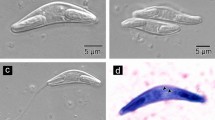
All internal organs were checked under a microscope for a parasitological survey of a total of 19 specimens, of which 11 specimens (7 females and 4 males) were infected by myxospores located in the GbW connective tissues. Myxospores were morphologically similar to the bivalvulids of the genus Sphaerospora Thélohan, 1892. The myxospores were agglutinated forming pseudocyst structures (Pcls) with a variable number of myxospores located in the mucosa of GbW (Fig. 1a–c). These groups of myxospores formed Pcls with variable dimensions were in direct contact with the host tissues (Fig. 1b, c), some of which are open contacting with the Gb lumen content (Fig. 1a and inset). LM observations of isolated mature myxospores show its morphological aspects, mainly the position of the two polar capsules located side by side (Fig. 1d).
Light micrographs of the infected gallbladder wall (GbW) of the teleostean Eugerres brasilianus from Brazil showing several myxospores of Kudoa eugerres n. sp. located in pseudocyst structures within the internal GbW tissues near the lumen. a Semi-thin section (stained with toluidine blue-Azur II) of a pseudocyst located in the GbW tissues (H), near the lumen (Lu), showing several myxospores (arrows), sectioned at different levels. Inset: detail of the different myxospore sections. b Fresh portion of the GbW showing some pseudocyst structures containing myxospores (*), organized among the Gb tissue layers (H). c Details of some myxospores (arrows) among host tissues (H), showing the two polar capsules. d Group of myxospores (arrows) randomly distributed in the host tissue
Systematic position
Phylum Cnidaria Hatschek, 1888
Sub-phylum Myxozoa Grassé, 1970;
Class Myxosporea Bütschli, 1881;
Order Multivalvulida Shulman, 1959
Diagnosis: Amended (bold): Spores are radially symmetrical, the posterior face is flat or semispherical. Spores’ wall comprising 2–13 soft valves (frequently 4) that overlap, making the suture line often indistinct. One polar capsule per valve situated at the apex of spore; their tips are covered by the valve and they discharge apically. Plasmodia are histozoic and develop, usually, in the muscle tissues of marine fishes. The pansporoblast formation has not been observed.
Family Kudoidae Meglitsch, 1960
Diagnosis: Amended (bold) after Whipps et al. 2004: Spores with 4–13 valves (rarely 2) and 4–13 polar capsules (rarely 2). Two sporoplasm cells, one inside the other, or a single binucleated sporoplasm.
Genus Kudoa Meglitsch, 1947
Diagnosis: Amended (bold) after Whipps et al. 2004: Spores stellate, quadrate, or subpherical to ovoid in apical view. Four or more (rarely 2) ellipsoids to pyriform polar capsules, usually of equal-size, corresponding to the number of valves.
Species Kudoa eugerres n. sp.
Description of the species
Type host: Eugerres brasilianus Cuvier, 1830 (Teleostei, Gerreidae).
Type locality: The Atlantic coast (09° 44′ S/35° 49′ W), near the city of Maceió (Alagoas State), Brazil.
Site of infection: The myxospores were agglutinated forming groups with a variable number of myxospores located among the GbW tissues.
Prevalence of infection: Eleven out of 19 fish examined (~ 47%): 7 females (7/19) (~ 37%) and 4 males (4/19) (~ 21%).
Type of specimens: One glass slide with semi-thin sections of the infected tissue of a Gb, containing myxospores of the hapantotype, was deposited in the Myxozoa (Cnidaria)-type collection at the “Instituto Nacional de Pesquisa da Amazônia” (“INPA”), Manaus, Brazil, under the acquisition number (46/2019). The SSU rDNA gene was deposited in GenBank under the accession number MH581487.
Etymology: The specific epithet “eugerres” is derived from the generic name of the host species name.
Description of the myxospores of Kudoa eugerres n. sp.
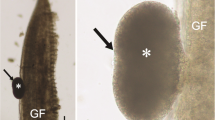
Some myxospores contained within the Pcls were in close contact with the thick collagen layer that surrounded these structures. At these sites, aspects of the degradation of the collagen fibers were evident (Fig. 1a, inset and Fig. 2a, b). Myxospores were pseudo-conical, measuring 5.2 ± 0.8 μm (4.5–6.0) (n = 30) in length, 4.3 ± 0.6 μm (3.8–4.7) (n = 25) in thickness, and 2.9 ± 0.2 μm (2.7–3.2) (n = 25) in width. The two ellipsoidal polar capsules (PC) were located obliquely side by side and opened close to the apical sutural line, each being 1.8 ± 0.4 × 1.2 ± 0.4 μm (n = 15) long and wide, respectively (Figs. 1c, d, and 2c, d). In the apical portion of the myxospores, the cap-like structure formed conical structures in continuity with the polar tubules (Fig. 2e). The polar tubule was isofilar and consisted of a single coil with five to six turns, organized obliquely to the PC axis (Fig. 2d, e). The two valves were thin and formed a prominent sutural line with extensive overlap (Fig. 2d, f). Internally, there was a binucleated sporoplasm localized in the basal region of myxospore. Diagrammatic illustration of the myxospore, based on ML and TEM observations, is present in Fig. 3.
Ultrastructural aspects of the myxospores of Kudoa eugerres n. sp. infecting the GbW of the teleostean Eugerres brasilianus in Brazil. a Pseudocyst structure composed mainly by numerous disorganized collagen fibers (H) and showing different sections of the myxospores (S). b Detail of a similar aspect of the anterior figure showing a myxospore (S) in close contact with the collagen fibers (arrows). Inset: detail of longitudinal sections of collagen fibers. c Transverse section of a myxospore showing the wall united along a suture line (SL), the two polar capsules (PC), and the cross-sections of its polar tubule. d Longitudinal section of a polar capsule (PC) showing the polar capsule wall, with the orifice for extrusion of the polar tubule (*) located close to the suture line (arrows). e Detail of a longitudinal section of the apical region of the polar capsule (PC) showing the polar tubule (PT) and the orifice (*) through which it extrudes. f Detail of a cross-section of the valve wall showing the ultrastructural organization of the suture line (arrows)
Reclassification of two species
Sphaerospora dicentrarchi Sitjà-Bobadilla and Alvarez-Pellitero, 1992 is transferred to Kudoa dicentrarchi n. comb.
Sphaerospora sp. (Mugil curema) Fiala, 2006 is transferred to Kudoa sp. (Mugil curema) n. comb.
Molecular phylogenetic analysis
Purified PCR products for the SSU rDNA gene with an approximate size of 1450 nt (18e/MXF4R), 460 bp (18e/Kud6r), 1500 bp (MXF/18r), and 550 bp (MXF4F/18r) were directly sequenced, and then assembled into a sequence of consensus of 1676 bp, with a GC content of 47.1%, corresponding almost to the complete SSU rDNA gene. The assembled sequence was deposited in the GenBank database under the accession number MH581487.
A total of 80 rDNA sequences, including those with the highest BLAST scores, were aligned with Kudoa eugerres n. sp., resulting in 5013 positions after trimming of the 3′ ends. All BI and ML phylogenetic trees constructed for the selected SSU rDNA sequences revealed very similar topology and showed that K. eugerres n. sp. is sister to K. dicentrarchi (KT970638), as well as to the Kudoa sp. (DQ3777695) found in Mugil curema. This clustering was well supported by the posterior probability of 1.00 for BI and bootstrap values of 98% for ML. These three species clustered to form a subclade of the main marine clade, closely related to histozoic species belonging to the genus Kudoa, with support of posterior probability/bootstrap values of 1.00/100% (BI/ML) (Fig. 4). For pairwise comparisons, a second alignment was performed with some SSU rDNA gene sequences of species of Kudoa, including the two species transferred to genus Kudoa and K. eugerres n. sp. All ambiguous positions were removed, resulting in a total of 1824 positions in the final dataset. The minimum genetic distance (p distance) was 1.9% to the partial sequence (772 nt) of Kudoa sp. (DQ377695) and 2.9% to K. dicentrarchi (KT970639), corresponding to almost complete sequence (1666 nt). All other analyzed sequences presented genetic distances greater than 5.1% (Table 1). Comparing the SSU rDNA sequences with the sequence obtained in this study, there are a total of 15 and 48 different nucleotides to Kudoa sp. (DQ377695) and K. dicentrarchi (KT970639), respectively.
Histopathology
Some free mature myxospores were found floating in the bile. The Pcls located in the mucosa of the GbW, and containing mature myxospores, were formed by a thick layer of collagen fibers, among which no host cell was observed (Fig. 2a, b, and inset). Several ultrastructural aspects of the collagen fibers contacting directly to the myxospores were observed (Fig. 2a–f). The surface of the collagen fibers contacting the myxospores appeared disorganized and degraded, suggesting that the myxospores were later released into the Gb lumen.
Remarks
Comparison of the site of infection, morphology, and morphometry of the myxospores to other species previously described shows the new parasite presenting some similarity to two histozoic Kudoa spp.: more specifically, K. dicentrarchi (Stijà Bobadilla and Alvarez Pellitero 1992) and Kudoa sp. (Mugil curema) Fiala, 2006. The myxospores of Kudoa eugerres n. sp., K. dicentrarchi, and Kudoa sp. (Mugil curema) exhibit reduced dimensions, probably the smallest among Myxozoa. However, the specificity of some morphometric features, combined with the molecular data acquired, demonstrates that the parasite described here is a new species (Table 2). The main differences are the host (genus and family), as well as the geographic localization; the myxospore shape, namely because of K. eugerres n. sp. has a ratio length/thickness of 1.2, while K. dicentrarchi and Kudoa sp. (Mugil curema) have a ratio length/thickness of 0.85 and 0.80, respectively; and different number of polar tubule coils in relation to K. dicentrarchi. Despite these 3 species having the wall of the Gb as the site of infection, K. dicentrarchi infects predominantly the muscularis of the Gb and intestine (Sitjà-Bobadilla and Alvarez-Pellitero 1992), while K. eugerres n. sp. develops in the mucosa, close to the epithelial tissue. Histological observations were not performed for the Kudoa sp. (Mugil curema) reported by Fiala (2006), so it is not possible to accurately indicate the tissue of infection.
Discussion
According to the literature, Sphaerospora spp. are a group of parasites infecting marine, brackish, and freshwater fishes, typically displaying coelozoic development in the urinary system (Lom and Dyková 2006), such as in the renal corpuscles (Dyková and Lom 1997), renal tubules (El-Matbouli and Hoffman 1996; Dyková and Lom 1997; Liu et al. 2018), urinary bladder, and ureters (Lom and Dyková 2006; Gunter and Adlard 2010). Occurrences as histozoic parasites in the gills (Lom et al. 1983), testis (Sitjà-Bobadilla and Alvarez-Pellitero 1990), ovary (Xiao and Desser 1997), intestine, and Gb (Sitjà-Bobadilla and Alvarez-Pellitero 1990, 1992) have been also reported, although in a much smaller number. In the case of Gb infections, there are 2 species of the genus Sphaerospora known to have coelozoic development, both from marine fishes, and without molecular information. In Tasmania, S. aldrichetta was found in the teleostean Aldrichetta forsteri (Su and White 1994), and in the Yellow Sea of China; S. sebasta was described in Sebastes schlegelii (Zhao et al. 2015).
The singularity of the systemic parasite K. dicentrarchi has been reported in several studies that establish the parasite having histozoic development, usually developing in the muscularis of the gallbladder and intestine, and less frequently in the epithelial tissues of the gallbladder, intestine and trunk kidney, and the connective tissues of the gonads, kidney, pancreas, spleen, and swim bladder. This species has been reported from marine fishes of high commercial value: European seabass D. labrax (Sitjà-Bobadilla and Alvarez-Pellitero 1992, 1993; Fioravanti et al. 2004) and D. punctatus (Xavier et al. 2013). Posteriorly, in the Gb of Mugil curema from the Caribbean Sea, another histozoic parasite of the genus Kudoa was reported (Fiala 2006). Recently, the SSU rDNA sequence of an actinospore classified as a new tetractinomyxon and found in the Polychaete of the genus Capitella allowed to molecularly infer the life cycle of K. dicentrarchi (Rangel et al. 2016).
Despite these three parasites displaying similar morphology to Sphaerospora spp., namely by having 2 polar capsules positioned perpendicularly to the sutural line formed by 2 valves, they are significantly smaller than all others and have histozoic development in internal tissues of marine fishes. In turn, true Sphaerosporids are widely reported as being predominately freshwater coelozoic species displaying affinity to the excretory system (Patra et al. 2018). Furthermore, ultrastructural analyses show that both K. eugerres n. sp. and K. dicentrarchi have thin valves that overlap at the sutural line (Sitjà-Bobadilla and Alvarez-Pellitero 1992), following what has been commonly reported for Kudoa and Unicapsula spp. (Diamant et al. 2005; Al-Jufaili et al. 2015). On the other hand, the sporoplasm cell of these two uncommon Kudoa spp. is binucleated, contrarily to what has been reported for Sphaerospora spp., which have two or more uninucleated sporoplasm cells, and also Kudoa spp., which are characterized by two uninucleated sporoplasm cells, one being located within the other.
Several aspects of GbW rupture were observed, showing a possible direct contact of the myxospores with the bile, and consequent liberation of the myxospores, which also appeared floating in the bile. In more advanced stages of the disease, enteric Sphaerospora sp. infecting groupers of the genus Epinephelus in the South China Sea presented similar aspects, with complete degradation of the intestinal mucosa and submucosa resulting in the release of sporogonic stages into the organ cavity (Liu et al. 2018).
Analyzing the genetic distance between these 3 histozoic parasites, the new species described in this paper presents a significant number of different nucleotides, even to the small partial sequenced region of Kudoa sp. IF-2006 (DQ377695). Considering the genetic distances between Kudoa spp. differences of less than 1% have been reported between species, such as K. permulticapsula (AY078429), K. scomberomori (AY302737), K. alliaria (DQ182561), and K. rosenbuschi (AY623795) (Table 1).
The molecular and phylogenetic analysis performed here show K. eugerres n. sp., K. dicentrarchi, and Kudoa sp. IF-2006 clustering together to form a histozoic subclade of the largest marine monophyletic histozoic clade of multivalvulids species (the Kudoa clade). Thus, these 3 Kudoa spp. are phylogenetically distant to all other Sphaerosporids, including those that are also considered as Sphaerospora spp. s.l. The main freshwater clade houses 2 Sphaerospora spp. of the urinary system: S. elwhaiensis (Jones et al. 2011) and S. oncorhynchi (Kent et al. 1998). Finally, S. testicularis (Bartošová et al. 2011) is located within the main marine clade, being sister to the genera Gadimyxa and Parvicapsula. Like previous phylogenetic studies that focused on the evolution of Sphaerospora spp. (Fiala 2006; Bartošová et al. 2011, 2013), we also showed a very close relationship of histozoic Gb (K. dicentrarchi and Kudoa sp. IF-2006) and K. eugerres n. sp. with the great monophyletic clade of Kudoa spp. being in common the habitat and the proliferation in tissues.
Several phylogenetic studies have shown that the species considered to be true Sphaerosporids, the Sphaerospora s.s., cluster among each other to form a well-supported clade that is basal to all other Myxosporea. In turn, the species comprising the so-called Sphaerospora s.l. are scattered across several other clades of the Myxosporea, acknowledging the necessity of revising the taxonomy of this genus, either through the erection of new genera or through the transference of species to other more appropriate genera.
In the last years, as a result of the increasing availability of genetic information, it has been possible to verify that several species are poorly classified because taxonomy was established exclusively on the basis of morphology. Consequently, several taxonomic revisions of the Myxosporea have been performed in recent years. In 2014, Atkinson et al. created the genus Ceratonova to include the well-known freshwater species Ceratomyxa shasta and another one C. gasterosteus n. sp. Members of Ceratomyxa constitute one of the main monophyletic coelozoic group of the marine clade and parasitize the Gb or urinary system of marine teleosts and elasmobranchs. In turn, Ceratonova spp. infects the intestine of the freshwater fish Gasterosteus aculeatus and is phylogenetically distant from all other Ceratomyxa spp. (Fiala 2006; Atkinson et al. 2014; Fiala et al. 2015). On the other hand, the genus Polysporoplasma was extinguished and its 2 species transferred to the genus Sphaerospora since they group within the basal clade formed by Sphaerospora s.s., differing only by having multiple sporoplasms (Bartošová et al. 2013). Extinction of genera has also occurred within the order Multivalvulida. Based on phylogenetic analyses, the genera Pentacapsula, Hexacapsula, and Septemcapsula were synonymized with Kudoa, turning the latter into the largest monophyletic genus composed of marine and histozoic species (Whipps et al. 2004). Considering the great plasticity of the order Multivalvulida, which envelops representatives with 3 to 13 valves, each one with a polar capsule, it is justifiable the inclusion of some species that present some morphological similarities with bivalvulids (myxospores formed by 2 valves). In the future, there should be an attempt to reassess the taxonomic classification of the remaining 3 Sphaerospora s.l. (2 freshwater and 1 marine).
Conclusion
In this study, the morphology and ultrastructural aspects of the myxospore, associated with the 18S rDNA gene sequence analysis, confirm that this parasite constitutes a new myxosporean species. Considering that this species displayed mixed morphological characters of a histozoic Bivalvulida and Multivalvulida species, inhabits the marine environment and is phylogenetically closely related to the Kudoa clade, it was classified as Kudoa eugerres n. sp. Consequently, the 2 Sphaerospora s.l., Sphaerospora dicentrarchi, and Sphaerospora sp. (Mugil curema) should be transferred to Kudoa dicentrarchi n. comb. and Kudoa sp. (Mugil curema) n. comb. Finally, the diagnosis of the order Multivalvulida Shulman, 1959; Family Kudoidae Meglitsch, 1960; and genus Kudoa Meglitsch, 1947 was amended in order to include myxospores of 2–13 (mostly 4) polar capsules and valves, valves that are very thin and overlapping at the sutural line.
References
Al-Jufaili SH, Freeman MA, Machkevskyi VK, Al-Nabhani A, Palm HW (2015) Morphological, ultrasctrucutral, and molecular description of Unicapsula fatimae n. sp. (Myxosporea: Triplosporidae) of whitespotted rabbitfish (Siganus canaliculatus) in Omani waters. Parasitol Res 115:1173–1184. https://doi.org/10.1007/s00436-015-4851-y
Atkinson SD, Foott JS, Bartholomew JL (2014) Erection of Ceratonova n. gen. (Myxosporea: Ceratomyxidae) to encompass freshwater species C. gasterostea n. sp. from threespine stickleback (Gasterosteus aculeatus) and C. shasta n. comb. from salmonid fishes. J Parasitol 100:640–645. https://doi.org/10.1645/13-434.1
Bartošová P, Fiala I, Jirků M, Cinková M, Caffara M, Fioravanti MLM, Atkinson SD, Bartholomew JL, Holzer AS (2013) Sphaerospora sensu stricto: taxonomy, diversity and evolution of a unique lineage of myxosperans (Myxozoa). Mol Phylogenetic Evol 68:93–105. https://doi.org/10.1016/j.ympev.2013.02.026
Bartošová P, Freeman MA, Yokoyama H, Caffara M, Fiala I (2011) Phylogenetic position of Sphaerospora testicularis and Latyspora scomberomori n. gen. n. sp. (Myxozoa) within the marine urinary clade. Parasitology 138:381–393. https://doi.org/10.1017/S0031182010001381
Carriero MM, Adriano EA, Silva MRM, Ceccarelli PS, Maia AAM (2013) Molecular phylogeny of the Myxobolus and Henneguya genera with new South American species. PLoS One 8:e 73713. https://doi.org/10.1371/journal.pone.0073713
Diamant A, Ucko M, Paperna I, Colorni A, Lipshitz A (2005) Kudoa iwatai (Myxosporea: Multivalvulida) in wild and cultured fish in the Red Sea: redescription and molecular phylogeny. J Parasitol 91:1175–1189. https://doi.org/10.1645/GE-491R.1
Dyková I, Lom J (1997) Light and electron microscope observations on Sphaerospora ojiroveci n. sp. (Myxozoa) from the kidney of Pangasius sutchi (Teleostei). Europ J Protistol 33:444–451
El-Matbouli M, Hoffman RW (1996) Light and electron microscopic descriptions of Sphaerospora coregoni El-Matbouli, Hoffmann and Kern, 1995 (Myxosporea: Sphaerosporidae) from the kidney of whitefish (Coregonus lavaretus). Europ J Protistol 32:389–398. https://doi.org/10.1016/S0932-4739(96)80063-0
Eszterbauer E, Sipos D, Forró B, Bartošová P, Holzer AS (2013) Molecular characterization of Sphaerospora molnari (Myxozoa), the agent of gill sphaerosporosis in common carp Cyprinus carpio carpio. Dis Aquat Org 104:59–67. https://doi.org/10.3354/dao02584
Fiala I (2006) The phylogeny of Myxosporea (Myxozoa) based on small subunit ribosomal RNA gene analysis. Intern J Parasitol 36:1521–1534. https://doi.org/10.1016/j.ijpara.2006.06.016
Fiala I, Hlavničková M, Kodádková A, Freeman MA, Bartošová-Sojková P, Atkinson SD (2015) Evolutionary origin of Ceratonova shasta and phylogeny of the marine myxosporean lineage. Mol Phylogenet Evol 86:75–89. https://doi.org/10.1016/j.ympev.2015.03.004
Fioravanti ML, Caffara M, Florio D, Gustinelli A, Marcer F (2004) Sphaerospora dicentrarchi and S. testicularis (Myxozoa: Sphaerosporidae) in farmed European seabass (Dicentrarchus labrax) from Italy. Folia Parasitol 51:208–210
Gunter NL, Adlard RD (2010) The demise of Leptotheca Thélohan, 1895 (Myxozoa: Myxosporea: Ceratomyxidae) and assignment of its species to Ceratomyxa Thélohan, 1892 (Myxosporea: Ceratomyxidae), Ellipsomyxa Køie, 2003 (Myxosporea: Ceratomyxidae), Myxobolus Bütschli, 1882 and Sphaerospora Thélohan, 1892 (Myxosporea: Sphaerosporidae). Syst Parasitol 75:81–104. https://doi.org/10.1007/s11230-009-9227-1
Hallett SL, Diamant A (2001) Ultrastructure and small-subunit ribosomal DNA sequence of Henneguya lesteri n. sp. (Myxosporea), a parasite of sand whiting Sillago analis (Sillaginidae) from the coast of Queensland, Australia. Dis Aquat Org 46:197–212. https://doi.org/10.3354/dao046197
Hillis DM, Dixon MT (1991) Ribosomal DNA: molecular evolution and phylogenetic inference. Q Rev Biol 66:411–453
Holzer AS, Bartošová P, Pecková H, Tyml T, Atkinson S, Bartholomew J, Sipos D, Eszterbauer E, Dyková I (2013) “Who’s who” in renal sphaerosporids (Bivalvulida: Myxozoa) from common carp, Prussian carp and goldfish-molecular identification of cryptic species, blood stages and new members of Sphaerospora sensu stricto. Parasitology 140:46–60. https://doi.org/10.1017/S0031182012001175
Holzer AS, Bartošová-Sojková P, Born-Torrijos A, Lövy A, Hartigan A, Fiala I (2018) The joint evolution of the Myxozoa and their alternate hosts: a cnidarian recipe for success and vast biodiversity. Mol Ecol 27:1651–1666. https://doi.org/10.1111/mec.14558
Jirků M, Fiala I, Modry D (2007) Tracing the genus Sphaerospora: rediscovery, redescription and phylogeny of the Sphaerospora ranae (Morelle, 1929) n. comb. (Myxosporea, Sphaerosporidae), with emendation of the genus Sphaerospora. Parasitology 134:1727–1739. https://doi.org/10.1017/S0031182007003241
Jones S, Fiala I, Prosperi-Porta G, House M, Mumford S (2011) Sphaerospora elwhaiensis sp. n. (Myxosporea: Sphaerosporidae) from landlocked sockeye salmon Oncorhynchus nerka (Salmoniformes: Salmonidae) in Washington State, USA. Folia Parasitol 58:87–94
Kent ML, Khattra JS, Hervio DML, Devlin RH (1998) Ribosomal DNA sequence analysis of isolates of the PKX myxosporean and their relationship to members of the genus Sphaerospora. J Aquat Anim Health 10:12–21. https://doi.org/10.1577/1548-8667(1998)010<0012:RDSAOI>2.0.CO;2
Kumar S, Stecher G, Tamura K (2016) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33:1870–1874. https://doi.org/10.1093/molbev/msw054
Liu XH, Xu LW, Luo D, Zhao YL, Zhang QQ, Liu GF (2018) Outbreak of mass mortality of yearling groupers of Epinephelus (Perciformes, Serranidae) associated with the infection of a suspected new enteric Sphaerospora (Myxozoa: Myxosporea) species in South China Sea. J Fish Dis 41:663–672. https://doi.org/10.1111/jfd.12766
Lom J, Dyková I (1992) Myxosporidia (phylum Myxozoa). In: Lom J, Dyková I (eds) Protozoan parasites of fishes. Developments in Aquaculture and Fisheries Science, vol vol 26. Elsevier, Amsterdam, pp 159–235
Lom J, Dyková I (2006) Myxozoan genera: definition and notes on taxonomy, life-cycle terminology and pathogenic species. Folia Parasitol 53:1–36
Lom J, Dyková I, Pavlásková M, Grupcheva G (1983) Sphaerospora molnari n. sp. (Myxozoa, Myxosporea) an agent of gill, skin and blood sphaerosporosis of common carp in Europe. Parasitology 86:529–533. https://doi.org/10.1017/S003118200005071X
Patra S, Bartošová-Sojková P, Pecková H, Fiala I, Eszterbauer E, Holzer AS (2018) Biodiversity and host-parasite cophylogeny of Sphaerospora (sensu stricto) (Cnidaria: Myxozoa). Parasit Vectors 11:347. https://doi.org/10.1186/s13071-018-2863-z
Rangel LF, Castro R, Rocha S, Severino R, Casal G, Azevedo C, Cavaleiro F, Santos MJ (2016) Tetractinomyxon stages genetically consistent with Sphaerospora dicentrarchi (Myxozoa: Sphaerosporidae) found in Capitella sp. (Polychaeta: Capitellidae) suggest potential role of marine polychaetes in parasite’s life cycle. Parasitology 143:1067–1073. https://doi.org/10.1017/S0031182016000512
Rocha S, Casal G, Rangel L, Castro R, Severino R, Azevedo C, Santos MJ (2015) Ultrastructure and phylogeny of Ceratomyxa auratae n. sp. (Myxosporea: Ceratomyxidae), a parasite infecting the gilthead seabream Sparus aurata (Teleostei: Sparidae). Parasitol Int 64:305–313. https://doi.org/10.1016/j.parint.2015.04.002
Ronquist F, Huelsenbeck JP (2003) MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19:1572–1574
Sitjà-Bobadilla A, Alvarez-Pellitero P (1990) Sphaerospora testicularis sp. nov. (Myxosporea: Sphaerosporidae) in wild and cultured sea bass, Dicentrarchus labrax (L.), from the Spanish Mediterranean area. J Fish Dis 13:193–203. https://doi.org/10.1111/j.1365-2761.1990.tb00774.x
Sitjà-Bobadilla A, Alvarez-Pellitero P (1992) Light and electron microscopic description of Sphaerospora dicentrarchi n. sp. (Myxosporea: Sphaerosporidae) from wild and cultured sea bass, Dicentrarchus labrax L. J Protozool 39:273–281. https://doi.org/10.1111/j.1550-7408.1992.tb01314.x
Sitjà-Bobadilla A, Alvarez-Pellitero P (1993) Population dynamics of Sphaerospora dicentrarchi Sitjà-Bobadilla et Alvarez-Pellitero, 1992 and S. testicularis Sitjà-Bobadilla et Alvarez-Pellitero, 1990 (Myxosporea: Bivalvulida) infections in wild and cultured Mediterranean sea bass (Dicentrarchus labrax L.). Parasitology 106:39–45. https://doi.org/10.1017/S0031182000074795
Soares EC, Paiva AG, Santos EL, Pereira SM, Almeida EO, Silva TJ (2016) Potential of Carapeba (Eugerres brasilianus) for aquaculture production. Lat Am J Aquat Res 44:718–725. https://doi.org/10.3856/vol44-issue4-fulltext-7
Su X-q, White RWG (1994) New myxosporeans (Myxozoa: Myxosporea) from marine fishes of Tasmania, Australia. Acta Protozool 33:251–259
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680. https://doi.org/10.1093/nar/22.22.4673
Whipps CM, Adlard RD, Bryant MS, Lester RJG, Findlav V, Kent ML (2003) First report of three Kudoa species from Eastern Australia: Kudoa thyrsites from Mahi mahi (Coryphaena hippurus), Kudoa amamiensis and Kudoa minithyrsites n. sp. from sweeper (Pempheris ypsilychnus). J Eukaryot Microbiol 50:215–219. https://doi.org/10.1111/j.1550-7408.2003.tb00120.xb
Whipps CM, Grossel G, Adlard RD, Yokoyama H, Bryant MS, Munday BL, Kent ML (2004) Phylogeny of the Multivalvulidae (Myxozoa: Myxosporea) based on comparative ribosomal DNA sequence analysis. J Parasitol 90:618–622. https://doi.org/10.1645/GE-153R
Xavier R, Severino R, Pérez-Losada M, Cable J, Harris DJ (2013) First record of Sphaerospora dicentrarchi (Myxosporea, Sphaerosporidae) in Dicentrarchus punctatus. Bull Europ Assoc Fish Pathol 33:21–23
Xiao C, Desser S (1997) Sphaerospora ovophila n. sp. and Myxobolus algonquinensis n. sp. (Myxozoa, Myxosporea), ovarian parasites of fish from Algonquin Park, Ontario, Canada. J Eukaryot Microbiol 44:157–161. https://doi.org/10.1111/j.1550-7408.1997.tb05953.x
Zhao Y, Al-Farraj SA, Al-Rasheid KAS, Song W (2015) Data on ten new myxosporean parasites (Myxozoa, Myxosporea, Bivalvulida) from the Yellow Sea, China. Acta Protozool 54:305–323. https://doi.org/10.4467/16890027AP.15.026.3540
Acknowledgements
We would like to thank the anonymous reviewers for their comments and their suggestions that have helped to improve the text.
Funding
This work was partially supported by the Eng. A. Almeida Foundation (Proj. 2017-18) (Porto, Portugal); FAPEAL, project PPP no. 630000879/2011, and Laboratory of Genetic-CECA/UFAL (Maceió, Alagoas State), Brazil; and the FCT (Lisbon, Portugal) within the scope of the Ph.D. fellowship grant attributed to S. Rocha (SFRH/BD/92661/2013) through the programme QREN-POPH/FSE.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. This study carried out according to Brazilian laws (MMA-ICMbio, license MMA—56475-1 to LAQUA—UFAL).
Conflict of interest
All authors declare that they have no conflict of interest.
Additional information
Section Editor: Astrid Holzer
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Casal, G., Soares, E.C., Rocha, S. et al. Description of a new myxozoan Kudoa eugerres n. sp. and reclassification of two Sphaerospora sensu lato species. Parasitol Res 118, 1719–1730 (2019). https://doi.org/10.1007/s00436-019-06324-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-019-06324-8