Abstract
Liver fibrosis is an important process that occurs in most types of chronic liver diseases and often results in the end stage of liver diseases, such as cirrhosis, portal hypertension, and hepatocellular carcinoma. Sorafenib, a multiple tyrosine kinase inhibitor, has been shown to inhibit liver fibrosis in multiple experimental fibrosis mouse and rat models. The aim of this study was to test the therapeutic effect of sorafenib on liver fibrosis induced by infection with a parasite, Schistosoma japonicum, in mice. Mice were percutaneously infected through the abdomen with Schistosoma cercariae to develop a schistosomula liver fibrosis model. Eight weeks after infection, infected mice were treated with the anti-parasitic agent praziquantel for 2 days and sorafenib for 2 weeks. Hepatic histopathological changes were assessed using hematoxylin and eosin (HE) and Masson’s trichome staining. The hepatic expression levels of collagen I, collagen III, alpha-smooth muscle actin (α-SMA), platelet-derived growth factor (PDGF), and PDGF receptor-beta (PDGFR-β) were analyzed by immunohistochemistry and western blot. Praziquantel administration alone but not sorafenib reduced liver fibrosis, and the combination of praziquantel and sorafenib significantly attenuated liver fibrosis in S. japonicum-infected mice. Moreover, sorafenib plus praziquantel markedly decreased the hepatic deposition of collagen and expression of fibrogenic genes in these mice. In conclusion, the use of sorafenib following praziquantel treatment may represent a potential therapeutic strategy for liver fibrosis induced by S. japonicum in patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Liver fibrosis is an important and common process that occurs in most types of chronic liver diseases and results in the end stage of liver diseases including cirrhosis, portal hypertension, and hepatocellular carcinoma (HCC). Accumulation of extracellular matrix (ECM) proteins during the wound-healing response to chronic injury leads to disruption of the normal architecture of the liver, resulting in fibrosis progression and subsequent cirrhosis (Bataller and Brenner 2005). Hepatic stellate cells (HSCs) are known to play a major role in liver fibrogenesis. They are activated in response to inflammatory injury and acquire fibrogenic properties characterized by the expression of alpha-smooth muscle actin (α-SMA) and the secretion of excessive collagens and other ECM components (Moreira 2007; Sato et al. 2003). During activation of HSCs, platelet-derived growth factor (PDGF) was identified as the main mediator of proliferation, and transforming growth factor beta (TGF-β) was identified as the most important cytokine stimulating fibrogenesis in HSCs (Borkham-Kamphorst et al. 2004; George et al. 1999; Yoshiji et al. 2006).
The main causes of liver fibrosis are viral or parasitic infection, cholestasis, metabolic diseases, and alcohol abuse. Schistosomiasis is currently endemic in many countries and represents a major global public health problem. In China, schistosomiasis is usually caused by the trematode Schistosoma japonicum (Colley et al. 2014; Zhou et al. 2005). Hepatic schistosomiasis is characterized by typical pathological changes including egg granuloma and hepatic fibrosis at chronic and advanced stages, resulting in liver cirrhosis, portal hypertension, ascites, and splenomegaly (Andrade 2009). Praziquantel (PZQ) is an effective drug against all schistosome species and is used to treat schistosomiasis. However, although it acts against adult schistosome worms, it has poor activity against immature schistosome larvae and has only a limited effect on already developed liver and spleen lesions (Cioli et al. 2014; Doenhoff et al. 2008).
Sorafenib, an inhibitor of multiple tyrosine kinase and the vascular endothelial growth factor receptor, was approved for the treatment of advanced HCC in 2007 (Guan and He 2011; Hsu et al. 2014). Recently, several groups of investigators have demonstrated that sorafenib also can inhibit liver fibrosis in different rat and mouse models established by partial portal vein ligation (Mejias et al. 2009), common bile duct ligation (Hennenberg et al. 2011; Mejias et al. 2009; Su et al. 2015; Thabut et al. 2011) and dimethylnitrosamine (Liu et al. 2015) or CCl4 (Deng et al. 2013) injection. However, further studies are needed to test the hypothesis that sorafenib plays an anti-fibrotic role in natural models of liver fibrosis. In the present study, we investigated the effect of sorafenib on liver fibrosis induced by infection of S. japonicum in mice.
Materials and methods
Animals, parasite, and drugs
Female, specific-pathogen-free BALB/C mice at 8 weeks of age weighing 18–20 g were purchased from the Hubei Provincial Centre for Disease Control and Prevention, Hubei, China (permission no. SCXK 2008-0004). All animals were maintained according to the guidelines of the animal facility at the Hubei Provincial Academy of Preventive Medicine. All animal experiments were conducted in accordance with the Guide for the Care and Use of Laboratory and were approved by the Committee on the Ethics of Animal Experiments of Hubei Provincial Academy of Preventive Medicine (project no. M2014001). S. japonicum cercariae were kindly provided by Dr. Dong from the Department of Human Parasitology, Wuhan University, China. PZQ was purchased from Nanjing Pharmaceutical Factory (catalog no. 20130110) and sorafenib was purchased from Gene Operation, USA (IMA1044-0010MG).
Animal treatment
The animals were infected with S. japonicum according to a previous study (Chen et al. 2013). Briefly, the mice were percutaneously infected with S. japonicum by placing a coverslip carrying 20 ± 1 cercariae in non-chlorine water on their abdomens for 30 min. Mice treated with non-chlorine water containing no cercariae were used as uninfected controls (group A, n = 10). Eight weeks after infection, the liver fibrosis model was successfully established according to our preliminary experiment. Then, the infected mice were randomly divided into four groups: the infected and untreated group (group B, n = 12), the sorafenib-treated group (group C, n = 12), the PZQ-treated group (group D, n = 12), and the sorafenib plus PZQ-treated group (group E, n = 12). The mice in group C were treated with an equivalent amount of normal saline solution once a day for 2 days and then with sorafenib (2 mg/kg/day) once a day for 2 weeks. The mice in group D were treated with PZQ (150 mg/kg/day) for 2 days and then continued receiving an equivalent amount of normal saline solution once a day for 2 weeks. The mice in group E were first treated with PZQ at the same dose and duration as group D followed by treatment with sorafenib 2 mg/kg per day for 2 weeks. The normal control group and the infected control group were given an equivalent amount of normal saline solution from the beginning of treatment to the end of the experiment. All drugs were intragastrically administered to the mice. Sorafenib has a long half-life (24 to 48 h) and steady-state concentrations are reached after 7 days. The dose and duration of sorafenib administration were selected based on an earlier study (Mejias et al. 2009). All animals were sacrificed at the end of sorafenib treatment, and liver tissues and serum were collected. Venous blood was centrifuged, and the serum was stored at − 20 °C until use. Parts of the liver tissues were fixed in a 4% formaldehyde solution and the remaining tissues were stored at − 80 °C.
Serum alanine aminotransferase (ALT) and albumin (ALB) analysis
Mouse blood was collected and kept at room temperature for 1–2 h. The serum was separated by centrifugation at 3000 r/min for 15 min. The level of serum ALT was detected by Reit’s method (C009-2, Njjcbio, China), and ALB was detected using the bromo cresol green method (A028-2, Njjcbio, China) according to the manufacturer’s protocol.
Histological analysis
Fixed liver tissues in 4% formaldehyde were routinely embedded in paraffin and cut into 4-μm-thick sections. The tissue sections were stained with hematoxylin and eosin (HE) or Masson’s trichrome, respectively. The HE-stained liver sections were examined in all groups to count the mean number of hepatic granulomas. At least 15 liver sections from five to six mice in each group were assessed. For the same liver section, five successive fields (10 × 10) were assessed to calculate the mean number of hepatic granulomas. Masson’s trichrome-stained liver sections were used to estimate the hepatic deposition of collagen. At least five successive fields in each mouse and a total of five mice from each group were assessed. The hepatic deposition of collagen was quantified by the percentage of the area stained with blue color in all fields (10 × 10) using Image-Pro Plus 6.0 according to the manufacturer’s protocol.
Immunohistochemistry
The method was based on the manufacturer’s protocol. The liver tissue sections were dewaxed, dehydrated, washed in phosphate-buffered saline (PBS) three times × 3 min, and heated in a microwave oven for 10 min. Endogenous peroxidase was blocked with 3% hydrogen peroxide (H2O2). Then, the sections were incubated with 5% normal goat serum at room temperature for 10 min. The primary antibodies (rabbit α-SMA antibody, BM0002; rabbit type I collagen antibody, BA0325; rabbit type III collagen antibody, BA0326; rabbit PDGFR-b antibody, BA0276; Bosider, Wuhan, China) were diluted 500-fold and then applied and incubated overnight at 4 °C. The sections were then washed with PBS three times. HRP-polymer anti-mouse/rabbit immunoglobulin G (KIT-9710/9720/9730; MAIXIN-BIO, China) was added to the sections and incubated at room temperature for 15 min. After washing three times × 5 min with PBS, the diaminobenzidine (DAB) solution was applied as the chromogen. Negative controls were incubated with PBS without the primary antibody. Three or four random fields of portal tracts and the peripheries of granuloma tissues (the most serious areas of pathological damage) in each section were microscopically evaluated at ×100 magnification. The optical density (OD) of the target protein was measured with Image-Pro Plus 6.0. The OD was defined as the ratio of positive optical density (yellow) and total pixels, which represented the quantity of the targeted protein.
Western blot analysis
Frozen liver tissues (150 mg) were homogenized on ice in 0.5 mL of lysis buffer (RIPA/PMSF = 100:1). After placement on the ice for an hour, the homogenates were centrifuged at 12,000 r/min at 4 °C for 15 min. The protein concentration was determined using the bicinchoninic acid assay. The samples (40 μg protein in each lane) were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred onto a nitrocellulose membrane. Non-specific binding sites were blocked by incubation with Tris-buffered saline (TBS) containing 5% non-fat milk for 2 h at room temperature. The primary antibodies (rabbit α-SMA monoclonal antibody, ab32575, 1:2500; and rabbit PDGF monoclonal antibody, ab32570, 1:2500; Abcam, UK) were applied and incubated overnight at 4 °C. After washing three times × 10 min with 0.1% Tween–TBS, the secondary antibody (goat anti-rabbit IgG, 1:30,000) was applied, followed by incubation for 1 h at room temperature. Then, the membranes were washed three times × 10 min with 0.1% Tween-TBS. Electrochemiluminescence reagents were used for detection. Images were analyzed with Quantity One. The expression levels of the target proteins were defined as the ratio of target protein OD to β-actin OD.
Statistical analysis
Statistical analyses were performed using GraphPad Prism software version 5 (GraphPad Software Inc., San Diego, CA). Significant differences were analyzed using the unpaired Student t test and Mann–Whitney test. P values < 0.05 were considered significant.
Results
Sorafenib plus PZQ treatment attenuated hepatic inflammation and recovered liver synthetic function in S. japonicum-infected mice
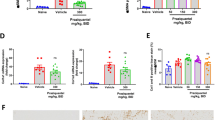
To evaluate inflammation and the synthetic function of the liver in S. japonicum-infected mice, we analyzed the serum levels of ALT and ALB in these mice. Compared to the uninfected mice, an increase in the serum level of ALT and a decrease in the serum level of ALB were observed in the S. japonicum-infected mice (Fig. 1). However, by comparing the serum levels of ALT and ALB in the groups of infected mice, we found that administration of PZQ alone but not sorafenib partially relieved inflammation and restored the synthetic function of the liver. Moreover, we found that the serum levels of ALT and ALB were totally recovered in the S. japonicum-infected mice treated with sorafenib plus PZQ (Fig. 1).
Sorafenib plus PZQ treatment attenuated hepatic inflammation and recovered liver synthetic function in S. japonicum-infected mice. The mice were infected with S. japonicum and treated with PBS (infected), sorafenib (infected + sor), PZQ (infected + PZQ), or sorafenib plus PZQ (infected + PZQ + sor) according to the “Materials and methods” section. Uninfected mice were used as normal controls (uninfected). The serum levels of ALT (a) and ALB (b) were analyzed to evaluate inflammation and the synthetic function of the liver in these mice. The bars represent the mean values and the standard errors of the means obtained from each group of mice. Asterisks mark significant differences between different groups (*p < 0.05, **p < 0.01, not significant (n.s.) indicates p > 0.05)
Sorafenib plus PZQ treatment attenuated the schistosomal hepatopathology in S. japonicum-infected mice
Liver samples were harvested 24 h after the last treatment. The livers from the S. japonicum-infected mice exhibited larger sizes and numerous miliary gray nodules on their surfaces (Fig. 2a). The livers from the infected mice treated with PZQ showed smaller sizes and smooth surfaces compared with the saline- or sorafenib-treated mice (Fig. 2a). To investigate the typical schistosomal hepatopathology in the S. japonicum-infected mice, the livers were fixed and stained with HE or Masson’s trichrome (Fig. 2b, c). Next, we investigated the mean numbers of hepatic egg deposition and granulomas in the liver tissues of infected and treated mice after staining with HE. Compared with the S. japonicum-infected and untreated mice, the infected mice treated with PZQ but not sorafenib showed fewer egg granulomas. Interestingly, administration of sorafenib after PZQ treatment further decreased the formation of hepatic egg granulomas (Fig. 2d). Fibrotic collagen deposition around the egg granulomas was assessed by Masson’s trichome staining, which stained collagen with blue color. Consistently, the infected mice treated with PZQ but not sorafenib showed reduced collagen deposition in the liver. Moreover, in the livers of the mice treated with sorafenib plus PZQ, collagen deposition was further decreased compared to that in the untreated or PZQ-treated mice (Fig. 2e).
Sorafenib plus PZQ treatment attenuated the schistosomal hepato-pathology in S. japonicum-infected mice. The mice were infected with S. japonicum and treated with PBS, PZQ, and sorafenib as described above. a Gross morphology of liver tissues in each group. b, c Representative images of liver tissues in different groups stained with HE (b) and Masson’s trichome (c). The original magnification is ×100 in (b) and ×200 in (c). d The number of hepatic egg granulomas in different groups of mice was calculated and expressed as per area of the liver (mm2). e Hepatic collagen deposition was quantified using Image-Pro Plus 6.0 according to the manufacturer’s protocol. Asterisks mark significant differences between different groups (*p < 0.05, **p < 0.01, not significant (n.s.) indicates p > 0.05)
Sorafenib plus PZQ treatment markedly decreased the expression of fibrogenic proteins in the livers of S. japonicum-infected mice
The hepatopathology study suggested that sorafenib plus anti-parasite treatment reduced hepatic inflammation and fibrosis in S. japonicum-infected mice. To further determine how sorafenib plus PZQ inhibited liver fibrosis in these mice, we conducted immunohistochemical studies and western blot to investigate the hepatic expression levels of pro-fibrotic proteins, such as collagen I, collagen III, α-SMA, PDGF, and PDGF receptor-beta (PDGFR-β).
Collagens I and III are two main ECM components produced by activated HSCs during chronic liver injury or inflammation. The expression levels of collagens I and III were remarkably high in the livers from S. japonicum-infected mice compared with those in uninfected mice (Fig. 3). Treatment with PZQ alone but not sorafenib reduced the expression levels of collagens I and III in the infected mice. Interestingly, sorafenib plus PZQ treatment caused a profound decrease in collagen expression in these mice, which was consistent with the reduced collagen deposition shown by Masson’s trichome staining.
Sorafenib plus PZQ treatment reduced collagen deposition in S. japonicum-infected mice. Mice were infected with S. japonicum and treated with PBS, PZQ, and sorafenib as described above. The hepatic expression of two main ECM components, collagens I (a) and III (b), was analyzed by IHC staining. The optical densities (ODs) of collagens I (c) and III (d) were measured with Image-Pro Plus 6.0 according to the manufacturer’s protocol. The original magnification is ×200. Asterisks mark significant differences between different groups (*p < 0.05, **p < 0.01, not significant (n.s.) indicates p > 0.05)
We also measured the protein expression of α-SMA, which is expressed by activated HSCs when they gain a myofibroblast-like phenotype in response to liver injury (Mejias et al. 2009). S. japonicum infection in mice led to increased expression of α-SMA in the liver (Fig. 4a, d), suggesting activation of HSCs. Anti-parasite treatment with PZQ alone partially reduced the hepatic expression of α-SMA. However, administration of sorafenib after PZQ treatment further decreased the expression of α-SMA, suggesting that this treatment inhibited HSC activation. These results were further supported by our findings that sorafenib plus PZQ attenuated the hepatic expression of PDGF (Fig. 4b, e) and its receptor PDGF receptor-beta (PDGFR-β) (Fig. 4c, f) in the S. japonicum-infected mice. PDGF, which is mainly produced by hepatic Kupffer cells, is the predominant mitogen for activated HSCs (Bataller and Brenner 2005). Sorafenib plus PZQ may inhibit activation of HSCs by decreasing the expression of PDGF and PDGFR-β in the liver. These immunohistochemical studies were supported and confirmed via western blot analysis showing that the hepatic expression of α-SMA and PDGF was decreased by treatment with sorafenib and PZQ in S. japonicum-infected mice (Fig. 5).
Sorafenib plus PZQ treatment decreased the hepatic expression of fibrogenic proteins in S. japonicum-infected mice. Mice were infected with S. japonicum and treated with PBS, PZQ, and sorafenib as described above. The expression of fibrogenic proteins α-SMA (a), PDGF (b), and PDGFR-β (c) were detected by IHC staining. The optical densities (ODs) of the target proteins (d–f) were measured with Image-Pro Plus 6.0 according to the manufacturer’s protocol. The original magnification is ×200. Asterisks mark significant differences between different groups (*p < 0.05, **p < 0.01, not significant (n.s.) indicates p > 0.05)
Sorafenib plus PZQ treatment decreased the hepatic expression of α-SMA and PDGF in S. japonicum-infected mice. Mice were infected with S. japonicum and treated with PBS, PZQ, and sorafenib as described above. The expression of pro-fibrogenic proteins α-SMA (a) and PDGF (b) was detected by western blot. Images were analyzed with Quantity One. The expression levels of the target proteins were defined as the ratio of target protein OD to β-actin OD. Asterisks mark significant differences between different groups (*p < 0.05, **p < 0.01, not significant (n.s.) indicates p > 0.05)
Discussion
In the present study, we investigated the antifibrotic effects of sorafenib in a natural liver fibrosis model by infecting mice with the parasite S. japonicum. The results show that sorafenib treatment alone without anti-parasite treatment had little effect on hepatic collagen deposition and the expression of profibrotic proteins, such as α-SMA, PDGF, and PDGFR-β. However, administration of the anti-parasite drug PZQ led to reductions in egg granuloma formation and liver fibrosis as shown by HE and Masson’s trichome staining. The expression levels of α-SMA, PDGF, and PDGFR-β were also reduced by PZQ treatment. Interestingly, after anti-parasite therapy, sorafenib treatment can further reduce egg granuloma formation and hepatic collagen deposition and also decrease the hepatic expression of profibrotic proteins.
The mechanisms of hepatic fibrosis induced by S. japonicum infection are unclear. However, activation of HSCs is the key process in liver fibrogenesis. Activated HSCs specifically express cytoskeletal protein α-SMA as the marker of their activation and produce extensive amounts of collagens, mostly types I and III, which deposit in the liver and contribute to liver fibrosis (Xu et al. 2014). The cytokine PDGF is the predominant mitogen for HSCs. The binding of PDGF and its receptor PDGFR-β leads to activation of intracellular signal pathways, such as PI3K/Akt and MAPK/ERK, which promote the activation and proliferation of HSCs (Parsons et al. 2007). In our study, we evaluated liver fibrosis by HE and Masson’s trichome staining and detected the activation and function of HSCs by immunohistochemically staining α-SMA, collagens, PDGF, and PDGFR-β.
The anti-schistosome drug PZQ has been used to treat human schistosomiasis for more than 30 years. In the S. japonicum-infected mice model, PZQ efficiently killed adult worms and alleviated schistosome egg accumulation in the liver (Zhu et al. 2015). In our study, we also found that PZQ treatment led to fewer egg granulomas and reduced collagen deposition in the liver as shown by HE and Masson’s trichome staining. In addition, PZQ exhibited antifibrotic effects by reducing collagen deposition and the expression levels of α-SMA, PDGF, and PDGFR-β in the liver, which is consistent with a recent study showing that PZQ inhibited the expression of α-SMA, TGF-β, MMP9, TIMP1, IL-4, IL-10, IL-13, and IFN-γ in the liver (Liang et al. 2011). Elimination of adult schistosome worms from mesenteric venules may contribute to the antifibrotic effects of PZQ. Adult worms produce numerous schistosome eggs, which reflux to the liver and mainly stimulate T cells to secret cytokines, such as IL-4, IL-10, IL-13, and IFN-γ. These cytokines lead to activation of HSCs and infiltration of macrophages and eosinophils in the liver (Colley and Secor 2014; Secor 2005). The activated macrophages and eosinophils surround the schistosome eggs and promote the formation of granulomas, reflecting a typical hepatopathological change and an important process during S. japonicum-induced liver fibrosis (Colley and Secor 2014).
Sorafenib, an inhibitor of multiple tyrosine kinase and the vascular endothelial growth factor receptor, has been shown to inhibit liver fibrosis in different rat and mouse models (Mejias et al. 2009; Su et al. 2015). Sorafenib inhibits liver fibrosis mainly by modulating the activation, proliferation, and apoptosis of HSCs. Sorafenib can directly inhibit the signal transduction of PDGF, which is the predominant mitogen for HSCs (Mejias et al. 2009). Furthermore, sorafenib reduced the proliferation but enhanced the apoptosis of HSCs by downregulating Cyclin D1 and Cyclin-dependent kinase 4 (Cdk-4) while increasing the expression of Caspase-3 and Bax (Wang et al. 2010). A recent study suggested that sorafenib and its derivative SC-1 exhibit antifibrotic effects by inhibiting the signal transducer and activator of transcription 3 (STAT3), which promotes HSC proliferation. Importantly, overexpression of phopho-STAT3 was found in chronic hepatitis B patients with advanced liver fibrosis and was positively correlated with the severity of liver fibrosis and the plasma IL-6 level (Su et al. 2015). In contrast, in our study, we found that sorafenib administration alone for 2 weeks did not result in an antifibrotic effect in our model. However, sorafenib treatment after eliminating worms using PZQ can further alleviate hepatic inflammation and decrease hepatic fibrosis, indicating that the persistence of parasites in the mesenteric venules blocked the antifibrotic effects of sorafenib. This finding emphasizes the importance of eliminating pathogens to achieve efficient antifibrotic therapy.
Several studies have also reported that two other tyrosine kinase inhibitors, imatinib and genistein, exerted antifibrotic effects and improved liver fibrosis induced by infection of mice with Schistosoma mansoni. Interestingly, unlike sorafenib, imatinib alone can directly inhibit liver fibrosis in the absence of PZQ treatment (El-Agamy et al. 2011). The direct anti-parasite effect of imatinib against S. mansoni may contribute to the prominent antifibrotic efficacy in this model (Beckmann and Grevelding 2010; Buro et al. 2014). Imatinib was found to inhibit the expression of fibrogenic genes, such as collagen I, α-SMA, and PDGFR-β in HSCs (Kim et al. 2012). In our study, sorafenib was also shown to inhibit the hepatic expression of these genes, which may indicate that the same mechanism is utilized by these two tyrosine kinase inhibitors. Two recent studies demonstrated that genistein significantly reduced the formation of egg granulomas and hepatic collagen deposition in mice infected with S. japonicum (Wan et al. 2017) and S. mansoni (Sobhy et al. 2018). In these studies, genistein treatment alone resulted in a reduced area of fibrosis; however, when combined with PZQ, genistein showed a stronger antifibrotic effect compared to that observed in the controls or with genistein administration alone. Further analysis revealed that genistein inhibited NF-κB signaling and subsequently decreased the expression of MCP-1, TNF-α, and IL-10 (Wan et al. 2017). Together with these studies, our study implied that the tyrosine kinase inhibitors may serve as antifibrotic agents against schistosomiasis.
In conclusion, the combination of sorafenib and PZQ reduced hepatic pathological damage and liver fibrosis in mice infected with S. japonicum. This study may provide a new strategy to treat hepatic fibrosis attributed to schistosomiasis, although further studies are needed to elucidate the mechanisms and side effects of sorafenib.
Abbreviations
- S. japonicum :
-
Schistosoma japonicum
- HE:
-
hematoxylin and eosin
- α-SMA:
-
alpha-smooth muscle actin
- PDGF:
-
platelet-derived growth factor
- PDGFR-β:
-
PDGF receptor-beta
- HCC:
-
hepatocellular carcinoma
- ECM:
-
extracellular matrix
- HSCs:
-
hepatic stellate cells
- TGF-β:
-
transforming growth factor beta
- PZQ:
-
praziquantel
- ALT:
-
alanine aminotransferase
- ALB:
-
albumin
- Schistosoma mansoni :
-
S. mansoni
References
Andrade ZA (2009) Schistosomiasis and liver fibrosis. Parasite Immunol 31:656–663. https://doi.org/10.1111/j.1365-3024.2009.01157.x
Bataller R, Brenner DA (2005) Liver fibrosis. J Clin Invest 115:209–218. https://doi.org/10.1172/JCI24282
Beckmann S, Grevelding CG (2010) Imatinib has a fatal impact on morphology, pairing stability and survival of adult Schistosoma mansoni in vitro. Int J Parasitol 40:521–526. https://doi.org/10.1016/j.ijpara.2010.01.007
Borkham-Kamphorst E, Herrmann J, Stoll D, Treptau J, Gressner AM, Weiskirchen R (2004) Dominant-negative soluble PDGF-beta receptor inhibits hepatic stellate cell activation and attenuates liver fibrosis. Lab Investig 84:766–777. https://doi.org/10.1038/labinvest.3700094
Buro C, Beckmann S, Oliveira KC, Dissous C, Cailliau K, Marhöfer RJ, Selzer PM, Verjovski-Almeida S, Grevelding CG (2014) Imatinib treatment causes substantial transcriptional changes in adult Schistosoma mansoni in vitro exhibiting pleiotropic effects. PLoS Negl Trop Dis 8:e2923. https://doi.org/10.1371/journal.pntd.0002923
Chen BL, Peng J, Li QF, Yang M, Wang Y, Chen W (2013) Exogenous bone morphogenetic protein-7 reduces hepatic fibrosis in Schistosoma japonicum-infected mice via transforming growth factor-beta/Smad signaling. World J Gastroenterol 19:1405–1415. https://doi.org/10.3748/wjg.v19.i9.1405
Cioli D, Pica-Mattoccia L, Basso A, Guidi A (2014) Schistosomiasis control: praziquantel forever? Mol Biochem Parasitol 195:23–29. https://doi.org/10.1016/j.molbiopara.2014.06.002
Colley DG, Secor WE (2014) Immunology of human schistosomiasis. Parasite Immunol 36:347–357. https://doi.org/10.1111/pim.12087
Colley DG, Bustinduy AL, Secor WE, King CH (2014) Human schistosomiasis. Lancet 383:2253–2264. https://doi.org/10.1016/S0140-6736(13)61949-2
Deng YR et al (2013) STAT3-mediated attenuation of CCl4-induced mouse liver fibrosis by the protein kinase inhibitor sorafenib. J Autoimmun 46:25–34. https://doi.org/10.1016/j.jaut.2013.07.008
Doenhoff MJ, Cioli D, Utzinger J (2008) Praziquantel: mechanisms of action, resistance and new derivatives for schistosomiasis. Curr Opin Infect Dis 21:659–667. https://doi.org/10.1097/QCO.0b013e328318978f
El-Agamy DS, Shebl AM, Said SA (2011) Prevention and treatment of Schistosoma mansoni-induced liver fibrosis in mice. Inflammopharmacology 19:307–316. https://doi.org/10.1007/s10787-011-0092-6
George J, Roulot D, Koteliansky VE, Bissell DM (1999) In vivo inhibition of rat stellate cell activation by soluble transforming growth factor beta type II receptor: a potential new therapy for hepatic fibrosis. Proc Natl Acad Sci U S A 96:12719–12724
Guan YS, He Q (2011) Sorafenib: activity and clinical application in patients with hepatocellular carcinoma. Expert Opin Pharmacother 12:303–313. https://doi.org/10.1517/14656566.2011.546346
Hennenberg M et al (2011) Hepatic and HSC-specific sorafenib effects in rats with established secondary biliary cirrhosis. Lab Investig 91:241–251. https://doi.org/10.1038/labinvest.2010.148
Hsu CH, Shen YC, Shao YY, Hsu C, Cheng AL (2014) Sorafenib in advanced hepatocellular carcinoma: current status and future perspectives. J Hepatocell Carcinoma 1:85–99. https://doi.org/10.2147/JHC.S45040
Kim Y, Fiel MI, Albanis E, Chou HI, Zhang W, Khitrov G, Friedman SL (2012) Anti-fibrotic activity and enhanced interleukin-6 production by hepatic stellate cells in response to imatinib mesylate. Liver Int 32:1008–1017. https://doi.org/10.1111/j.1478-3231.2012.02806.x
Liang YJ, Luo J, Yuan Q, Zheng D, Liu YP, Shi L, Zhou Y, Chen AL, Ren YY, Sun KY, Sun Y, Wang Y, Zhang ZS (2011) New insight into the antifibrotic effects of praziquantel on mice in infection with Schistosoma japonicum. PLoS One 6:e20247. https://doi.org/10.1371/journal.pone.0020247
Liu C, Yang Z, Wang L, Lu Y, Tang B, Miao H, Xu Q, Chen X (2015) Combination of sorafenib and gadolinium chloride (GdCl3) attenuates dimethylnitrosamine(DMN)-induced liver fibrosis in rats. BMC Gastroenterol 15:159. https://doi.org/10.1186/s12876-015-0380-5
Mejias M, Garcia-Pras E, Tiani C, Miquel R, Bosch J, Fernandez M (2009) Beneficial effects of sorafenib on splanchnic, intrahepatic, and portocollateral circulations in portal hypertensive and cirrhotic rats. Hepatology 49:1245–1256. https://doi.org/10.1002/hep.22758
Moreira RK (2007) Hepatic stellate cells and liver fibrosis. Arch Pathol Lab Med 131:1728–1734. https://doi.org/10.1043/1543-2165(2007)131[1728:HSCALF]2.0.CO;2
Parsons CJ, Takashima M, Rippe RA (2007) Molecular mechanisms of hepatic fibrogenesis. J Gastroenterol Hepatol 22(Suppl 1):S79–S84. https://doi.org/10.1111/j.1440-1746.2006.04659.x
Sato M, Suzuki S, Senoo H (2003) Hepatic stellate cells: unique characteristics in cell biology and phenotype. Cell Struct Funct 28:105–112
Secor WE (2005) Immunology of human schistosomiasis: off the beaten path. Parasite Immunol 27:309–316. https://doi.org/10.1111/j.1365-3024.2005.00778.x
Sobhy MMK, Mahmoud SS, El-Sayed SH, Rizk EMA, Raafat A, Negm MSI (2018) Impact of treatment with a Protein Tyrosine Kinase Inhibitor (Genistein) on acute and chronic experimental Schistosoma mansoni infection. Exp Parasitol 185:115–123. https://doi.org/10.1016/j.exppara.2018.01.013
Su TH et al (2015) Sorafenib and its derivative SC-1 exhibit antifibrotic effects through signal transducer and activator of transcription 3 inhibition. Proc Natl Acad Sci U S A 112:7243–7248. https://doi.org/10.1073/pnas.1507499112
Thabut D, Routray C, Lomberk G, Shergill U, Glaser K, Huebert R, Patel L, Masyuk T, Blechacz B, Vercnocke A, Ritman E, Ehman R, Urrutia R, Shah V (2011) Complementary vascular and matrix regulatory pathways underlie the beneficial mechanism of action of sorafenib in liver fibrosis. Hepatology 54:573–585. https://doi.org/10.1002/hep.24427
Wan C, Jin F, Du Y, Yang K, Yao L, Mei Z, Huang W (2017) Genistein improves schistosomiasis liver granuloma and fibrosis via dampening NF-kB signaling in mice. Parasitol Res 116:1165–1174. https://doi.org/10.1007/s00436-017-5392-3
Wang Y, Gao J, Zhang D, Zhang J, Ma J, Jiang H (2010) New insights into the antifibrotic effects of sorafenib on hepatic stellate cells and liver fibrosis. J Hepatol 53:132–144. https://doi.org/10.1016/j.jhep.2010.02.027
Xu J, Liu X, Koyama Y, Wang P, Lan T, Kim IG, Kim IH, Ma HY, Kisseleva T (2014) The types of hepatic myofibroblasts contributing to liver fibrosis of different etiologies. Front Pharmacol 5:167. https://doi.org/10.3389/fphar.2014.00167
Yoshiji H, Kuriyama S, Noguchi R, Ikenaka Y, Yoshii J, Yanase K, Namisaki T, Kitade M, Yamazaki M, Asada K, Akahane T, Tsujimoto T, Uemura M, Fukui H (2006) Amelioration of liver fibrogenesis by dual inhibition of PDGF and TGF-beta with a combination of imatinib mesylate and ACE inhibitor in rats. Int J Mol Med 17:899–904
Zhou XN et al (2005) The public health significance and control of schistosomiasis in China—then and now. Acta Trop 96:97–105. https://doi.org/10.1016/j.actatropica.2005.07.005
Zhu D, Song K, Chen J, Wang J, Sun X, Qian H, Gu X, Zhang L, Qin Y, Duan Y (2015) Expression of Septin4 in Schistosoma japonicum-infected mouse livers after praziquantel treatment. Parasit Vectors 8:19. https://doi.org/10.1186/s13071-015-0640-9
Acknowledgements
We thank Dr. Yuanyuan Cao from State Key Laboratory of Virology, Modern Virology Research Center, College of Life Sciences, Wuhan University for excellent technical assistance.
Funding
This work was supported by grants from the National Science Foundation, China (81401663) and grants from Health and Family Planning Commission of HuBei Province, China (XF2012-22).
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: YX, ZYM, and XL. Performed the experiments: ZYM, XL, HFD, and LXW. Analyzed the data: ZYM, YX, XL, HFD, YC, and DX. Contributed reagents/materials/analysis tools: ZYM, YX, XL, HFD, and DX. Wrote the paper: ZYM, XL, YC, and YX. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All animals were maintained according to the guidelines of the animal facility at the Hubei Provincial Academy of Preventive Medicine. All animal experiments were conducted in accordance with the Guide for the Care and Use of Laboratory and approved by the Committee on the Ethics of Animal Experiments of Hubei Provincial Academy of Preventive Medicine (project number M2014001).
Consent for publication
Not applicable.
Availability of the data and materials
The datasets supporting the conclusions of this article are included within the article.
Competing interests
The authors declare that they have no competing interests.
Additional information
Section Editor: Christoph G. Grevelding
Rights and permissions
About this article
Cite this article
Ma, Z., Liu, X., Dong, H. et al. Sorafenib and praziquantel synergistically attenuate Schistosoma japonicum-induced liver fibrosis in mice. Parasitol Res 117, 2831–2839 (2018). https://doi.org/10.1007/s00436-018-5972-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-018-5972-x