Abstract
Ascaris lumbricoides is the largest roundworm known from the human intestine while Ascaris suum is an internal parasite of pigs. Ascariasis, caused by Ascaris lumbricoides, has a worldwide distribution. Here, we have provided the first molecular identification of Ascaris eggs and adults recovered from humans and pigs in Thailand, Lao PDR, and Myanmar. We amplified and sequenced nuclear ribosomal DNA (ITS1 and ITS2 regions) and mitochondrial DNA (cox1 gene). Sequence chromatograms of PCR-amplified ITS1 region revealed a probable hybrid genotype from two human ascariasis cases from Chiang Mai Province, northern Thailand. All complete ITS2 sequences were identical and did not differ between the species. Phylogenetic trees and haplotype analysis of cox1 sequences showed three clusters with 99 haplotypes. Forty-seven samples from the present study represented 14 haplotypes, including 7 new haplotypes. To our knowledge, this is the first molecular confirmation of Ascaris species in Thailand, Lao PDR, and Myanmar. Zoonotic cross-transmission of Ascaris roundworm between pigs and humans probably occurs in these countries.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ascaris lumbricoides is the largest nematode (roundworm) parasitizing the human intestine and it is one of the principal soil-transmitted helminthic human infections worldwide, infecting an estimated 819 million people (Pullan et al. 2014; Holland 2013). Ascariasis has been considered as a neglected tropical disease by WHO and is highly prevalent in poor urban and rural areas (Dold and Holland 2010). Ascaris suum is an intestinal roundworm of pigs but can cause human ascariasis (Arizono et al. 2010). The two species are similar morphologically and closely related. Indeed, there is debate as to whether they are distinct species (Leles et al. 2012; Shao et al. 2014). Ascaris-infected pigs have been reported as a reservoir host for humans in both endemic and non-endemic areas such as in China (Zhou et al. 2012; Cavallero et al. 2013; Dunn et al. 2016). Ascaris lumbricoides (identified using mitochondrial DNA sequences) has been found in chimpanzees and gibbons in a zoological garden in southwest China (Xie et al. 2013). There have also been reports of the pig Ascaris from chimpanzees (Nejsum et al. 2006, 2010). However, cross-transmission of A. lumbricoides and A. suum between humans and pigs is still debated (Nejsum et al. 2010, 2017a). A pig farmer in northwest Italy was found to be infected with hybrid Ascaris suum/lumbricoides. This was determined using PCR-RFLP analysis of the ribosomal nuclear ITS regions (Dutto and Petrosillo 2013). Hybrid genotypes were also found in 32% (30/93) of Ascaris adult worms obtained from pigs in the USA (Jesudoss Chelladurai et al. 2017). Experimental cross-transmission of Ascaris species between humans and pigs is known (Betson et al. 2014). This implies that control of these parasites requires attention to pig infections as well as human infections.
Several molecular epidemiological investigations, using polymorphic markers (internal transcribed spacer 1; ITS1, mitochondrial cytochrome C oxidase subunit 1; cox1, NADH dehydrogenase subunit 1; nad1 and microsatellite markers), have explored the specificity of the two Ascaris species for their respective hosts and their taxonomic status (Cavallero et al. 2013; Iñiguez et al. 2012; Leles et al. 2009). Furthermore, knowledge on transmission dynamics and hybridization of Ascaris species has been gained by DNA sequencing, polymerase chain reaction restriction fragment length polymorphism (PCR-RFLP), and microsatellite marker techniques (Arizono et al. 2010; Cavallero et al. 2013; Jesudoss Chelladurai et al. 2017; Betson et al. 2014).
In Thailand, Lao PDR, and Myanmar, adjacent countries in Southeast Asia, this soil-transmitted helminth is common and poses a public-health problem (Dunn et al. 2016; Laymanivong et al. 2014; Jex et al. 2011; Hlaing et al. 1984). However, molecular confirmation of the identities of Ascaris spp. in these areas is still lacking. To fill this gap in our knowledge, we sequenced partial ITS1, complete ITS2 (these are the internal transcribed spacers of the nuclear ribosomal gene repeat), and partial mitochondrial cox1 sequences from Ascaris samples from these countries and prepared a phylogenetic tree and haplotype network of cox1 sequences. Comparisons with sequences in public databases provide a better understanding of the genetic diversity and identities of Ascaris populations in Thailand, Lao PDR, and Myanmar.
Materials and methods
Ascaris samples and ethics approvals
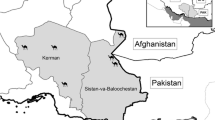
The study used mainly pre-existing material recovered from diagnostic specimens acquired during routine laboratory investigations, and from fecal specimens from patients who had visited the hospital for treatment: documented informed consent was not required by the ethics committee. Thirty-eight human fecal samples containing Ascaris eggs were received from Thailand (n = 18), Lao PDR (n = 8), and Myanmar (n = 12). An additional five (human = 2, pigs = 3) and four (pigs = 4) Ascaris adult worms were collected from Thailand and Lao PDR, respectively (Fig. 1 and Table 1). Samples were kept in 70% alcohol and stored at − 20 °C until used. This study was approved by the Human Ethics Committee of Khon Kaen University (Reference no. HE591403). Patient anonymity was maintained. Specimens of Ascaris adult worms from pigs obtained from local slaughterhouses do not correspond to the criteria for observational and field studies, and therefore, a separate ethics clearance was not required for these. No vertebrate specimens were used.
Map showing collection sites of Ascaris samples from Thailand, Lao PDR, and Myanmar. This map was modified from a map in The World Factbook, published by the Central Intelligence Agency (Central Intelligence Agency [US] (2017)
DNA extraction
Genomic DNA was extracted from a portion of tissue from each adult worm. The tissue was homogenized and then processed using a NucleoSpin® tissue kit (Macherey-Nagel GmbH & Co., Düren, Germany) according to the manufacturer’s protocol. Ascaris eggs were collected from fecal sediment after processing with a Mini Parasep® SF fecal parasite concentrator (Apacor, Wokingham, England). Ascaris eggs (n = 30 per sample) were homogenized and DNA extracted using a QIAamp® DNA stool mini kit (Qiagen, Hilden, Germany) according to the manufacturer’s protocol. Genomic DNA was eluted in 50 μl of elution buffer and stored at − 20 °C until used.
Polymerase chain reaction amplification and DNA sequencing
Three specific primer sets were used to amplify ITS1 (Ishiwata et al. 2004), ITS2 (newly designed), and cox1 (Peng et al. 2005) regions (Table 2). Optimum conditions for amplification with each set are summarized in Table 2. The PCR was done using a Gene Amp® PCR System 9700 (Applied Biosystems (ABI), Singapore). Each reaction contained 2.5 μl of 10× high fidelity PCR buffer, 2.0 μl of MgCl2 (25 mM), 0.5 μl of each deoxyribonucleotide triphosphate (dNTP) mixed (10 mM), 0.5 μl of each primer (10 μM) (Sigma-Aldrich, St. Louis, MO), 0.125 units of Taq high-fidelity PCR system (Roche Applied Science, Mannheim, Germany), and 2 μl of DNA template. Deionized water was added to a final volume of 25 μl. PCR products (2 μl) were separated by electrophoresis using 1% agarose gels, stained with ethidium bromide, and the fragment sizes determined by comparison with a 100 bp DNA ladder (Promega™). DNA sequencing was done by First BASE Laboratories Sdn Bhd (Selangor, Malaysia) using the BigDye terminator v3.1 cycle-sequencing kit (ABI). PCR primers were used as sequencing primers and PCR products were sequenced in both directions.
Sequence alignment, network, and phylogenetic tree analyses
Ascaris nucleotide sequences were aligned using the multiple sequence alignment program ClustalW (Thompson et al. 1994) available within BioEdit (http://www.mbio.ncsu.edu/bioedit/bioedit.html) (Hall 1999). Sequence comparisons of the ITS1 region were used for differentiation of A. lumbricoides and A. suum (Zhu et al. 1999). The complete ITS2 nucleotide sequences (272 bp) were compared with those of other Ascaris spp. from the GenBank database. The cox1 sequence dataset included 47 sequences generated in this study and 156 from the GenBank database. Phylogenetic tree reconstruction of the cox1 alignment was done using Bayesian inference in the program MrBayes v3.2 (Ronquist et al. 2012). The substitution models for cox1 dataset were chosen using the Bayesian Information Criterion (BIC) in MEGA7 software: the lowest BIC score is considered to best describe the substitution pattern (Kumar et al. 2016). For the cox1 sequence alignment, the Hasegawa-Kishino-Yano (HKY) model with non-uniformity of evolutionary rates among sites (+G) was selected. Analysis of the cox1 alignment was run for three million generations (2 runs, each of four chains), by which time the standard deviation of split frequencies had fallen below 0.01. Trees were sampled every 1000 generations. Maximum-likelihood (ML) and neighbor-joining (NJ) methods were implemented in MEGA7 (Kumar et al. 2016). The HKY + G model was used for the ML method, while the Tamura-Nei (TN93 + G) model was used for the NJ method (Tamura and Nei 1993). Branch support was provided for ML and NJ trees by bootstrapping with 1000 replications (Kumar et al. 2016). Anisakis simplex (JN786328) and Toxocara vitulorum (FJ664617) from GenBank database were included in the analysis to serve as out-groups. A median-joining network was based on cox1 gene sequences of Ascaris using an alignment trimmed to the shortest sequence (327 bp) available in the public database (Supplementary Table S1). Haplotypes were identified in DnaSP version 5.10 (Librado and Rozas 2009). DnaSP was used to prepare an input file for Network software version 5.0.0.1 (Fluxus Technology Ltd., www.fluxus-engineering.com) (Bandelt et al. 1999). The latter was used to generate a median-joining haplotype network.
Results
ITS1 sequence analysis
Partial sequences of the ribosomal ITS1 region (~ 452 bp) were used to distinguish between A. lumbricoides and A. suum in Thailand, Lao PDR, and Myanmar. The two species differ at six positions in this spacer region (Table 3). Differences at positions 133 and 248 were of most use to distinguish between the species (Fig. 2 and Table 3). All samples from pigs were identified as A. suum on this basis. Similarly, almost all human samples were A. lumbricoides. However, two human-derived Ascaris samples from Chiang Mai Province, Thailand, showed double peaks in chromatograms at positions 133 (Fig. 2a and Table 3) and 248 (Fig. 2b and Table 3), indicating probable hybrids between A. suum and A. lumbricoides. Representative ITS1 sequences (n = 16) have been submitted to the GenBank database (Table 3).
Sequence chromatograms of PCR-amplified ITS1 region from two cases (H 20N-THA (accession no. MF358960) and H 50N-THA) of ascariasis from the northern part of Thailand. Probable hybrid genotypes of Ascaris species are indicated by double peaks (boxed), with C or G and A or T at positions 133 (a) and 248 (b), respectively. H, human; N, northern, THA, Thailand; H 20N-THA, collected from human No. 20 from northern Thailand and H 50N-THA, collected from human No. 50 from northern Thailand
Analyses of ITS2 regions
Our newly designed ITS2 primer pair (Table 2) amplified a 550 bp product encompassing the 5.8 s, ITS2 and 28 s rDNA gene regions. The complete ITS2 sequences are 272 bp in length. This region is not informative for differentiation between the two species. Representative ITS2 sequences have been submitted to the GenBank database (GenBank accession nos. MF358936, MF358939, MF358942, MF358946, MF358953-MF358958, MF358961, and MF358962).
Phylogenetic tree and network analysis of the cox1 region
A 431 bp PCR product was obtained from all 47 samples sequenced as part of this study. The alignment used was trimmed to 327 bp (the length of the shortest sequence) and contained our new sequences and 156 representative sequences from GenBank (Supplementary Table S1). Representative cox1 sequences (n = 32) from Thailand, Lao PDR, and Myanmar have been submitted to the GenBank database (GenBank accession nos. MF358904 - MF358935). The median-joining network (Fig. 3) shows three clusters (A, B, and C) corresponding very closely to those in the phylogenetic tree (figure not shown). Ninety-nine haplotypes were recognized in this analysis (haplotype diversity 0.9599) (Fig. 3 and Supplementary Table S1). More Ascaris samples fell into cluster A than into clusters B and C. Our new sequences represented 14 haplotypes, seven of which have been previously reported (H27, H29, H44, H47, H55, H58, and H77), and seven of which are new (H93–99). Our new haplotype 97, from Luang Prabang, Lao PDR, was grouped in cluster B, a cluster in which Ascaris spp. from natural hosts (chimpanzee and gibbon) are also placed. The remaining six new haplotypes were in cluster A.
Median-joining network of cox1 sequences of Ascaris spp. Ascaris cox1 sequences from this study (n = 47) and GenBank sequences (n = 156) were distributed in three clusters (A, B, and C). Nucleotide differences are indicated by hatch marks across the connecting lines (each hatch mark representing a single nucleotide difference). The seven new haplotypes (H93–H99) are shown in black bold text. Accession numbers of sequences from the present study are shown in Supplementary Table S1
The most common haplotype among our sequences was H27 (25.53%; 12/47). Six of our H27 sequences came from Thailand (and included one sample of hybrid genotype), four from Lao PDR, and two from Myanmar. Seven samples were from humans and five from pigs, consistent with previous reports from the USA, Japan, United Kingdom, Philippines, and Uganda (Supplementary Table S1). Ascaris eggs from humans recovered from Myanmar (n = 5) and Thailand (n = 1) were classified in H29, along with published human sequences (n = 12) from Brazil, Angola, Tanzania, Zambia, Kenya, Bangladesh, and Uganda and pig sequences (n = 5) from USA, Brazil, Uganda, China, and Thailand (from the present study). Haplotype H44 consisted only of sequences from Ascaris recovered from humans (n = 8) from China (n = 1), Thailand (n = 1), Lao PDR (n = 5), and Myanmar (n = 1).
Discussion
Ascaris lumbricoides and A. suum are among the most common soil-transmitted helminths and cause serious health and socioeconomic problems in humans and loss of production in pigs. Evidence of cross-transmission of these species between humans and pigs has been reported (Cavallero et al. 2013), and there is no doubt that the two species share a recent common ancestor (Betson et al. 2013). The host-preference of the common ancestor and the timing and geographical location(s) of the split have been much debated, but the evolutionary histories of human and pig Ascaris are likely to be complex and interwoven (Betson et al. 2013). Molecular epidemiology must be used to distinguish and classify these parasites and to clarify the extent of zoonotic transmission. Despite the importance of ascariasis in Thailand, Lao PDR, and Myanmar, there has been no previous work on molecular species identification. Here, we examined the identities of Ascaris samples collected from humans and pigs based on sequences of three gene regions. Partial ITS1 nucleotide sequences have two positions that can be used to distinguish between A. lumbricoides and A. suum (Fig. 2 and Table 3). Two Ascaris egg samples from humans in northern Thailand yielded ITS1 sequences suggesting that they were from probable hybrid worms. Previous workers have mentioned the same two variable positions and have detected likely hybrids (Leles et al. 2009; Zhu et al. 1999; Peng et al. 2003). Among our samples, 38 from humans corresponded to the G1 genotype (human-associated genotype), two from humans corresponded to the G5 genotype (probable hybrid genotype), and all 7 samples from pigs represented the G3 genotype (pig-associated genotype, Peng et al. 2003). Additional differences between A. suum and A. lumbricoides in the ITS1 region include a tract near the 5′ end in which there is a run of up to 13 consecutive “A”s. In human-associated Ascaris, we found the number of “A”s to always be 10, as also noted by Zhu et al. (1999). In pig-associated worms, the number varies from 11 to 13 but Zhu et al. (1999) showed 12 consecutive “A”s. We prefer to place no importance on this region in attempting to discriminate between A. suum and A. lumbricoides. Two of these “A”s correspond to positions 20 and 21 in Table 2 of Peng et al. (2003). The question arises as to whether hybrids between the two Ascaris species are fertile. If they are as fertile and fecund as the two parent species, gene flow between the species might simply cause them to merge into a single gene pool. Søe et al. (2016) have argued that the gene pools remain largely distinct and that hybrids likely have low, or no, fertility (Søe et al. 2016). In such cases, hybrids would not transmit genes to future generations and would be of little epidemiological significance. On the other hand, occasional fertile hybrids might lead to introgression between the gene pools, with possible outcomes as discussed by Charles and Criscione (2013).
ITS1 sequence data from Ascaris worms from pigs in Iowa, USA, indicated that transmission had occurred from humans to pigs (Jesudoss Chelladurai et al. 2017). Some hybrid Ascaris have been found in humans from China, Guatemala, Europe, and Nepal, and among worms from pigs in China, Europe, and Guatemala (Zhou et al. 2012; Betson et al. 2014). Complete ITS2 nucleotide sequences of Ascaris samples from our study showed 100% similarity with A. lumbricoides from humans (e.g., GenBank no. AB571301) or A. suum from pigs (e.g., GenBank no. AB571302).
The phylogenetic tree constructed from cox1 gene sequences showed three clusters (A, B, and C) that were the same as those depicted in the median-joining network (Fig. 3). Many haplotypes in these clusters have a wide distribution, suggesting frequent movement of Ascaris species around the world. This could be via infected people, or possibly pigs. Interestingly, seven new haplotypes were found, suggesting that further genetic diversity remains to be discovered in Ascaris. One new haplotype, from Lao PDR (haplotype 97), was grouped in cluster B with Ascaris spp. from chimpanzee and gibbon. The remaining six of our new haplotypes were in cluster A with Ascaris spp. from human in Thailand and Lao PDR. This new diversity is another piece of the evolutionary jigsaw of Ascaris: such population markers are informative in monitoring worm dynamics during ongoing control efforts (Betson et al. 2011; Sparks et al. 2015). Cluster C was regarded by Nejsum et al. (2017b) as an early-diverging mitochondrial lineage, which has only been identified in pigs and humans from Slovakia, United Kingdom, Denmark, Italy, Uganda, and Tanzania. Probable hybrid Ascaris from Thailand (cox1 haplotypes H27 and H58) as well as many Ascaris samples from Thailand, Lao PDR, and Myanmar fell into cox1 cluster A. In the USA, 32% of Ascaris hybrids were in cluster A (Jesudoss Chelladurai et al. 2017). Moreover, Ascaris spp. from humans and pigs in Brazil shared common haplotypes in two widely separated geographical regions (Iñiguez et al. 2012). Cox1 sequences from these probable hybrids exhibited haplotypes 27 and 58 (Fig. 3 and Supplemental Table S1), haplotypes that are known to occur in samples from pigs and from humans. The taxonomic status of both species remains contentious: do A. lumbricoides and A. suum constitute two species or one (Leles et al. 2012; Betson and Stothard 2016; da Silva Alves et al. 2016)?
On a cautionary note, we must point out that the present study used a pool of Ascaris eggs from each host for DNA extraction and sequencing. So, it is possible that eggs may have come from different female worms, perhaps representing both pure A. lumbricoides and A. suum. This possibility needs to be tested empirically in the future.
Conclusions
To our knowledge, our study is the first molecular identification at the species level of Ascaris in Thailand, Lao PDR, and Myanmar. Also, this is the first report of probable hybrids between A. suum and A. lumbricoides in the region. Thus, zoonotic cross-transmission between humans and pigs, as revealed in Chiang Mai Province, northern Thailand, may occur. This is particularly likely at pig farms, where people are in frequent contact with pigs and use their manure as fertilizer. Farmers in this part of Thailand need to be made aware of hygiene precautions when handling pigs or their manure.
References
Arizono N, Yoshimura Y, Tohzaka N, Yamada M, Tegoshi T, Onishi K, Uchikawa R (2010) Ascariasis in Japan: is pig-derived Ascaris infecting humans? Jpn J Infect Dis 63:447–448
Bandelt HJ, Forster P, Röhl A (1999) Median-joining networks for inferring intraspecific phylogenies. Mol Biol Evol 16:37–48
Betson M, Stothard JR (2016) Ascaris lumbricoides or Ascaris suum: What’s in a name? J Infect Dis 213:1355–1356
Betson M, Halstead FD, Nejsum P, Imison E, Khamis IS, Sousa-Figueiredo JC, Rollinson D, Stothard JR (2011) A molecular epidemiological investigation of Ascaris on Unguja, Zanzibar using isoenyzme analysis, DNA barcoding and microsatellite DNA profiling. Trans R Soc Trop Med Hyg 105:370–379
Betson M, Nejsum P, Stothard JR (2013) From the twig tips to the deeper branches: new insights into evolutionary history and phylogeography of Ascaris. In: Holland C (ed) Ascaris: the neglected parasite. Elsevier Inc., Amsterdam, pp 265–285
Betson M, Nejsum P, Bendall RP, Deb RM, Stothard JR (2014) Molecular epidemiology of ascariasis: a global perspective on the transmission dynamics of Ascaris in people and pigs. J Infect Dis 210:932–941
Cavallero S, Snabel V, Pacella F, Perrone V, D’Amelio S (2013) Phylogeographical studies of Ascaris spp. based on ribosomal and mitochondrial DNA sequences. PLoS Negl Trop Dis 7:e2170. https://doi.org/10.1371/journal.pntd.0002170
Central Intelligence Agency [US] (2017). The World Factbook. https://www.cia.gov/library/publications/theworld-factbook/geos/bm.html. Accesed 28 May 2017
Charles D, Criscione (2013) Genetic Epidemiology of Ascaris: Cross-transmission between Humans and Pigs, Focal Transmission, and Effective Population Size. In: Holland C (ed) Ascaris: The neglected parasite. Elsevier Inc, Amsterdam, pp 203–226
da Silva Alves EB, Conceição MJ, Leles D (2016) Ascaris lumbricoides, Ascaris suum, or "Ascaris lumbrisuum". J Infect Dis 213:1355. https://doi.org/10.1093/infdis/jiw027
Dold C, Holland CV (2010) Ascaris and ascariasis. Microbes Infect 13:632–637
Dunn JC, Turner HC, Tun A, Anderson RM (2016) Epidemiological surveys of, and research on, soil-transmitted helminths in Southeast Asia: a systematic review. Parasit Vectors 9:31. https://doi.org/10.1186/s13071-016-1310-2
Dutto M, Petrosillo N (2013) Hybrid Ascaris suum/lumbricoides (ascarididae) infestation in a pig farmer: a rare case of zoonotic ascariasis. Cent Eur J Public Health 21:224–226
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for windows 95/98/NT. Nucleic Acids Symp Ser 41:95–98
Hlaing T, Than S, Htay Htay A, Myint L, Thein Maung M (1984) Epidemiology and transmission dynamics of Ascaris lumbricoides in Okpo village, rural Burma. Trans R Soc Trop Med Hyg 78:497–504
Holland C (2013) Ascaris: the neglected parasite. London. In: UK
Iñiguez AM, Leles D, Jaeger LH, Carvalho-Costa FA, Araújo A, Amazonas Research Group (2012) Genetic characterisation and molecular epidemiology of Ascaris spp. from humans and pigs in Brazil. Trans R Soc Trop Med Hyg 106:604–612
Ishiwata K, Shinohara A, Yagi K, Horii Y, Tsuchiya K, Nawa Y (2004) Identification of tissue-embedded ascarid larvae by ribosomal DNA sequencing. Parasitol Res 92:50–52
Jesudoss Chelladurai J, Murphy K, Snobl T, Bader C, West C, Thompson K, Brewer MT (2017) Molecular epidemiology of Ascaris infection among pigs in Iowa. J Infect Dis 215:131–138
Jex AR, Lim YA, Bethony JM, Hotez PJ, Young ND, Gasser RB (2011) Soil-transmitted helminths of humans in Southeast Asia--towards integrated control. Adv Parasitol 74:231–265
Kumar S, Stecher G, Tamura K (2016) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33:1870–1874
Laymanivong S, Hangvanthong B, Keokhamphavanh B, Phommasansak M, Phinmaland B, Sanpool O et al (2014) Current status of human hookworm infections, ascariasis, trichuriasis, Schistosomiasis mekongi and other trematodiases in Lao People’s Democratic Republic. Am J Trop Med Hyg 90:667–669
Leles D, Araújo A, Vicente AC, Iñiguez AM (2009) Molecular diagnosis of ascariasis from human feces and description of a new Ascaris sp. genotype in Brazil. Vet Parasitol 163:167–170
Leles D, Gardner SL, Reinhard K, Iñiguez A, Araujo A (2012) Are Ascaris lumbricoides and Ascaris suum a single species? Parasit Vectors 5:42
Librado P, Rozas J (2009) DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics 25:1451–1452
Nejsum P, Grøndahl C, Murrell KD (2006) Molecular evidence for the infection of zoo chimpanzees by pig Ascaris. Vet Parasitol 139:203–210
Nejsum P, Bertelsen MF, Betson M, Stothard JR, Murrell KD (2010) Molecular evidence for sustained transmission of zoonotic Ascaris suum among zoo chimpanzees (Pan troglodytes). Vet Parasitol 171:273–276
Nejsum P, Betson M, Stothard R (2017a) Analysis of ribosomal DNA cannot unequivocally assign Ascaris to species level or identify hybrids. J Infect Dis 216:616–617. https://doi.org/10.1093/infdis/jix300
Nejsum P, Hawash MB, Betson M, Stothard JR, Gasser RB, Andersen LO (2017b) Ascaris phylogeny based on multiple whole mtDNA genomes. Infect Genet Evol 48:4–9
Peng W, Yuan K, Zhou X, Hu M, Abs EL-Osta YG, Gasser RB (2003) Molecular epidemiological investigation of Ascaris genotypes in China based on single-strand conformation polymorphism analysis of ribosomal DNA. Electrophoresis 24:2308–2315
Peng W, Yuan K, Hu M, Zhou X, Gasser RB (2005) Mutation scanning-coupled analysis of haplotypic variability in mitochondrial DNA regions reveals low gene flow between human and porcine Ascaris in endemic regions of China. Electrophoresis 26:4317–4326
Pullan RL, Smith JL, Jasrasaria R, Brooker SJ (2014) Global numbers of infection and disease burden of soil transmitted helminth infections in 2010. Parasit Vectors 7:37. https://doi.org/10.1186/1756-3305-7-37
Ronquist F, Teslenko M, van der Mark P et al (2012) MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol l61:539–542
Shao C-C, Xu M-J, Alasaad S, Song H-Q, Peng L, Tao J-P, Zhu XQ (2014) Comparative analysis of microRNA profiles between adult Ascaris lumbricoides and Ascaris suum. BMC Vet Res 10:99. https://doi.org/10.1186/1746-6148-10-99
Søe MJ, Kapel CM, Nejsum P (2016) Ascaris from humans and pigs appear to be reproductively isolated species.PLoS Negl Trop Dis;10 (9):e0004855. https://doi.org/10.1371/journal.pntd.0004855
Sparks AM, Betson M, Oviedo G, Sandoval C, Cooper PJ, Stothard JR (2015) Characterization of Ascaris from Ecuador and Zanzibar. J Helminthol 89:512–515
Tamura K, Nei M (1993) Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol Biol Evol 10:512–526
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Xie Y, Niu L, Zhao B et al (2013) Complete mitochondrial genomes of chimpanzee- and gibbon-derived Ascaris isolated from a zoological garden in Southwest China. PLoS One 8(12):e82795. https://doi.org/10.1371/journal.pone.0082795
Zhou C, Li M, Yuan K, Deng S, Peng W (2012) Pig Ascaris: an important source of human ascariasis in China. Infect Genet Evol 12:1172–1177
Zhu X, Chilton NB, Jacobs DE, Boes J, Gasser RB (1999) Characterisation of Ascaris from human and pig hosts by nuclear ribosomal DNA sequences. Int J Parasitol 29:469–478
Acknowledgments
We would like to thank David Blair for valuable suggestions and assistance with the presentation of this paper through Khon Kaen University Publication Clinic. This study was supported by the TRF Senior Research Scholar Grant, Thailand Research Fund grant no. RTA5880001; LS was partially supported by the Faculty of Medicine, Khon Kaen University (IN60209). OS were supported by Scholarship under the Post-Doctoral Training Program from Research Affairs and Graduate School, Khon Kaen University (grant no. 58101).
Funding
This study was supported by a TRF Senior Research Scholar Grant, Thailand Research Fund grant no. RTA5880001; LS was partially supported by the Faculty of Medicine, Khon Kaen University (IN60209). OS were supported by Scholarship under the Post-Doctoral Training Program from Research Affairs and Graduate School, Khon Kaen University (grant no. 58101).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
This study was approved by the Human Ethics Committee of Khon Kaen University (Reference no. HE591403). Patient anonymity was maintained. Specimens of Ascaris adult worms from pigs obtained from local slaughterhouses do not correspond to the criteria for observational and field studies, and therefore, a separate ethics clearance was not required for these. No vertebrate specimens were used.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Section Editor: Xing-Quan Zhu
Electronic supplementary material
ESM 1
(DOCX 44 kb)
Rights and permissions
About this article
Cite this article
Sadaow, L., Sanpool, O., Phosuk, I. et al. Molecular identification of Ascaris lumbricoides and Ascaris suum recovered from humans and pigs in Thailand, Lao PDR, and Myanmar. Parasitol Res 117, 2427–2436 (2018). https://doi.org/10.1007/s00436-018-5931-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-018-5931-6