Abstract
The skin is the first organ to be infected by the parasite in canine visceral leishmaniasis. The enzyme matrix metalloproteinase (MMP) acts towards degradation of the extracellular matrix (ECM) and modulation of the inflammatory response against many kinds of injuries. The aims of this study were to evaluate the expression of MMP-2 and MMP-9 through immunohistochemistry and zymography on the skin (muzzle, ears, and abdomen) of dogs that were naturally infected by Leishmania spp. and to compare these results with immunodetection of the parasite and with alterations to the dermal ECM. Picrosirius red staining was used to differentiate collagen types I and III in three regions of the skin. The parasite load, intensity of inflammation, and production of MMP-2 (latent) and MMP-9 (active and latent) were higher in the ear and muzzle regions. MMP-9 (active) predominated in the infected group of dogs and its production was significantly different to that of the control group. Macrophages, lymphocytes, and plasma cells predominated in the dermal inflammation and formed granulomas in association with degradation of mature collagen (type I) and with discrete deposition of young collagen (type III). This dermal change was more pronounced in dogs with high parasite load in the skin. Therefore, it was concluded that the greater parasite load and intensity of inflammation in the skin led consequently to increased degradation of mature collagen, caused by increased production of MMPs, particularly active MMP-9, in dogs with visceral leishmaniasis. This host response profile possibly favors systemic dissemination of the parasite.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In visceral leishmaniasis (VL), the skin is the first target of the vector (Lutzomyia longipalpis) of Leishmania infantum in dogs and humans (Brasil 2014). In dogs, one of the main findings is skin lesions. However, in dogs without macroscopic cutaneous lesions (asymptomatic dogs), high parasite loads have also been reported (Solano-Gallego et al. 2004; Madeira et al. 2009).
The microscopic lesions observed in the skin of dogs with VL are due to inflammation of the superficial dermis. These lesions are composed of parasitized or non-parasitized macrophages, followed by plasmocytes, lymphocytes, and rare neutrophils (Solano-Gallego et al. 2004; Reis et al. 2009). Likewise, type I collagen reduction and type III collagen increase, due to the deleterious effects of the inflammatory process, have also been described (Giunchetti et al. 2006).
Matrix metalloproteinases (MMPs) are zinc-dependent endopeptidases. They are associated with physiological and with acute and chronic pathological processes. MMPs have importance in relation to dissemination of neoplasia because of their capacity to degrade the extracellular matrix (ECM) (Cawston and Wilson 2006; Kupai et al. 2010).
The gelatinases MMP-2 and MMP-9 degrade the basement membrane and collagen fibers and allow carcinoma cells to migrate to blood vessels (Nagase and Woessner Jr 1999; Gutierrez et al. 2008; Kupai et al. 2010). MMP-2 arises through constitutive expression in healthy tissues and is unresponsive to the majority of stimuli. However, MMP-9 is inducible and is considered to be a systemic inflammation marker in animals (Opdenakker et al. 2001a).
In cases of infection with Leishmania mexicana, loosening or detachment of the connective tissue matrix of the dermis, allowing establishment of infection, has been reported. The parasite has receptors for type I collagen, which facilitates penetration of the parasite in the connective tissue of the dermis, before being phagocytosed by macrophages, thus enabling migration to other organs of the host (Lira et al. 1997). However, the pathogeny of this interaction with the ECM remains unclear and the role of MMPs in this has been little studied.
The aim of the present study was to evaluate MMP-2 and MMP-9 enzyme expression in different areas of canine cutaneous tissue (muzzle, ears, and abdomen), in order to determine which cells produce these gelatinases and to quantify their active and inactive isoforms. These findings were compared with the parasite load and dermal changes in infected dogs.
Material and methods
Animals
Thirty-six dogs infected with Leishmania infantum (infected group) were used, without preference for age, breed, or gender. They were obtained from the Zoonosis Control Center of the municipality of Araçatuba, São Paulo State, Brazil, a region that is endemic for VL. The animals were euthanized using an intravenous (IV) overdose of barbiturate, followed by IV administration of potassium chloride (decree number 51.838 of the Brazilian Ministry of Health and Resolution number 714, of June 20, 2002, of the Federal Veterinary Medicine Council). The necropsy on the dogs was performed immediately after euthanasia.
A control group composed of four dogs was recruited from within routine care at the Department of Veterinary Pathology, Jaboticabal-SP, Brazil, which is in an area that is non-endemic for VL (Oliveira et al. 2008). The infected and control dogs were selected for presence or absence of disease, respectively, by means of the ELISA test (Lima et al. 2005). The dogs of the infected and control groups were further selected through obtaining a negative result for Ehrlichia canis, through cytological analysis on bone marrow.
Histopathological and collagen fiber analysis
For histopathological analysis, skin samples measuring approximately 2 × 2 cm were collected from the muzzle (transition area between the muzzle and the skin), ear (middle third of the auricular pavilion), and abdomen (glabrous ventral abdominal area, next to the linea alba). These are regions in which there is greater probability of contact with the vector insect of VL. These samples were analyzed regardless of the presence of cutaneous lesions (ulcers, scabs, or scaling). The skin fragments were fixed in 10% phosphate-buffered formalin solution (pH 7.2), for 12 h. They were then processed, embedded in paraffin, sectioned at 5 μm, and stained with hematoxylin and eosin. The cutaneous inflammatory response, and its distribution and intensity, was evaluated in the dogs with VL.
The dermal collagen fiber composition was analyzed using skin samples that had been embedded in paraffin and stained with Sirius red dissolved in saturated solution of picric acid. A polarization filter was used, thus allowing differentiation between collagen fiber type I (mature) and type III (neoformed). The proportion of each type of collagen fiber per animal was evaluated. This proportion was determined through scores in three categories: mild (1), moderate (2), and severe (3) collagen formation.
Parasite load and MMP-2 and MMP-9 immunolabeling
The immunohistochemical analysis to determine the parasite load in the three skin areas was performed using the streptavidin-biotin-peroxidase complex (LSAB kit; code K0690, Dako Cytomation, CA, USA).
For immunolabeling of the amastigote forms, hyperimmune serum from a leishmaniasis-positive dog (titer of 1:40,000, by means of the ELISA test) was used at a dilution of 1:1.000 (Moreira et al. 2010, modified from Tafuri et al. 2004). For metalloproteinase immunolabeling, polyclonal antibodies for detection of MMP-2 (ABCAM, code AB79781, dilution 1:250) and MMP-9 (ABCAM, code AB38899, dilution 1:4500) were used. The cell types in the dermal inflammatory infiltrate were identified using the antibodies CD138 (plasma cells, Spring code E4564, dilution 1:25), CD3 (T lymphocytes, Dako code M7254, dilution 1:200), and MCA387 (macrophages, AbD Serotec code batch 1209, dilution 1:3500).
Antigen retrieval was performed in a water bath at 95 °C for 30 min. Peroxidase blocking was performed by using a 12% methanol and hydrogen peroxide solution, for 10 min. To block nonspecific sites, a commercial product was used (Protein Block; code X0909, Dako Cytomation, CA, USA) for 20 min. The chromogen was diaminobenzidine (DAB; code K3468, Dako Cytomation, CA, USA). Negative controls were produced using the antibody diluent (Dako Cytomation, reference S302283-2) to replace the primary antibody.
Expression of MMP-2 and MMP-9 through zymography
To investigate the presence of the MMP-2 and MMP-9 enzymes in the skin samples, a pool of subcutaneous tissue measuring about 1.5 × 1 cm, including the three areas analyzed (muzzle, ears, and abdomen), was subjected to zymographic analysis. The skin fragments were frozen in liquid nitrogen and stored in a freezer at − 80 °C for further analysis of enzymatic activity.
During the analysis, the skin samples were macerated together with a lysis buffer (50 mM of Tris-HCl (pH 7.6), 150 mM of NaCl, 5 mM of CaCl2, 0.05% Brij-35®, and distilled water qsp to complete up to 1000 mL). Afterwards, this homogenized mix was centrifuged (12,000×g) for 15 min at 4 °C and the supernatant was stored in 50 uL aliquots at − 20 °C for further analysis. All the steps of this procedure were performed with the samples immersed in ice (4 °C).
Skin total protein was quantified by means of the bicinchoninic acid method (BCA, 23225, Pierce Biotechnology, Rockford, IL, USA). This analysis was performed to standardize the protein concentration used in the zymogram gels.
The enzymatic activity of these samples was detected by means of SDS-PAGE (sodium dodecyl sulfate-polyacrylamide gel electrophoresis) zymography, as described by Ihara et al. (2001) and modified by Machado et al. (2010). The polyacrylamide gels (10%) were used together with gelatin (G8150-100G, Sigma-Aldrich). Samples with previously standardized protein concentration (10 μL) were put together with a buffer solution (10 μL), containing 125 mM of Tris-HCl (pH 6.8), glycerol 20% (v/v), SDS 4% (w/v), and bromophenol blue 0.2% (w/v). The samples were subjected to electrophoresis at 4 °C for 3 h. Subsequently, the gels were placed in Triton X-100 (2.5% v/v) for 30 min, for SDS removal, and were incubated with enzymatic activation buffer containing 50 mM of Tris, 200 mM of NaCl, 5 mM of CaCl2 and Brij-35 (0.2% w/v), at pH 7.5, for 20 h at 37 °C, under gentle agitation. After the incubation, the gels were stained with Coomassie brilliant blue R-250 (0.5% w/v), methanol (45% v/v), and glacial acetic acid (10% v/v) for 30 min. The mixture was subsequently destained using 45% methanol and glacial acetic acid (10% v/v) for 30 min. The MMP gelatinolytic activity in the samples was shown by the appearance of light bands highlighted in a dark blue background. The positive control for the zymography was a sample of female dog mammary adenocarcinoma, as previously standardized (Machado et al. 2010).
The gel images were captured by means of optical densitometry (ImageQuant LAS 4000; GE Healthcare Bio-Sciences, Uppsala, Sweden) and the results were analyzed through the public-domain ImageJ 1.45 s software (Wayne Rasband, National Institutes of Health; http://rsb.info.nih.gov/ij).
Statistical analysis
The number of cells immunolabeled by antibodies (MMP-2, MMP-9 and parasite load) was determined from five high-power fields (× 40 objective lens), thus finding the average number of immunolabeled cells per animal.
The analysis on the average number of cells immunolabeled by MMP-2 and MMP-9 per animal was performed through the Kruskal-Wallis nonparametric test. The comparison between groups (infected and control), in each area (muzzle, ear, and abdomen), was performed through Dunn’s multiple comparison test. The same test was used to show the predominance of latent versus active forms of MMP-2 and MMP-9 in the same groups. The gelatinolytic activity in the pool from the skin regions (muzzle, ear, and abdomen) in the infected and control groups was determined through the Mann-Whitney nonparametric test. The comparison between parasite load in skin areas (muzzle, ear, and abdomen) of the infected group was performed through Dunn’s multiple comparison test. The statistical software used was GraphPad Prism (version 5.00; 2007) and differences were considered significant at p < 0.05.
Results
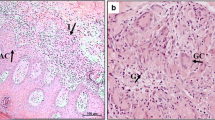
In the histopathological analysis, the infected group showed cutaneous inflammation distributed diffusely (Fig. 1a) or multifocally, predominantly around cutaneous adnexa (Fig. 1b) in the dermis from the ear, muzzle, and abdomen areas. In the infected dogs, the inflammation and parasite load were observed to present lower intensity in the abdominal region. This infiltrate was composed of plasmocytes (Fig. 1c), lymphocytes (Fig. 1d), parasitized or non-parasitized macrophages (Fig. 1e), and mast cells (detail, Fig. 1b), forming granulomas with poorly defined edges. Macrophages were the predominant cell type in the inflammatory infiltrate. When the inflammation was composed of highly parasitized macrophages, there was severe dissociation of the dermal collagen fibers, due to the diffuse distribution of the granulomatous inflammatory infiltrate.
Photomicrograph of skin from dogs with visceral leishmaniasis. a Ear: diffuse inflammatory infiltrate (*; × 10 obj. lens). Detail: predominant macrophage infiltrate (black arrow) followed by plasma cells (yellow arrow) and lymphocytes (blue arrow; × 40 obj. lens); hematoxylin and eosin. b Multifocal inflammation around the cutaneous adnexa (*; × 10 obj. lens), hematoxylin and eosin. Detail: note mast cells (red arrow) in association with parasitized macrophages (black arrow; × 40 obj. lens), toluidine blue. c Immunostaining of plasma cells (CD138) in inflammatory infiltrate around cutaneous adnexa (* red; × 40 obj. lens); in detail, note immunostaining cytoplasmic membrane of the cell (× 100 obj. lens); peroxidase-linked polymer complex. d Immunostaining of T cells (CD3) in inflammatory infiltrate around cutaneous adnexa (* red); in detail, note immunodetection of the lymphocytes among parasitized macrophages (× 40 obj. lens); peroxidase-linked polymer complex. e Immunostaining of macrophages (MCA387) in cutaneous inflammatory infiltrate without parasites (black arrow) among parasitized macrophages (red arrow) (× 40 obj. lens); peroxidase-linked polymer complex. f Sirius red staining with halogen light. g Sirius red staining with polarized light. Note intense collagen degradation associated with the inflammatory infiltrate around annexes (*; × 10 obj. lens)
It was noted that the greatest intensity of inflammation was in the ear area (Fig. 1a), followed by the muzzle. Additionally, acanthosis, hyperkeratosis, ulceration, and crusts in the epidermis were observed.
In cases of diffuse inflammation that were rich in parasitized macrophages, the infiltrate extended to deeper layers of the skin, such as adipose tissue. In these situations, it was common to observe complete degradation of the dermal collagen.
Dermal mature collagen degradation was observed at the same intensity and in the same locations as the cutaneous inflammatory infiltrate (Fig. 1f). The dogs infected with gross skin lesions (n = 26) had higher intensity of inflammation, and consequently, the destruction of mature collagen was severe (Fig. 2b). This degradation was characterized by absence of mature collagen fibers in the areas where the inflammatory infiltrate was located (Fig. 1g). The proportion of young collagen (type III) was low in all infected dogs. The dogs infected without gross skin lesions (n = 10) showed dermal inflammation and presence of parasitized macrophages in the inflammatory infiltrate (2/10).
Photomicrographs of muzzle skin from dogs infected with Leishmania infantum or the control group. a Macrophages immunostained for Leishmania infantum (arrow) and detail (yellow arrow) in the inflammatory infiltrate of the superficial dermis (infected group, × 20. obj. lens; detail × 40. obj. lens). b Note the higher parasitized macrophage density (arrow and detail) and evident dissociation of the collagen fibers in the deep dermis (infected group, × 20 obj. lens and × 40. obj. lens). c Immunostaining for MMP-2 (*) in the inflammatory infiltrate of the superficial dermis (infected group, × 20 obj. lens). d Absence of immunostaining for MMP-2 in dermis (*; control group, × 20 obj. lens). e Immunostaining for MMP-9 in granulomatous inflammation around adnexa (*; infected group, × 20. obj. lens). Detail: note macrophages positive for MMP-9 (arrow; × 40 obj. lens). f Absence of immunostaining for MMP-9 in dermis (*; control group, × 20 obj. lens). Polymer complex linked to peroxidase
In dogs with high density of parasitized macrophages, the dermal mature collagen degradation was intense. In these areas of the skin, the proportion of young collagen fibers (type III collagen), which are thinner and slightly birefringent, was lower.
The immunohistochemical technique showed that immunodetection of the parasite was restricted to intracytoplasmic amastigote forms in macrophages (Fig. 2a, b) and in fibroblasts of the superficial dermis. In the infected group, it was observed that the ears and muzzle were the areas that presented the highest parasite density. The abdomen was the region that presented the lowest intensities of inflammation and parasitized macrophages (Fig. 3).
Immunolabeling for MMP-2 (Fig. 2c) and MMP-9 (Fig. 2e) was observed in the cytoplasm of macrophages, lymphocytes, mast cells, and plasmocytes of the inflammatory infiltrate. Likewise, lower intensity of immunolabeling was seen in fibroblasts, collagen fibers, epidermis, hair follicle, and chondrocytes of the ear cartilage. In the control group, immunostaining of MMP-2 (Fig. 2d) and MMP-9 (Fig. 2f) was absent or rare and located near the cutaneous adnexa.
The highest proportion of parasitized cells occurred in the ears and muzzle. However, there were no significant differences between the areas (p = 0.1798; Fig. 3).
The zymographic analysis revealed gelatinolytic activity relating to enzymes with molecular weight of 92 kDa (pro-MMP-9), 86 (active MMP-9), 72 (pro-MMP-2), and 66 (active MMP-2), which were detected in the pool of skin areas from the infected dogs and control dogs (Fig. 4).
Representative zymogram of the skin from dogs with visceral leishmaniasis presenting gelatinolytic bands corresponding to pro-MMP-9 (92 kDa), active MMP-9 (86 kDa), pro-MMP-2 (72 kDa), and active MMP-2 (66 kDa). a Molecular weight (mammary adenocarcinoma used as positive control). b Infected dogs. c Non-infected dogs (control group)
The gelatinolytic activity was evaluated in the three skin areas of the infected group, in which a significant difference in the expression of active MMP-9 (p = 0.0122) was observed in comparison with the control animals (Fig. 5a). When these areas were analyzed separately, greater expression of latent MMP-9 (p = 0.0409), active MMP-9 (p = 0.0067), and active MMP-2 (p = 0.0384) was observed in the ears of the infected group, in comparison with the control group (Fig. 5b). Greater expression of latent MMP-9 (p = 0.0106), active MMP-9 (p = 0.0115), and active MMP-2 (p = 0.0249; Fig. 5c) was also seen in the muzzle. In the abdomen, there was no significant difference in the expression of MMPs between the infected dogs and the control group (Fig. 5d).
Gelatinolytic activity of skin from dogs with visceral leishmaniasis. a Significant difference in expression of active MMP-9 (p = 0.0122) was observed in the three areas of the skin from dogs with VL, compared with the control group. b Skin from ear area: significant differences in latent MMP-9 (p = 0.0409), active MMP-9 (p = 0.0067), and active MMP-2 (p = 0.0384), in comparison with the control animals. c Skin from muzzle area: significant difference in latent MMP-9 (p = 0.0106), active MMP-9 (p = 0.0115), and active MMP-2 (p = 0.0249), in comparison with the control animals. d Skin from abdomen area. There was no significant difference in MMP expression in the animals with VL, in comparison with the control animals (p > 0.05). The results are shown as means and standard errors, from Mann-Whitney test. The values were expressed as arbitrary units (AU)
Discussion
In the present study, the cutaneous inflammatory process was predominantly around the adnexa, with multifocal to coalescent distribution. In the cases with greater numbers of parasitized cells, this distribution was diffuse, extending as far as the subcutaneous tissue. The inflammatory infiltrate was distributed diffusely and sometimes only around the hair follicles (Giunchetti et al. 2006; Xavier et al. 2006; Moreira et al. 2013).
Macrophages were the predominant cell type in the inflammatory infiltrate that was observed in the three skin areas, similar to what had been observed in other studies (Xavier et al. 2006; Moreira et al. 2013). Giunchetti et al. (2006) found predominance of plasma cells in the dermal infiltrate of dogs with VL.
Plasma cells were also evident in the cutaneous inflammatory infiltrate, with a proportion similar to that of the macrophages in the infected group of dogs. These cells may reflect the humoral response profile, given that the presence of plasma cells with Russell bodies (Mott cells) was a common finding. Dogs with severe VL lesions may present elevated antibody titers, and this may worsen the disease through deposition of immunocomplexes in several tissues, such as the kidneys (Feitosa et al. 2000; Barbiéri 2006).
The structural profile of the dermal granulomas of the infected dogs was characterized by poorly defined edges, with predominance of macrophages with variable parasite load. Classically, granulomas are divided into Th1 or Th2, according to the predominant cytokine profile and the macrophage activation pathway through CD4 T cells, such as the Arg-1 pathway (L-arginine, Th2) or the iNOS pathway (Th1) (Ackermann 2011). In dogs with high numbers of parasitized macrophages, the diffuse granulomas suggest a Th2 cytokine profile.
In this study, the areas with greater lesion intensity (ears and muzzle) coincided with the findings from other studies (Giunchetti et al. 2006; Moreira et al. 2013). The predominance of cutaneous lesions on the head is because this is the preferential access site for the vector of the protozoon (Giunchetti et al. 2006). This hypothesis is supported by the finding of lesions of greater severity in the cervical lymph nodes of dogs with spontaneous VL. These lymph nodes drain the head area (Lima et al. 2004).
In cases of diffuse inflammation that was rich in parasitized macrophages, the infiltrate extended to the adipose tissue. This suggested that parasitized macrophages could invade the vascular bed in order to reach the peripheral lymph nodes. Moreira et al. (2010) found severe lesions in the popliteal lymph nodes of dogs that were symptomatic for VL, with predominance of parasitized macrophages and lymphoid atrophy. Similar to what was observed by Xavier et al. (2006), dogs without macroscopic cutaneous lesions also presented inflammation of the dermis and some of them showed presence of parasitized macrophages. Animals with no clinically visible signs of VL are also important sources of contamination for humans and dogs.
Immunostaining of Leishmania sp. was observed in macrophages and also in fibroblasts in the three skin areas (ears, muzzle, and abdomen) of the infected dogs. Macrophages are the parasite target cells. However, fibroblasts produce proteins for the ECM, cytokines, MMPs, and chemokines that regulate the extracellular microenvironment composition under physiological and pathological conditions (Ackermann 2011). In the present study, the parasitized fibroblasts or fibroblasts that were stimulated by mediators released at the inflammation site may also have contributed towards the dermal alterations, especially through release of gelatinolytic enzymes (MMPs).
In the current study, the presence of accentuated degradation of mature collagen in the dermis was associated with inflammatory infiltrate. Therefore, the production of collagenolytic enzymes (MMPs) by macrophages, fibroblasts, and mast cells in the dermis may contribute towards ECM degradation in VL (Cawston and Wilson 2006).
The formation of young collagen was low in relation to the proportion of it that was degraded in the infected group. Dermal collagen evaluations in dogs with VL in other studies showed that in dogs with intense inflammatory infiltrate and presence of high numbers of parasitized macrophages, type III collagen (young) predominated. These changes may be related to deleterious effects in the inflammatory infiltrate (Giunchetti et al. 2006) or to mechanisms for evasion of the host’s immune response that the parasite induces (Lira et al. 1997). On the other hand, Kondo et al. (2009) found that type I collagen was gradually replaced by type III collagen in the popliteal lymph nodes of dogs with VL.
In the present study, greater degradation of type I collagen was confirmed by detection of MMP-2 and MMP-9 in the skin (inflammatory infiltrate, fibroblasts, and collagen), which was seen in the groups of infected dogs. Similar results were described by Cardoso et al. (2017), who observed greater degradation of mature collagen (type I) and lower deposition of young collagen (type III) in symptomatic dogs, possibly through these dogs’ loss of capacity to remodel the extracellular matrix within the chronic inflammatory process. However, these authors highlighted that there was greater deposition of young collagen in the oligosymptomatic dogs. That result differed from what was seen in the present study, since the infected dogs always presented a lower proportion of young collagen. The reduction in mature collagen probably favored migration of the parasitized macrophages, thus contributing towards systemic dissemination of the infection.
In canine VL, there have not been any reports of MMP-2 and MMP-9 immunolabeling in the skin of dogs naturally infected with Leishmania infantum. However, these MMPs were evaluated in the brain (Machado et al. 2010) and cerebrospinal fluid (CSF) (Marangoni et al. 2011) of dogs with VL. The authors concluded, respectively, that there was positive latent MMP-2 and MMP-9 immunolabeling in resident cells and in inflammatory infiltrate cells in the brain and that there were high levels of MMP-9 in the CSF, which could participate in disruption of the blood-brain barrier. A previous study with methodology similar to the latter study was conducted on dogs with mammary tumors and showed high detection of MMP-2 and MMP-9, which correlated with the malignant behavior of these tumors (Loukopoulos et al. 2003). In human mucosal leishmaniasis, neutrophils were found to contribute towards lesion expansion through MMP-9 production (Boaventura et al. 2010). Similarly, in human cutaneous leishmaniosis, high levels of MMP-9 were observed in skin lesions, compared with healthy patients. Monocytes and MMP-9 may play key roles in tissue destruction through L. braziliensis infection (Campos et al. 2014).
In the present study, the collagen degradation was possibly induced by macrophages, fibroblasts, and active mast cells, which contributed towards MMP-2 and MMP-9 production. In the same way, the proportion of the active form of MMP-9 was greater in the infected group, thus suggesting that this enzyme is a good marker for active inflammation. On the other hand, MMP-2 showed greater expression in the latent form in the same group. This gelatinase (MMP-2) has constitutive expression in tissues, whereas MMP-9 is inducible (Opdenakker et al. 2001b). In the present study, MMP-9 production was induced by cells of the dermal inflammatory infiltrate in dogs with VL. It is possible that fibroblasts activated by the parasite also contributed towards higher expression of this enzyme.
It can be hypothesized that MMP-2 is released in greater concentrations in the initial phase of the infection and that MMP-9 becomes predominant through chronic evolution of the disease. This hypothesis could not be confirmed under the conditions of the present study, because dogs with spontaneous disease were used. TIMPs are considered to be the main tissue regulators of MMPs (Dzwonek et al. 2004). In the future, it would be interesting to evaluate the role of these MMP regulators in the infected groups.
In summary, the infected dogs showed that the intensity of gross lesions in the skin was proportional to the intensity of inflammation, the parasite load, and the degradation of mature collagen fibers. Changes to the dermal collagen composition were induced by MMP enzymes, especially active MMP-9, and these changes favored the spread of parasitized macrophages to the blood vessels, thus also favoring systemic dissemination of the parasite and maintenance of persistent infection.
Conclusions
Active MMP-9 was significantly present in the dogs of the infected group, in the ear and muzzle regions, coinciding with areas of higher parasite load and cutaneous inflammatory infiltrate. The intensity of degradation of mature collagen fibers was associated with the inflammation and this was perhaps one of the factors that favored migration of parasitized macrophages to the vascular bed and led to systemic dissemination.
References
Ackermann MR (2011) Chronic inflammation and wound healing. In: McGavin MD, Zachary JF (eds) Pathologic basis of veterinary disease, 5th edn. Elsevier, St Louis, pp 153–191
Barbiéri CL (2006) Immunology of canine leishmaniasis. Parasite Immunol 28:329–337. https://doi.org/10.1111/j.1365-3024.2006.00840.x
Boaventura VS, Santos CS, Cardoso CR, Andrade J, Santos WLC, Clarêncio J, Silva JS, Borges VM, Barral-Netto M, Brodskyn CI, Barral A (2010) Human mucosal leishmaniasis: neutrophils infiltrate areas of tissue damage that express high levels of Th17-related cytokines. Eur J Immunol 40:2830–2836. https://doi.org/10.1002/eji.200940115
Brasil (2014) Ministério da Saúde. Secretaria de Vigilância em Saúde. Departamento de Vigilância Epidemiológica. Manual de vigilância e controle da leishmaniose visceral. Ministério da Saúde, Brasília, pp 120
Campos TM, Passos ST, Novais FO, Beiting DP, Costa RS, Queiroz A, Mosser D, Scott P, Carvalho EM, Carvalho LP (2014) Matrix metalloproteinase 9 production by monocytes is enhanced by TNF and participates in the pathology of human cutaneous leishmaniasis. PLoS Negl Trop Dis 8(11):e3282. https://doi.org/10.1371/journal.pntd.0003282
Cardoso JMO, Ker HG, Aguiar-Soares RDO, Moreira ND, Mathias FAZ, Reis LES, Roatt BM, Vieira PMA, Coura-Vital W, Carneiro CM, Reis AB (2017) Association between mast cells, tissue remodelation and parasite burden in the skin of dogs with visceral leishmaniasis. Vet Parasitol 243:260–266. https://doi.org/10.1016/j.vetpar.2017.05.028
Cawston TE, Wilson AJ (2006) Understanding the role of tissue degrading enzymes and their inhibitors in development and disease. Best Pract Res Clin Rheumatol 20:983–1002. https://doi.org/10.1016/j.berh.2006.06.007
Dzwonek J, Rylski M, Kaczmarek L (2004) Matrix metalloproteinases and their endogenous inhibitors in neuronal physiology of the adult brain. FEBS Lett 567:129–135. https://doi.org/10.1016/j.febslet.2004.03.070
Feitosa MM, Ikeda FA, Luvizotto MCR, Perri SHV (2000) Aspectos clínicos de cães com leishmaniose visceral no município de Araçatuba—São Paulo (Brasil). Clin Vet 5:36–44
Giunchetti RC, Mayrink W, Genaro O, Carneiro CM, Corrêa-Oliveira R, Martins-Filho OA, Marques MJ, Tafuri WL, Reis AB (2006) Relationship between canine visceral leishmaniosis and the Leishmania (Leishmania) chagasi burden in dermal foci. J Comp Pathol 135:100–107
Gutierrez FRS, Lalu MM, Mariano FS, Milanezi CM, Cena J, Gerlach RF, Santos JET, Torres-Dueñas D, Cunha FQ, Schulz R, Silva JS (2008) Increased activities of cardiac matrix metalloproteinases MMP-2 and MMP-9 are associated with mortality during the acute phase of experimental Trypanosoma cruzi infection. J Infect Dis 197:1468–1476
Ihara M, Tomimoto H, Kinoshita M, Oh J, Noda M, Wakita H, Akiguchi I, Shibasaki H (2001) Chronic cerebral hypoperfusion induces MMP-2 but not MMP-9 expression in the microglia and vascular endothelium of white matter. J Cereb Blood Flow Metab 21:828–834
Kondo KRJ, Fonseca CC, Matta SLP, Viloria MIV (2009) Análise histomorfométrica da matriz extracelular do linfonodo poplíteo de cães naturalmente infectados por Leishmania (L.) chagasi. Pesq Vet Bras 29:610–616
Kupai K, Szucs G, Cseh S, Hajdu I, Csonka C, Csont T, Ferdinandy P (2010) Matrix metalloproteinases activity assays: importance of zymography. J Pharmacol Toxicol Methods 65:205–209
Lima WG, Michalick MSM, Melo MN, Tafuri WL, Tafuri WL (2004) Canine visceral leishmaniasis: a histopathological study of lymph nodes. Acta Trop 92:43–53
Lima VMF, Biazzono L, Silva AC, Correa APFL, Luvizotto MCR (2005) Serological diagnosis of visceral leishmaniasis by an enzyme immunoassay using protein A in naturally infected dogs. Pesq Vet Bras 25(4):215–218
Lira R, Rosales-Encina JL, Arguelos C (1997) Leishmania mexicana: binding of promastigotes to type I collagen. Exp Parasitol 85:149–157
Loukopoulos P, Mungall BA, Straw RC, Thornton JR, Robinson WF (2003) Matrix metalloproteinase-2 and -9 involvement in canine tumors. Vet Pathol 40:382–394
Machado GF, Melo GD, Moraes OC, Souza MS, Marcondes M, Perri SHV, Vasconcelos RO (2010) Differential alterations in the activity of matrix metalloproteinases within the nervous tissue of dogs in distinct manifestations of visceral leishmaniasis. Vet Immunol Immunopathol 136:340–345
Madeira MF, Figueiredo FB, Pinto AG, Nascimento LD, Furtado M, Mouta-Confort E, Paula CC, Bogio A, Gomes MCA, Bessa AMS, Passos SRL (2009) Parasitological diagnosis of canine visceral leishmaniasis: is intact skin a good target? Res Vet Sci 87:260–262
Marangoni NR, Melo GD, Moraes OC, Souza MS, Perri SHV, Machado GF (2011) Levels of matrix metalloproteinase-2 and metalloproteinase-9 in the cerebrospinal fluid of dogs with visceral leishmaniasis. Parasite Immunol 11(33):330–334
Moreira PRR, Vieira LM, Andrade MMC, Bandarra MB, Machado GF, Munari DP, Vasconcelos RO (2010) Immune response pattern of the popliteal lymph nodes of dogs with visceral leishmaniasis. Parasitol Res 107:605–613. https://doi.org/10.1007/s00436-010-1902-2
Moreira PRR, Bandarra MB, Magalhães GM, Munari DP, Machado GF, Prandini MM, Alessi AC, Vasconcelos RO (2013) Influence of apoptosis on the cutaneous and peripheral lymph node inflammatory response in dogs with visceral leishmaniasis. Vet Parasitol 192:149–157. https://doi.org/10.1016/j.vetpar.2012.09.029
Nagase H, Woessner Jr JF (1999) Matrix metalloproteinases. J Biol Chem 274:21491–21494
Oliveira TMFS, Furuta PI, Carvalho D, Machado RZ (2008) A study of cross-reactivity in serum samples from dogs positive for Leishmania sp, Babesia canis and Ehrlichia canis in enzyme-linked immunosorbent assay and indirect fluorescent antibody test. Ver Bras Parasitol Vet 17:7–11
Opdenakker G, Van den Steen PE, Dubois B, Nelissen I, Coillie EV, Masure S, Proost P, Van Damme J (2001a) Gelatinase B functions as regulator and effector in leukocyte biology. J Leukoc Biol 69:851–859
Opdenakker G, Van den Steen PE, Van Damme J (2001b) Gelatinase B: a tuner and amplifier of immune functions. Trends Immunol 22:571–579
Reis AB, Martins-Filho OA, Teixeira-Carvalho A, Giunchetti RC, Carneiro CM, Mayrink W, Tafuri WL, Corrêa-Oliveira R (2009) Systemic and compartmentalized immune response in canine visceral leishmaniasis. Vet Immunol Immunopathol 128:87–95
Solano-Gallego L, Fernández-Bellon H, Morrell P, Fondevila D, Alberola J, Ramis A, Ferrer L (2004) Histological and immunohistochemical study of clinically normal skin of Leishmania infantum-infected dogs. J Comp Pathol 130:7–12
Tafuri WL, Santos RL, Arantes RME, Gonçalves R, Melo MN, Michalick MSM, Tafuri WL (2004) An alternative immunohistochemical method for detecting Leishmania amastigotes in paraffin-embedded canine tissues. J Immunol Methods 292:17–23
Xavier SC, Chiarelli IM, Lima WG, Gonçalves R, Tafuri WL (2006) Canine visceral leishmaniasis: a remarkable histopathological picture of one asymptomatic animal reported from Belo Horizonte, Minas Gerais, Brazil. Arq Bras Med Vet Zootec 58(6):994–1000
Acknowledgements
The authors wish to acknowledge the assistance given by the Zoonosis Control Center of Araçatuba, which provided animals for this study.
Funding
Financial assistance was provided by FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo; procedural number 2009/07815-4). Ana Paula Prudente Jacintho was a FAPESP grantee (2009/11687-1).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical standards
The design for this study was approved by the Ethics and Animal Welfare Committee (CEBEA no. 020373/09) of FCAV/UNESP, Jaboticabal, state of São Paulo, Brazil.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Section Editor: Tobili Sam-Yellowe
Rights and permissions
About this article
Cite this article
Jacintho, A.P.P., Melo, G.D., Machado, G.F. et al. Expression of matrix metalloproteinase-2 and metalloproteinase-9 in the skin of dogs with visceral leishmaniasis. Parasitol Res 117, 1819–1827 (2018). https://doi.org/10.1007/s00436-018-5868-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-018-5868-9