Abstract
We tested whether biogeographic patterns characteristic of species diversity and composition may also apply to community assembly by investigating geographic variation in the pattern (PSA) (aggregation versus segregation) and strength of species associations (SSA) in flea and mite communities harbored by small mammalian hosts in Western Siberia. We asked whether (a) there is a relationship between latitude and PSA or SSA and (b) similarities in PSA or SSA follow a distance decay pattern or if they are better explained by variation in environmental factors (altitude, amount of vegetation, precipitation, and air temperature). We used a sign of a co-occurrence metric (the C-score) as an indicator of PSA and its absolute standardized value as a measure of SSA. We analyzed data using logistic and linear models, generalized dissimilarity modeling (GDM), and a logistic version of the multiple regression on distance matrices (MRM). The majority of the C-scores of the observed presence/absence matrices indicated a tendency to species aggregation rather than segregation. No effect of latitude on PSA or SSA was found. The dissimilarity in PSA was affected by environmental dissimilarity in mite compound communities only. A relatively large proportion of the deviance of spatial variation in SSA was explained by the GDMs in infracommunities, but not component communities, and in only three (of seven) and two (of eight) host species of fleas and mites, respectively. The best predictors of dissimilarity in SSA in fleas differed between host species, whereas the same factor (precipitation) was the best predictor of dissimilarity in SSA in mites. We conclude that PSA and SSA in parasite communities rarely conform to biogeographic rules. However, when a biogeographic pattern is detected, its manifestation differs among hosts and between ectoparasite taxa.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The spatial variation of biological communities is one of the central themes in biogeography. Patterns of this variation have been repeatedly studied in various taxa, across various geographic regions, and in various environments (see Brown 1995, 2014; Gaston 2000; Hubbell 2001; Ricklefs and Jenkins 2011 and references therein). These studies revealed that biological communities do not vary in space in a random fashion, but rather, this variation is often governed by certain rules. Among these rules, the most persistent are those associated with latitude and geographic distance (Pianka 1966; Rohde 1992; Nekola and White 1999; Soininen et al. 2007; Morlon et al. 2008; Astorga et al. 2012; Brown 2014; Schemske and Mittelbach 2017). For example, a well-known increase in the number of species in an assemblage from polar to tropical regions (= latitudinal gradient) is an almost ubiquitous pattern (e.g., Brown 2014). A decrease in compositional and/or phylogenetic similarity between communities with an increase in geographic distance between them (= distance decay of similarity) is also well-documented (Nekola and White 1999; Soininen et al. 2007; Saito et al. 2015).
The absolute majority of studies on the latitudinal gradient and distance decay of similarity in biological communities focused on either species composition or species diversity or both. Community assembly rules or processes shaping biological assemblages have received less attention (but see Bertness and Ewanchuk 2002; Lortie and Callaway 2006; Henriques-Silva et al. 2013; Qiao et al. 2015). Indeed, species composition and/or diversity are not the only facets of community structure. One of the most important aspects of community structure is whether an assemblage represents a random set of species or whether it is organized by certain rules such as Diamond’s assembly rules (Diamond 1975), species nestedness (Patterson and Atmar 1986), and core-satellite organization (Hanski 1982). Every assembly rule suggests that species in a community are associated non-randomly. If these associations are positive, then the frequency of species co-occurrence is greater than expected from random associations, and thus, the community is structured aggregatively. If, however, these associations are negative, then the frequency of species co-occurrence is smaller than expected from random associations, and thus, the community is structured segregatively. The results of recent studies on plant communities suggested that species interactions within a community tend to be predominantly negative in favorable environments and predominantly positive in more stressful environments (Callaway et al. 2002; Maestre et al. 2009). As a result, a latitudinal gradient in the pattern of species associations may occur. In addition, variation in the strength of species associations along a latitudinal gradient may be expected because an increase in species richness at low latitudes may result in an increased degree of niche overlap and competition and thus an increased strength of negative species associations from north to south (Vázquez and Stevens 2004) in the Northern Hemisphere. Another, not necessarily alternative, pattern may arise due to latitudinal variation in the degree of environmental filtering with a north–south shift from stronger to weaker environmentally filtered communities and thus an increased strength of positive species associations from south to north (Qiao et al. 2012, 2015).
Furthermore, not every species in a community may be associated (positively or negatively) with other species. Instead, some pairs of species may mainly contribute to a general pattern of non-randomness, whereas other species are randomly associated (e.g., Gotelli and Ulrich 2010), so that the non-random structure arises from non-random associations of only a subset of, rather than all, species. Consequently, and given that the species composition of communities often varies predictably across space (see above), the patterns and strength of species associations may also conform to these rules because variation in species composition may likely be reflected in variation in the pattern of these species’ interactions (Qiao et al. 2015).
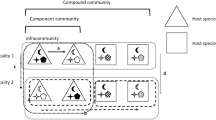
Studying spatial variation in the community structure of free-living organisms is sometimes methodologically difficult due to ambiguity in the identification of community boundaries because these communities exist in a spatial continuum (Loreau 2000). Studies of parasite communities allow us to avoid, or at least diminish, this difficulty because parasite communities are fragmented among host individuals (infracommunities), among populations of conspecific hosts within a locality (component communities), and among host communities in different localities (compound communities) (Holmes and Price 1986; Bush et al. 1997; Poulin 2007). Both a latitudinal gradient in species diversity and a distance decay of compositional similarity have been demonstrated for communities of many, albeit not all, parasite taxa (see reviews in Bordes et al. 2010; Poulin and Krasnov 2010; Rohde 2010).
Here, we tested whether biogeographic patterns documented for species composition and diversity in parasite communities can also be applied to species associations in these communities, and studied the pattern (i.e., segregation versus aggregation) and the strength of these associations in fleas and gamasid mites parasitic on small mammals in Western Siberia. Fleas are obligatory hematophagous at the imago stage with the majority of species alternating periods spent on the bodies and the nests/burrows of their hosts. Pre-imaginal stages are mostly non-parasitic and develop mostly off-host (see Krasnov 2008). The range of lifestyles in gamasid mites is enormous from obligate via facultative hematophagy to phoresy, and many (if not the absolute majority) species spend most of their life off-host (Radovsky 1985). We estimated the pattern and strength of species associations (see “Materials and methods” for details) at two hierarchical scales. At a lower scale, we estimated species associations in infracommunities (i.e., across host individuals) harbored by hosts belonging to the same species, that is, for each component community, and then tested for spatial patterns across these component communities (= across populations of the same host species). At a higher scale, we estimated species associations in component communities (i.e., across host species), that is, for each compound community, and then tested for spatial patterns across these compound communities (= across locations).
First, we asked whether there is a relationship between latitude and the pattern (positive or negative) and strength of species associations. We expected to find predominantly negative associations at lower latitudes and predominantly positive ones at higher latitudes because low ambient temperatures at the latter are likely stressful for both taxa (Kozlova 1983; Krasnov 2008) (see above). However, we chose not to propose a specific prediction regarding the relationship between latitude and the strength of species association. This is because an increase in species richness with a decrease in latitude (see Krasnov et al. 2007 for fleas) may result in stronger interspecific competition (see above; Vázquez and Stevens 2004) and an increased strength of negative associations to the south, whereas a latitudinal gradient in environmental filtering (see above; Qiao et al. 2015) may cause an increased strength of positive associations to the north.
Second, we asked whether similarities in the pattern and strength of species associations follow a distance decay pattern or whether they are better explained by variation in environmental factors. Earlier, we demonstrated that environmental dissimilarity had a much stronger effect than geographic distance on compositional dissimilarity in both flea and mite communities (Vinarski et al. 2007; Krasnov et al. 2010a). Consequently, we predicted that similarity in the pattern and strength of flea and mite species associations would be better explained by variation in environmental variables rather than by mere geographic distance.
Materials and methods
Ectoparasite community composition
Data on fleas and mites recorded on the bodies of their small mammalian hosts (rodents and shrews) were compiled from the database of the Omsk Research Institute of Natural Foci Infections (Omsk, Russia). The database comprised data collected from 1960 to 2013 by various researchers in 109 localities across Western Siberia (see the map in Fig. S1 of Electronic Supplementary Material). Mammals were sampled in late spring–early fall using either snap-traps or pitfall traps with drift fences. Snap traps were deposited in the late evening and checked at dawn to minimize the number of parasites abandoning dead hosts. Pitfall traps were not filled with any fixation liquid. Captured animals were placed individually in cloth bags, transferred to a laboratory, and parasitologically examined under a stereoscopic microscope. The collected ectoparasites were then identified. To calculate metrics of co-occurrence (see below) in infracommunities (i.e., at the scale of component community), we included in the analysis only those host species that were represented in a locality by at least six individuals harboring at least three flea or mite species. To calculate metrics of co-occurrence in component communities (i.e., at the scale of compound community), we included in the analysis localities in which at least three host species were found to harbor at least two flea or mite species. This selection resulted in calculations of co-occurrence metrics (a) for infracommunities in component communities of fleas and mites in six and eight host species, respectively, and (b) for component communities in compound communities of fleas and mites in 44 and 47 localities, respectively.
Environmental data
The latitudinal and longitudinal positions of the locality centers were determined using ArcGIS 10.6. Environmental data for each locality included altitude; the amount of green vegetation (normalized difference vegetation indices (NVDI) separately for autumn, winter, spring, and summer); mean, maximum, and minimum air temperatures as well as annual and monthly ranges; and precipitation (separately for autumn, winter, spring, and summer), averaged across the area of a 2.5-km radius around the geographic position of the locality center. Altitude data were obtained using ArcGIS 10.6. NDVI data were taken from the VEGETATION Program (http://free.vgt.vito.be) for the period 1999–2018. Air temperature and precipitation data were taken from WORLDCLIM (BIOCLIM) 2.0 package (Fick and Hijmans 2017) for the period 1970–2000.
Calculation of co-occurrence metrics
The data for each component or compound community were organized as a presence/absence matrix with rows representing flea or mite species and columns representing either host individuals (for component communities) or host species (for compound communities). In the analyses of parasite co-occurrences, incidence (i.e., presence/absence) matrices are more appropriate than abundance matrices because measurements of parasite occurrences are more reliable than measurements of their abundances (Gotelli and Rohde 2002), especially due to the aggregated character of parasite distribution among host individuals (Poulin 2007).
Then, we applied the null model analyses implemented in the program EcoSim Professional 1.2d (Acquired Intelligence, Inc., and Pinyon Publishing; http://www.garyentsminger.com/ecosim/index.htm), and we used the C-score (the average number of checkerboard units found for each species pair; Stone and Roberts 1990) as a metric of co-occurrence of flea or mite species in each component or compound community [i.e., parasites × hosts (individuals or species, respectively) a matrix]. The C-score is one of the most commonly used metrics of species co-occurrence (Gotelli and McCabe 2002; Gotelli and Rohde 2002; Krasnov et al. 2006; Tello et al. 2008; Gotelli and Ulrich 2010; Kohli et al. 2018). A C-score was calculated for each present/absent matrix (observed C-score) and compared with the C-scores calculated for 5000 randomly assembled null matrices (expected C-scores); the tail probability that the observed index was larger or smaller than expected by chance was measured. A C-score is an inverse indicator of the co-occurrence frequency, so that an observed C-score larger than the average of expected C-scores (observed (O) > expected (E)) indicates negative co-occurrences (i.e., species are segregated), while an observed C-score smaller than the average of expected C-scores (O < E) indicates positive co-occurrences (i.e., species are aggregated) (Gotelli and Graves 1996; Gotelli 2000). We assembled null matrices by Monte Carlo procedures using a fixed-equiprobable (FE) algorithm, which does not constrain the number of parasite species on a host individual or species assuming that different host individuals or species are equivalent in their probability to harbor a particular number of ectoparasite species. Krasnov et al. (2006, 2010b, 2011) presented a biological justification for using the FE algorithm for analyzing the community structure of ectoparasites harbored by small mammals.
Then, we calculated the standardized effect size (SES) for each component or compound community matrix. SES measures the number of standard deviations that the observed index is above or below the mean index of simulated matrices (Gotelli and McCabe 2002) and is calculated as the difference between an observed index and a mean of simulated indices divided by the standard deviation of simulated indices. Approximately 95% of the observed SES values are expected to fall between − 2.0 and 2.0 under the assumption of a normal distribution of deviations. The sign of the SES of a C-score thus indicates a pattern of species association (segregation or aggregation), whereas its absolute value is a measure of association strength (Stone and Roberts 1990; Kohli et al. 2018). In many communities, the observed indices did not differ significantly from the null expectations (see “Results”). Nevertheless, independently of whether the observed indices differed significantly from the metrics calculated for simulated matrices, we considered communities with negative SES values as tending to be aggregated and communities with positive SES values as tending to be segregated.
Data analyses
To test for latitudinal variation in the pattern or strength of species associations, we applied either logistic or linear, respectively, models using the R package “stats” (R Core Team 2018). A response variable in the linear models was the absolute value of SES corrected for matrix size where necessary (see below). For a binomial response variable in the logistic models, we assigned zero or one to the communities with a tendency to demonstrate either segregative or aggregative, respectively, patterns of species co-occurrence. McFadden pseudo-r2 values for logistic regressions were calculated using the package “pscl” (Jackman 2017) implemented in the R Statistical Environment (R Core Team 2018). We applied both models separately for fleas and mites and across either component (e.g., within a host species) or compound (e.g., across localities) communities.
Then, we analyzed the effects of geographic distance and environmental dissimilarity on the dissimilarity in the pattern and strength of species associations in infra- and component communities of fleas and mites across component and compound communities, respectively, from different localities. The effects of geographic distance and environmental dissimilarity on the dissimilarity in the pattern of species associations were analyzed using multiple regression on distance matrices (MRM; Manly 1986; Legendre and Legendre 1998; Lichstein 2007), whereas these effects on the strength of species associations were analyzed using generalized dissimilarity modeling (GDM; Ferrier 2002; Ferrier et al. 2002; Ferrier et al. 2007). This difference in the analyses was necessary because GDM could not be applied to a binary response matrix (see below).
To test for the effects of geographic distance and dissimilarity in altitude and environmental factors on the dissimilarity in the patterns of co-occurrence, we constructed a matrix of pairwise binary distance measures assigning each pair of communities a distance of zero if they demonstrated the same pattern of co-occurrence (both segregative or both aggregative) or one if they demonstrate contrasting patterns. MRM is an extension of a partial Mantel analysis aimed at investigating relationships between a multivariate response (distance) matrix and a number of explanatory (distance) matrices. The significance of the model and regression coefficients is tested by permuting (10,000 permutations) a response matrix while holding the explanatory matrices constant. The rows and corresponding columns in the response matrices are permuted simultaneously, and the coefficient of determination of the model and regression coefficients are calculated for each permutation to generate a null distribution (Legendre and Legendre 1998; Lichstein 2007). Geographic distances between localities where ectoparasite communities occurred were calculated using Vincenty’s formula for distance on an ellipsoid implemented in the R package “geosphere” (Hijmans 2017). A dissimilarity matrix for altitude was constructed as absolute differences in altitude between each pair of these localities. The NDVI and climatic dissimilarities were computed between each pair of rows of data matrices (e.g., localities) using the Euclidean distance measure with the function “dist” of the R package “stats” (R Core Team 2018). MRM analyses were performed using the function “MRM” from the R package “ecodist” (Goslee and Urban 2007) modified by Pilosof et al. (2015).
The GDM analyses of the effects of geographic distance and environmental (altitude, NDVI, and climate) dissimilarities on the dissimilarity in the strength of species associations among ectoparasite communities followed van der Mescht et al. (2018). GDM represents an extension of matrix regression designed to take non-linearity into account. This is because (a) the relationship between ecological pairwise dissimilarity among communities or localities and the environmental or geographic distance between them is definitely curvilinear at high values of dissimilarity (because dissimilarity may vary only from 0 to 1), and (b) the rate of change of, for example, community structure along geographic distance or an environmental gradient may not be constant (Ferrier et al. 2007). To overcome (a), GDM transforms the linear predictor variable via a link function that defines the relationship between pairwise dissimilarities in structure among communities (constrained to the range 0–1) and a scaled combination of pairwise distances between communities based on environmental or geographical variables (see Ferrier et al. 2007 for details). To overcome (b), GDM transforms each predictor variable using an iterative maximum-likelihood estimation and I-splines (i.e., monotone piecewise functions). A given I-spline indicates the importance of a given predictor, while all other predictors are held constant (so that I-splines are partial regression fits). The maximum height of an I-spline represents the total amount of change along a given gradient, while its slope demonstrates the rate of change and its variation along this gradient (Ferrier et al. 2007).
To run GDMs, we used the “gdm” package (Manion et al. 2018) implemented in R. Dissimilarity matrices of the strength of species associations in flea or mite infra- or component communities were constructed using absolute pairwise differences in standardized C-scores (i.e., SES) between the component or compound communities, respectively, of either fleas or mites. The size of a community (i.e., matrix size; number of rows × number of columns) can significantly affect the absolute value of SES (Gotelli and McCabe 2002). This indeed appeared to be the case for all six component communities of fleas (r2 = 0.23–0.77, F = 4.21–9.67; p < 0.05 for all) and three of the eight component communities of mites (communities of Sorex araneus, Myodes rufocanus, and Apodemus agrarius; r2 = 0.20–0.80, F = 5.59–24.51; p < 0.05 for all), as well as for the compound communities of both ectoparasite taxa (r2 = 0.61, F = 65.01 for fleas and r2 = 0.43, F = 33.65 for mites; p < 0.01 for both). No relationship between matrix size and the absolute value of SES was found for the remaining component communities of mites (r2 = 0.02–0.18, F = 0.68–3.06; p > 0.10 for all). Consequently, prior to calculating dissimilarity matrices on the strength of species association, we substituted the original values of SES with their residual deviations from their regression on matrix size (for communities in which the relationships between these variables were significant). Then, we scaled the values of either absolute SES values or the abovementioned residuals to a 0–1 range and constructed dissimilarity matrices from these scaled values.
Before further analyses, we applied a principal component analysis separately to NDVI and climatic (air temperature and precipitation) variables. We extracted a single principal component from four NDVI variables (further referred to as a NDVI factor). This component explained 85.3% of total variation in NDVI, and its scores correlated negatively with four original (= seasonal) NDVI variables (r ranged from − 0.98 to − 0.85). A principal component analysis of climatic variables resulted in two principal components (climatic factor 1 and climatic factor 2) that explained 77.03% of the total variation. Climatic factor 1 correlated positively with spring, summer, and fall precipitation and negatively with the annual range of air temperature (r = 0.81–0.94 and r = − 0.74), whereas climatic factor 2 correlated positively with the remaining temperature variables (r = 0.73–0.95).
For each of the abovementioned factors, as well as for the altitude, environmental dissimilarity matrices were constructed using Euclidean distances between each pair of component or compound communities by the internal function of the “gdm” package. Then, we used the default setting of three I-splines to fit the GDMs. The response matrix in the main GDM was pairwise dissimilarity in the strength of flea or mite species associations, whereas the predictor matrices were pairwise dissimilarity in altitude, NDVI factor, climatic factors 1 and 2, and geographic distance between the localities of component and compound communities.
We did not adjust the alpha-level for multiple comparisons because this may increase the probability of a type II error, which has been severely criticized by both statisticians and biologists (Perneger 1998; Nakagawa 2004).
Results
The majority of the C-scores of the observed present/absent matrices indicated a tendency to species aggregation rather than segregation in infra- and component communities of both fleas and mites (Table 1). The C-scores of the observed matrices were significantly smaller than those of the simulated matrices for 86 of 244 component communities and 27 of 122 compound communities (Table 1). The C-scores of the observed matrices were significantly larger than the C-scores of the simulated matrices for only two component communities (fleas in infracommunities of Myodes glareolus and mites in infracommunities of Microtus oeconomus) (Table 1).
There was no effect of latitude on the pattern of species associations (McFadden pseudo-r2 = 0.0002–0.12, p > 0.23 for coefficients in all models). The same was true for the strength of species associations in a community (i.e., absolute value of SES) for both fleas and mites in either component (r2 = 0.0006–0.19, F = 0.01–2.91 for fleas and r2 = 0.01–0.15, F = 0.01–3.13 for mites; p > 0.09 for all) or compound (r2 = 0.1, F = 0.68 for fleas and r2 = 0.02, F = 1.02 for mites; p > 0.32 for both) communities.
The results of the logistic MRM analysis of the effects of geographic distance and environmental variables on dissimilarity in the pattern of species co-occurrence (segregative versus aggregative) are presented in Table 2. No effect of either geographic distance or environmental dissimilarity on the dissimilarity of the pattern of species co-occurrence in infracommunities (within a component community) was found in either fleas or mites. The same was true for component communities within a compound community of fleas. However, dissimilarity in the pattern of mite species co-occurrence appeared to be affected by dissimilarity in the amount of green vegetation (Table 2). The probability of the pattern of species co-occurrence in a pair of communities to be different increased with an increase of dissimilarity in NDVI (Fig. 1).
Total deviance explained by the full GDMs ranged from 0.88 to 58.49% for the strength of species associations in flea infracommunities and from 2.27 to 47.18% in mite infracommunities (Tables 3 and 4). A relatively large proportion of total deviance was explained by GDMs in only three host species for fleas (Apodemus agrarius, M. glareolus, and Myodes rufocanus) and two host species for mites (Microtus gregalis and M. rufocanus) (Tables 3 and 4). The best predictors of dissimilarity in the strength of flea species associations differed between host species, with the NDVI factor for A. agrarius, geographic distance for M. glareolus, and climatic factor 1 for M. rufocanus (Table 3). In the two former host species, dissimilarity in the strength of species associations was the greatest between communities in localities with the lowest amount of green vegetation (note the negative correlation between the observed values of NDVI and the NDVI factor) or those that were situated at the farthest distances, whereas in the latter, species dissimilarity in the strength of species associations sharply increased in localities with lower precipitation and a high annual range of air temperature (see “Materials and methods”) and then stabilized (Fig. 2). Climatic factor 1 was the best predictor of dissimilarity in the strength of mite species associations in infracommunities harbored by both M. gregalis and M. rufocanus. In both host species, the pattern of the rate of change in the strength of species associations along this gradient was similar to that found for flea infracommunities in the latter species (Fig. 3).
Generalized dissimilarity model–fitted I-splines (partial regression fits) of NDVI factor (negative correlation with seasonal NDVI), geographic distances, and climatic factor 1 (positive correlation with seasonal precipitation and negative correlation with the annual range of air temperatures) as predictors of the dissimilarity in the strength of species associations in infracommunities of fleas harbored by Apodemus agrarius, Myodes glareolus, and Myodes rufocanus. The steeper slope of the transformed relationship on a given section of the gradient indicates a greater rate of changes in the strength of species associations
Generalized dissimilarity model–fitted I-splines (partial regression fits) of climatic factor 1 (positive correlation with seasonal precipitation and negative correlation with the annual range of air temperatures) as a predictor of the dissimilarity in the strength of species associations in infracommunities of mites harbored by Microtus gregalis and Myodes rufocanus. The steeper slope of the transformed relationship on a given section of the gradient indicates a greater rate of changes in the strength of species associations
The proportion of total deviance explained by the full GDMs for dissimilarity in the strength of species associations in flea and mite component communities was low and constituted only 0.04% and 0.16%, respectively.
Discussion
In general, the results of this study did not support the majority of our expectations. First, we did not find any effect of either latitude or environmental variables on either the pattern or the strength of species associations. Second, dissimilarity in the pattern and strength of species associations between parasite communities did not depend on geographic distance (except for flea communities harbored by M. glareolus) but was affected by dissimilarity in environmental factors, although this was found for component communities of some but not other host species and for compound communities of mites but not fleas.
One of the reasons for the lack of the effect of latitude on community assembly is that latitude is not a factor that can directly affect species relationships but is rather a proxy for many factors such as, for example, climate and soil and vegetation structure, as well as their interactions (Hawkins and Diniz-Filho 2004; Morand 2015). However, the direct effects of the NDVI and climatic variables on either the pattern or the strength of species associations also remain undiscovered. This can be associated with the relatively short latitudinal range in our study (from the highest 68.04°N to the lowest 50.09°N) and the concomitant weak latitudinal variation in bioclimatic factors. Moreover, many fleas and mites spend most of their lives in host burrows/nests with a relatively stable microclimate that varies spatially and temporally much more weakly than does the surrounding environment (Shenbrot et al. 2002). The microclimatic stability of burrows does not even force rodents to construct deeper burrows in more northern regions because of the insulation provided by soil and snow (e.g., Van Vuren and Ordeñana 2012). Consequently, the within-burrow environment has likely not been perceived by ectoparasites as more benign in the south or harsher in the north. As a result, the combination of the stress gradient and the latitudinal gradient of community assembly hypotheses (Qiao et al. 2015) cannot hold for nidicolous ectoparasites, at least, for the latitudinal range considered in our study. In addition, the latitudinal gradient in the parasite community structure, including species richness and composition, as well as the traits of individual species, appeared not to be universal (Vinarski et al. 2007; Blasco-Costa et al. 2015; van der Mescht et al. 2018).
Similar to latitude, geographic distance per se is not a real factor, but it rather reflects the dissimilarity in biotic and abiotic conditions among localities. As a result, compositional dissimilarity in ectoparasite communities has been found to be affected mainly by environmental dissimilarity rather than geographic distance (Vinarski et al. 2007; Krasnov et al. 2010a; Maestri et al. 2017). Indeed, we found an effect of geographic distance on the dissimilarity in the strength of flea (but not mite) species associations in one host species only (M. glareolus). Moreover, the differences in altitude and the amount of vegetation between localities also affected the dissimilarity in the strength of species associations in flea communities harbored by this host. It is thus possible that geographic distance was correlated with some other environmental variables important for flea communities of M. glareolus that have not been measured. We, however, admit that this explanation is speculative.
Despite relatively homogeneous environmental conditions across our study area, environmental differences between localities were sufficiently pronounced for their effects on the pattern or strength of species associations to be detected. However, the effect of environmental dissimilarity on the pattern of species co-occurrence has been found in only one case—across compound communities of mites—although the proportion of communities with contrasting patterns of co-occurrences did not differ between the two ectoparasite taxa. The most obvious reason for the general lack of any relationship between dissimilarity in the pattern of associations and environmental dissimilarity or geographic distance is merely the relative invariance in this pattern. Indeed, an absolute majority of communities were characterized by positive species associations. This is in agreement with the results of our earlier studies on fleas at different hierarchical scales (Krasnov et al. 2006, 2010b, 2011). Predominantly positive species co-occurrences in fleas have been explained by apparent facilitation (Levine 1999) among species mediated via the host due to immunosuppression in a host subjected to multiple challenges (Cox 2001). However, support for this mechanism from experimental manipulations with a pair of flea species was equivocal, and the outcome of species interactions depended on the identities of both fleas and hosts (Khokhlova et al. 2015 versus Khokhlova et al. 2016). Another, not necessarily alternative, reason for the positive co-occurrences of flea and mite species on host bodies can be the microclimatic conditions in host burrows that are favorable for many ectoparasite species. Coupled with a large amount of organic matter serving as food resources for flea larvae and immature and mature stages of many mite species, these conditions likely result in the co-occurrence of multiple species in the hosts’ burrow/nest, which is further translated into their co-occurrence on the hosts’ bodies (Krasnov et al. 2004).
Nevertheless, a relationship between the dissimilarity in the amount of green vegetation and the probability of the pattern of species co-occurrence in a pair of mite communities to be different was found. This suggests that vegetation structure may somehow affect mite species interactions. The most likely mechanism behind this is the effect of the structure of the host’s nest on interactions between mite species because vegetation structure affects the selection of burrow/nest sites and the composition of nests in small mammals (e.g., Kuroe et al. 2007). In addition, nidicolous ectoparasites searching for a host may move from the nest to the entrance of the host’s burrow and even outside the burrow (Humphries 1969; Tagiltsev and Tarasevich 1982; Cox et al. 1999; Burdelov et al. 2007) and thus be directly affected by vegetation structure. However, life history details are unknown for the majority of mite species, so it is difficult to propose a more detailed explanation.
Changes in the strength of species associations along environmental gradients were detected across component communities. These changes were mainly affected by gradients in the amount of green vegetation and precipitation (indicated via climatic factor 1). In general, this supports the idea that environmental factors may enhance or suppress the intensity of both positive and negative species interactions (Kraft et al. 2014). Environmental mediation of species interactions has long been known (Hutchinson 1961) and has often been reported for free-living species (see review in Brooker et al. 2008 for plants). For example, the intensity of interspecific competition between two bird species varied due to habitat productivity reflected in food availability (Dhondt 2010). Water temperature was found to affect the intensity and outcome of interspecific interactions in fish (Milazzo et al. 2012). Choler et al. (2001) demonstrated that facilitation among alpine plants increased with increasing altitude. To the best of our knowledge, the effect of environment on species interactions has never been specifically studied in parasites. However, studies on co-infection patterns suggest that this effect undoubtedly exists (see review in Viney and Graham 2013). Proximate mechanisms for the effect of environment on the outcome of species interactions may include similar environmental preferences of interacting species that ultimately result in the co-occurrence of a certain set of species in a particular environment (= environmental filtering; see Krasnov et al. 2015 for fleas). These mechanisms may also be associated with differential responses to environmental factors causing species segregation among localities (see Krasnov et al. 2001 for fleas parasitic on the same host species).
As mentioned above, positive parasite co-occurrences may arise from the processes acting in a host body (immunosuppression due to multiple challenges) or processes acting in a host burrow (favorable conditions for multiple ectoparasite species) or both. We found the presence of relationships between environmental factors and the strength of ectoparasite species associations (predominantly positive) in the infracommunities of some but not other host species. One explanation for this could be among-host differences in the extent of spatial or environmental variation in immunocompetence or burrow structure. Although the study of the eco-immunology of wild small mammals is in its infancy, and their immune abilities are poorly known (Viney and Riley 2017), among-species differences in immunocompetence have been shown (Goüy de Bellocq et al. 2006; Previtali et al. 2012), and thus, differences in the response of this trait to environmental variation are likely. For example, regarding burrows, it is known that burrow architecture (depth, tunnel length, number of entrances) varies among habitats in some small mammal species more than in others (Kucheruk 1983).
In conclusion, the results of our study demonstrate that the pattern and strength of species associations in ectoparasite communities rarely conform to biogeographic rules. Nevertheless, when a biogeographic pattern in community assembly is found, its manifestation differs among host species and between ectoparasite taxa.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Astorga A, Oksanen J, Luoto M, Soininen J, Virtanen R, Muotka T (2012) Distance decay of similarity in freshwater communities: do macro- and microorganisms follow the same rules? Glob Ecol Biogeogr 21:365–375. https://doi.org/10.1111/j.1466-8238.2011.00681.x
Bertness M, Ewanchuk P (2002) Latitudinal and climate-driven variation in the strength and nature of biological interactions in New England salt marshes. Oecologia 132:392–401. https://doi.org/10.1007/s00442-002-0972-y
Blasco-Costa I, Rouco C, Poulin R (2015) Biogeography of parasitism in freshwater fish: spatial patterns in hot spots of infection. Ecography 38:301–310. https://doi.org/10.1111/ecog.01020
Bordes F, Morand S, Krasnov BR, Poulin R (2010) Parasite diversity and latitudinal gradients in terrestrial mammals. In: Morand S, Krasnov BR (eds) The biogeography of host-parasite interactions. Oxford Univ Press, Oxford, pp 89–98
Brooker RW, Maestre FT, Callaway RM, Lortie CL, Cavieres LA, Kunstler G, Liancourt P et al (2008) Facilitation in plant communities: the past, the present, and the future. J Ecol 96:18–34. https://doi.org/10.1111/j.1365-2745.2007.01295.x
Brown JH (1995) Macroecology. Univ Chicago Press, Chicago
Brown JH (2014) Why are there so many species in the tropics? J Biogeogr 41:8–22. https://doi.org/10.1111/jbi.12228
Burdelov SA, Leiderman M, Khokhlova IS, Krasnov BR, Degen AA (2007) Locomotor response to light and surface angle in three species of desert fleas. Parasitol Res 100:973–982. https://doi.org/10.1007/s00436-006-0383-9
Bush AO, Lafferty KD, Lotz JM, Shostak AW (1997) Parasitology meets ecology on its own terms: Margolis et al. revisited. J Parasitol 83:575–583. https://doi.org/10.2307/3284227
Callaway RM, Brooker RW, Choler P, Kikvidze Z, Lortiek CJ, Michalet R, Paolini L, Pugnaireq FI, Newingham B, Aschehoug ET, Armas C, Kikodze D, Cook B (2002) Positive interactions among alpine plants increase with stress. Nature 417:844–848. https://doi.org/10.1038/nature00812
Choler P, Michalet R, Callaway RM (2001) Facilitation and competition on gradients in alpine plant communities. Ecology 82:3295–3308. https://doi.org/10.1890/0012-9658(2001)082[3295:FACOGI]2.0.CO;2
Cox FEG (2001) Concomitant infections, parasites and immune responses. Parasitology 122:S23–S38. https://doi.org/10.1017/S003118200001698X
Cox R, Stewart PD, Macdonald DW (1999) The ectoparasites of the European badger, Meles meles, and the behavior of the host-specific flea, Paraceras melis. J Insect Behav 12:245–265. https://doi.org/10.1023/A:1020923001987
Dhondt AA (2010) Effects of competition on great and blue tit reproduction: intensity and importance in relation to habitat quality. J Anim Ecol 79:257–265. https://doi.org/10.1111/j.1365-2656.2009.01624.x
Diamond JM (1975) Assembly of species communities: chance or competition. In: Cody ML, Diamond JM (eds) Ecology and evolution of communities. Harvard Univ Press, Cambridge, pp 342–444
Ferrier S (2002) Mapping spatial pattern in biodiversity for regional conservation planning: where to from here? Syst Biol 51:331–363. https://doi.org/10.1080/10635150252899806
Ferrier S, Drielsma M, Manion G, Watson G (2002) Extended statistical approaches to modeling spatial pattern in biodiversity in north-east New South Wales: II. Community- level modeling. Biodivers Conserv 11:2309–2338. https://doi.org/10.1023/A:1021374009951
Ferrier S, Manion G, Elith J, Richardson K (2007) Using generalized dissimilarity modelling to analyse and predict patterns of beta diversity in regional biodiversity assessment. Divers Distrib 13:252–264. https://doi.org/10.1111/j.1472-4642.2007.00341.x
Fick SE, Hijmans RJ (2017) WorldClim 2: new 1-km spatial resolution climate surfaces for global land areas. Int J Climatol 37:4302–4315. https://doi.org/10.1002/joc.5086
Gaston KJ (2000) Global patterns in biodiversity. Nature 405:220–227. https://doi.org/10.1038/35012228
Goslee SC, Urban DL (2007) The ecodist package for dissimilarity-based analysis of ecological data. J Stat Softw 22:1–19. https://doi.org/10.18637/jss.v022.i07
Gotelli NJ (2000) Null model analysis of species co-occurrence patterns. Ecology 81:2606–2621. https://doi.org/10.1890/0012-9658(2000)081[2606:NMAOSC]2.0.CO;2
Gotelli NJ, Graves GR (1996) Null models in ecology. Smithsonian Institution Press, Washington and London
Gotelli NJ, McCabe DJ (2002) Species co-occurrence: a meta-analysis of J. M. Diamond’s assembly rules model. Ecology 83:2091–2096. https://doi.org/10.1890/0012-9658(2002)083[2091:SCOAMA]2.0.CO;2
Gotelli NJ, Rohde K (2002) Co-occurrence of ectoparasites of marine fishes: a null model analysis. Ecol Lett 5:86–94. https://doi.org/10.1046/j.1461-0248.2002.00288.x
Gotelli NJ, Ulrich W (2010) The empirical Bayes approach as a tool to identify non-random species associations. Oecologia 162:463–477. https://doi.org/10.1007/s00442-009-1474-y
Goüy de Bellocq J, Krasnov BR, Khokhlova IS, Pinshow B (2006) Temporal dynamics of a T-cell mediated immune response in desert rodents. Comp Biochem Physiol A 145:554–559. https://doi.org/10.1016/j.cbpa.2006.08.045
Hanski I (1982) Communities of bumblebees: testing the core-satellite hypothesis. Ann Zool Fenn 19:65–73
Hawkins BA, Diniz-Filho FAJ (2004) ‘Latitude’ and geographic patterns in species richness. Ecography 27:268–272. https://doi.org/10.1111/j.0906-7590.2004.03883.x
Henriques-Silva R, Lindo Z, Peres-Neto PR (2013) A community of metacommunities: exploring patterns in species distributions across large geographical areas. Ecology 94:627–639. https://doi.org/10.1890/12-0683.1
Hijmans RJ (2017) Geosphere: spherical trigonometry. R package version 1.5–7. https://CRAN.R-project.org/package=geosphere
Holmes JC, Price PW (1986) Communities of parasites. In: Kittawa J, Anderson DJ (eds) Community ecology: pattern and process. Blackwell, Oxford, pp 187–213
Hubbell S (2001) The unified neutral theory of biodiversity and biogeography. Princeton Monogr Popul Biol, Princeton Univ Press. Princeton
Humphries DA (1969) Behavioral aspects of the ecology of the sandmartin flea Ceratophyllus styx jordani smit (Siphonaptera). Parasitology 59:311–334. https://doi.org/10.1017/S0031182000082287
Hutchinson GE (1961) The paradox of the plankton. Am Nat 95:137–145. https://doi.org/10.1086/282171
Jackman S (2017) pscl: classes and methods for R developed in the political science computational laboratory. US Studies Centre, Univ Sydney. Sydney, New South Wales, Australia. R package version 1.5.2. URL https://github.com/atahk/pscl/
Khokhlova IS, Dlugosz EM, Krasnov BR (2015) Fitness responses to co-infestation in fleas exploiting rodent hosts. Parasitology 142:1535–1542. https://doi.org/10.1017/S0031182015001006
Khokhlova IS, Dlugosz EM, Krasnov BR (2016) Experimental evidence of negative interspecific interactions among imago fleas: flea and host identities matter. Parasitol Res 115:937–947. https://doi.org/10.1007/s00436-015-4818-z
Kohli BA, Terry RC, Rowe RJ (2018) A trait-based framework for discerning drivers of species co-occurrence across heterogeneous landscapes. Ecography 41:1921–1933. https://doi.org/10.1111/ecog.03747
Kozlova RG (1983) The effect of air moisture on development, survival and behaviour of the mite Haemogamasus nidi (Gamasoidea, Haemogamasidae). Parazitologiya 17:293–298 (in Russian)
Kraft NGB, Adler PB, Godoy O, James EC, Fuller S, Levine JM (2014) Community assembly, coexistence and the environmental filtering metaphor. Funct Ecol 29:592–599. https://doi.org/10.1111/1365-2435.12345
Krasnov BR (2008) Functional and evolutionary ecology of fleas. In: A model for ecological parasitology. Cambridge Univ Press, Cambridge
Krasnov BR, Khokhlova IS, Fielden LJ, Burdelova NV (2001) The effect of air temperature and humidity on the survival of pre-imaginal stages of two flea species (Siphonaptera: Pulicidae). J Med Entomol 38:629–637. https://doi.org/10.1603/0022-2585-38.5.629
Krasnov BR, Shenbrot GI, Khokhlova IS (2004) Sampling fleas: the reliability of host infestation data. Med Vet Entomol 18:232–240. https://doi.org/10.1111/j.0269-283X.2004.00500.x
Krasnov BR, Stanko M, Morand S (2006) Are ectoparasite communities structured? Species co-occurrence, temporal variation and null models. J Anim Ecol 75:1330–1339. https://doi.org/10.1111/j.1365-2656.2006.01156.x
Krasnov BR, Shenbrot GI, Khokhlova IS, Poulin R (2007) Geographical variation in the “bottom-up” control of diversity: fleas and their small mammalian hosts. Glob Ecol Biogeogr 16:179–186. https://doi.org/10.1111/j.1466-8238.2006.00273.x
Krasnov BR, Mouillot D, Shenbrot GI, Khokhlova IS, Poulin R (2010a) Deconstructing spatial patterns in species composition of ectoparasite communities: the relative contribution of host composition, environmental variables and geography. Glob Ecol Biogeogr 19:515–526. https://doi.org/10.1111/j.1466-8238.2010.00529.x
Krasnov BR, Matthee S, Lareschi M, Korallo-Vinarskaya NP, Vinarski MV (2010b) Co-occurrence of ectoparasites on rodent hosts: null model analyses of data from three continents. Oikos 116:120–128. https://doi.org/10.1111/j.1600-0706.2009.17902.x
Krasnov BR, Shenbrot GI, Khokhlova IS (2011) Aggregative structure is the rule in communities of fleas: null model analysis. Ecography 34:751–761. https://doi.org/10.1111/j.1600-0587.2010.06597.x
Krasnov BR, Shenbrot GI, Khokhlova IS, Stanko M, Morand S, Mouillot D (2015) Assembly rules of ectoparasite communities across scales: combining patterns of abiotic factors, host composition, geographic space, phylogeny and traits. Ecography 38:184–197. https://doi.org/10.1111/ecog.00915
Kucheruk VV (1983) Mammal burrows: their structure, topology and use. Fauna and Ecology of Rodents 15:5–54 (in Russian)
Kuroe M, Ohori S, Takatsuki S, Miyashita T (2007) Nest-site selection by the harvest mouse Micromys minutus in seasonally changing environments. Acta Theriol 52:355–360. https://doi.org/10.1007/BF03194233
Legendre P, Legendre L (1998) Numerical ecology, 2nd Engl edn. Elsevier, Amsterdam
Levine JM (1999) Indirect facilitation: evidence and predictions from a riparian community. Ecology 80:1762–1769. https://doi.org/10.1890/0012-9658(1999)080[1762:IFEAPF]2.0.CO;2
Lichstein JW (2007) Multiple regression on distance matrices: a multivariate spatial analysis tool. Plant Ecol 188:117–131. https://doi.org/10.1007/s11258-006-9126-3
Loreau M (2000) Are communities saturated? On the relationship of α, β and γ diversity. Ecol Lett 3:73–76. https://doi.org/10.1046/j.1461-0248.2000.00127.x
Lortie CJ, Callaway RM (2006) Re-analysis of meta-analysis: support for the stress-gradient hypothesis. J Ecol 94:7–16. https://doi.org/10.1111/j.1365-2745.2005.01066.x
Maestre FT, Callaway RM, Valladares F, Lortie CJ (2009) Refining the stress-gradient hypothesis for competition and facilitation in plant communities. J Ecol 97:199–205. https://doi.org/10.1111/j.1365-2745.2008.01476.x
Maestri R, Shenbrot GI, Krasnov BR (2017) Parasite beta diversity, host beta diversity and environment: application of two approaches to reveal patterns of flea species turnover in Mongolia. J Biogeogr 44:1880–1890. https://doi.org/10.1111/jbi.13025
Manion G, Lisk M, Ferrier S, Nieto-Lugilde D, Mokany K, Fitzpatrick MC (2018). gdm: generalized dissimilarity modeling. R package version 13.11. https://CRAN.R-project.org/package=gdm
Manly BF (1986) Randomization and regression methods for testing for associations with geographical, environmental and biological distances between populations. Res Popul Ecol 28:201–218. https://doi.org/10.1007/BF02515450
Milazzo M, Mirto S, Domenici P, Gristina M (2012) Climate change exacerbates interspecific interactions in sympatric coastal fishes. J Anim Ecol 82:468–477. https://doi.org/10.1111/j.1365-2656.2012.02034.x
Morand S (2015) (macro-) Evolutionary ecology of parasite diversity: from determinants of parasite species richness to host diversification. Int J Parasitol Parasites Wildl 4:80–87. https://doi.org/10.1016/j.ijppaw.2015.01.001
Morlon H, Chuyong G, Condit R, Hubbell S, Kenfack D, Thomas D, Valencia R, Green JL (2008) A general framework for the distance–decay of similarity in ecological communities. Ecol Lett 11:904–917. https://doi.org/10.1111/j.1461-0248.2008.01202.x
Nakagawa S (2004) A farewell to Bonferroni: the problems of low statistical power and publication bias. Behav Ecol 15:1044–1045. https://doi.org/10.1093/beheco/arh107
Nekola JC, White PS (1999) The distance decay of similarity in biogeography and ecology. J Biogeogr 26:867–878. https://doi.org/10.1046/j.1365-2699.1999.00305.x
Patterson BD, Atmar W (1986) Nested subsets and the structure of insular mammalian faunas and archipelagos. Biol J Linn Soc 28:65–82. https://doi.org/10.1111/j.1095-8312.1986.tb01749.x
Perneger TV (1998) What’s wrong with Bonferroni adjustments. Br Med J 316:1236–1238. https://doi.org/10.1136/bmj.316.7139.1236
Pianka ER (1966) Latitudinal gradients in species diversity: a review of concepts. Am Nat 100:33–46. https://doi.org/10.1086/282398
Pilosof S, Morand S, Krasnov BR, Nunn CL (2015) Potential parasite transmission in multi-host networks based on parasite sharing. PLoS One 10:e0117909. https://doi.org/10.1371/journal.pone.0117909
Poulin R (2007) Evolutionary ecology of parasites: from individuals to communities, 2nd edn. Princeton University Press, Princeton
Poulin R, Krasnov BR (2010) Similarity and variability of parasite assemblages across geographic space. In: Morand S, Krasnov BR (eds) The biogeography of host-parasite interactions. Oxford Univ Press, Oxford, pp 115–128
Previtali MA, Ostfeld RS, Keesing F, Jolles AE, Hanselmann R, Martin LB (2012) Relationship between pace of life and immune responses in wild rodents. Oikos 121:1483–1492. https://doi.org/10.1111/j.1600-0706.2012.020215.x
Qiao X, Tang Z, Wang S, Liu Y, Fang J (2012) Effects of community structure on the species–area relationship in China’s forests. Ecography 35:1117–1123. https://doi.org/10.1111/j.1600-0587.2012.00050.x
Qiao X, Jabot F, Tang Z, Jiang M, Fang J (2015) A latitudinal gradient in tree community assembly processes evidenced in Chinese forests. Glob Ecol Biogeogr 24:314–323. https://doi.org/10.1111/geb.12278
R Core Team (2018) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/
Radovsky FJ (1985) Evolution of mammalian mesostigmatid mites. In: Kim KC (ed) Coevolution of parasitic arthropods and mammals. Wiley, New York, pp 441–504
Ricklefs RE, Jenkins DG (2011) Biogeography and ecology: towards the integration of two disciplines. Philos Trans R Soc Lond 366:2438–2448. https://doi.org/10.1098/rstb.2011.0066
Rohde K (1992) Latitudinal gradients in species diversity: the search for the primary cause. Oikos 65:514–527. https://doi.org/10.2307/3545569
Rohde K (2010) Marine parasite diversity and environmental gradients. In: Morand S, Krasnov BR (eds) The biogeography of host-parasite interactions. Oxford Univ Press, Oxford, pp 73–88
Saito VS, Soininen J, Fonseca-Gessner AA, Siqueira T (2015) Dispersal traits drive the phylogenetic distance decay of similarity in Neotropical stream metacommunities. J Biogeogr 42:2101–2111. https://doi.org/10.1111/jbi.12577
Schemske DW, Mittelbach GG (2017) “Latitudinal gradients in species diversity”: reflections on Pianka’s 1966 article and a look forward. Am Nat 189:599–603. https://doi.org/10.1086/691719
Shenbrot G, Krasnov B, Khokhlova I, Demidova T, Fielden L (2002) Habitat-dependent differences in architecture and microclimate of the burrows of Sundevall’s jird (Meriones crassus) (Rodentia: Gerbillinae) in the Negev Desert, Israel. J Arid Environ 51:265–279. https://doi.org/10.1006/jare.2001.0945
Soininen J, McDonald R, Hillebrand H (2007) The distance decay of similarity in ecological communities. Ecography 30:3–12. https://doi.org/10.1111/j.0906-7590.2007.04817.x
Stone L, Roberts A (1990) The checkerboard score and species distributions. Oecologia 85:74–79. https://doi.org/10.1007/BF00317345
Tagiltsev AA, Tarasevich LN (1982) Nidicolous arthropods in natural foci of arboviral diseases. Nauka, Novosibirsk, USSR (in Russian).
Tello JS, Stevens RD, Dick CW (2008) Patterns of species co-occurrence and density compensation: a test for interspecific competition in bat ectoparasite infracommunities. Oikos 117:693–702. https://doi.org/10.1111/j.0030-1299.2008.16212.x
van der Mescht L, Warburton EM, Khokhlova IS, Stanko M, Vinarski MV, Korallo-Vinarskaya NP, Krasnov BR (2018) Biogeography of parasite abundance: latitudinal gradient and distance decay of similarity in the abundance of fleas and mites parasitic on small mammals in the Palearctic at three spatial scales. Int J Parasitol 48:857–866. https://doi.org/10.1016/j.ijpara.
Van Vuren DH, Ordeñana MA (2012) Factors influencing burrow length and depth of ground-dwelling squirrels. J Mammal 93:1240–1246. https://doi.org/10.1644/12-MAMM-A-049.1
Vázquez DP, Stevens RD (2004) The latitudinal gradient in niche breadth: concepts and evidence. Am Nat 164:E1–E19. https://doi.org/10.1086/421445
Vinarski MV, Korallo NP, Krasnov BR, Shenbrot GI, Poulin R (2007) Decay of similarity of gamasid mite assemblages parasitic on Palaearctic small mammals: geographic distance, host-species composition or environment. J Biogeogr 34:1691–1700. https://doi.org/10.1111/j.1365-2699.2007.01735.x
Viney ME, Graham AL (2013) Patterns and processes in parasite co-infection. Adv Parasitol 82:321–370. https://doi.org/10.1016/B978-0-12-407706-5.00005-8
Viney M, Riley EM (2017) The immunology of wild rodents: current status and future prospects. Front Immunol 8:1481. https://doi.org/10.3389/fimmu.2017.01481
Acknowledgements
We thank two anonymous referees for their helpful comments on the earlier version of the manuscript. This is publication no. 1006 of the Mitrani Department of Desert Ecology.
Funding
This study was partly supported by the Israel Science Foundation (grant no. 149/17 to BRK and ISK) and the Russian Ministry of Education and Science (grant no. 6.1352.2017/4.6 to MVV and NPKV). EMW received financial support from the Blaustein Center for Scientific Cooperation. LVDM received financial support from the Blaustein Center for Scientific Cooperation and the French Associates Institute for Agriculture and Biotechnology of Drylands.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All sampling procedures were in accordance with the national laws and guidelines of USSR and Russian Federation at the time of sampling.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: Julia Walochnik
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 4963 kb)
Rights and permissions
About this article
Cite this article
Krasnov, B.R., Shenbrot, G.I., Korallo-Vinarskaya, N.P. et al. Do the pattern and strength of species associations in ectoparasite communities conform to biogeographic rules?. Parasitol Res 118, 1113–1125 (2019). https://doi.org/10.1007/s00436-019-06255-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-019-06255-4