Abstract
Sulfadoxine-pyrimethamine (SP) is the recommended drug for intermittent preventive treatment of malaria in pregnancy in most of sub-Saharan Africa. Resistance to SP is related to mutations in the dhfr and dhps gene of Plasmodium falciparum. This study determined the prevalence of Pfdhfr and Pfdhps polymorphisms found in asymptomatic pregnant women attending antenatal care in Calabar, Nigeria. From October 2013 to November 2014, asymptomatic pregnant women attending antenatal care clinics were enrolled after obtaining informed consent. Malaria diagnosis testing was done using thick and thin smears. Dried blood spot filter papers were collected. Parasite DNA was extracted from the filter papers using a chelex extraction. Extraction was followed by nested PCR and restriction enzyme digestion. P. falciparum infection was detected by microscopy in 7% (32/459) participants. Twenty-eight P. falciparum isolates were successfully genotyped. In the Pfdhfr gene, the triple mutation was almost fixed; S108N mutation was (100%), N51I (93%) and C59R mutations (93%), whereas the I164L mutation was absent. The prevalence of Pfdhps S436A, A437G, A581G and A613S mutations was 82.1% (23/28), 96.4% (27/28), 71.4% (20/28) and 71.4% (20/28) respectively. The K540E mutation was absent. The prevalence of the Pfdhfr triple mutation IRNI was 92.9% (26/28). The efficacy of SP as IPTp in Southeast Nigeria may be severely threatened. The continuous monitoring of SP molecular markers of resistance is required to assess thresholds. The evaluation of alternative preventive treatment strategies and drug options for preventing malaria in pregnancy may be necessary.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Malaria remains a leading cause of death predominantly in sub-Saharan Africa and East Asia. The latest World Health Organization (WHO) statistics report an estimated 438,000 deaths attributable to malaria in 2015 alone. The Democratic Republic of the Congo and Nigeria together accounted for more than 35% of this global total of estimated malaria deaths (World Health Organization 2015). Plasmodium falciparum malaria disproportionately affects pregnant women and children aged under 5 years in high transmission areas, particularly in poor and disadvantaged areas. Over 32 million pregnant women are estimated to be at risk of P. falciparum malaria in sub-Saharan Africa annually (Dellicour et al. 2010). Malaria infection adversely affects the outcome of pregnancy. The foetus is at increased risk of premature and stillbirth, intrauterine growth retardation, low birth weight, anaemia and congenital malaria (Brabin 1991; Desai et al. 2007; McGready et al. 2012).
In Nigeria, malaria incidence is estimated to have decreased by less than 50% between 2000 and 2015 (World Health Organization 2015). Sadly, malaria remains a significant challenge, as malaria-related deaths still account for up to 11, 25 and 20% maternal, infant and under-five mortality respectively (National Population Commission 2012).
The WHO recommends a package of interventions to prevent the adverse effects of malaria during pregnancy in areas with stable transmission in sub-Saharan Africa including the use of insecticide-treated nets (ITNs), intermittent preventive treatment (IPTp) and effective case management of malaria and anaemia. Sulfadoxine-pyrimethamine (SP) is the recommended drug for IPTp (World Health Organization 2004). The WHO now recommends a dose of SP at each scheduled antenatal care (ANC) visit, beginning as early as possible in the second trimester, and with each dose at least a month apart for areas of moderate-to-high malaria transmission (World Health Organization 2012).
In Nigeria, there has been an overall increase in access to and ownership of ITNs, but geographical variations still exist (National Population Commission 2012; Nigeria Demographic Health Survey 2014). IPTp utilisation remains low with only 13% of pregnant women receiving the recommended preventive treatment (≥ 2 doses of SP) during ANC (National Population Commission 2012).
The development of drug resistance remains one of the most significant challenges of malaria control programmes. While SP is recommended for IPTp, in stable transmission areas, increasing resistance has been reported and is compromising the beneficial effects of SP on birth weight and anaemia (McGready et al. 2011; Cottrell et al. 2015). IPTp with SP causes placental proliferation of resistant parasites in pregnant women (Harrington et al. 2011). Pregnant women with asymptomatic parasitaemia could constitute a reservoir of parasites for the inoculation of mosquitoes (Kern et al. 2011) and may play a role in increasing drug resistance. This growing resistance has enormous implications for malaria control efforts, and thus monitoring of SP resistance remains an important task.
The efficacy of SP is dependent on some mutations which may accumulate in P. falciparum dhfr and dhps genes which code for the proteins DHFR and DHPS respectively (Kublin et al. 2002). Pyrimethamine and sulfadoxine selectively inhibit dihydrofolate reductase (DHFR) and dihydropteroate synthetase (DHPS) which are both essential for folate biosynthesis in the malaria parasites. P. falciparum resistance to pyrimethamine has been associated with specific point mutations (A16V, C50R, N51I, C59R, S108N/T, V140L and I164L) in the dhfr gene (Peterson et al. 1990; Plowe et al. 1997) and in the dhps gene (I431V, S436A/F, A437G, K540E, A581G and A613S/T) (Triglia and Cowman 1994; Sutherland et al. 2009; Chauvin et al. 2015).
Higher SP resistance is associated with an increasing number of mutations in both the Pfdhfr and Pfdhps genes. In sub-Saharan Africa, the so-called Pfdhfr/Pfdhps quintuple mutation which is a combination of a triple Pfdhfr mutation (51I-59R-108N) and the Pfdhps double mutation (437G-540E) are predictive of SP treatment failure (Duraisingh et al. 1998; Vinayak et al. 2010; Happi et al. 2005).
Given the central role of SP in the current IPTp policy recommendations, there is the need for continuous molecular surveillance for SP resistance to inform the National Malaria Control Programmes on where SP can still be used effectively and cases where it needs replacing (Naidoo and Roper 2013). In the present study, we investigated the prevalence of dhfr and dhps point mutations in P. falciparum isolates collected from asymptomatic pregnant women who had not received IPTp-SP.
Methods
Study area, subjects and sample collection
The study was carried out at the General Hospital situated in Calabar. The General Hospital is the largest government-owned secondary health facility in the city and caters to the health needs of the majority of the inhabitants. Since August 2009, pregnant women and children under 5 years of age receive free medical care as part of a funded welfare program by the Cross River State government. The average annual antenatal clinic attendance and births at the hospital are 16,550 and 3100 respectively (Ekpo A. personal communication). The climate in Calabar is tropical-humid with wet and dry seasons, with average temperatures ranging between 15 and 30 °C and the annual rainfall between 1300 and 3000 mm. The vegetation in Calabar is mangrove swamp forest. Malaria transmission in this area is intense and perennial but with a peak in the rainy season, and P. falciparum is the predominant malaria-causing species (World Health Organization 2015; National Population Commission 2012; Oduwole et al. 2011).
Blood samples were collected as part of a clinical trial (PACTR201308000543272) on the effectiveness of intermittent screening and treatment for malaria prevention in pregnancy. All samples were collected from asymptomatic pregnant women attending ANC. Briefly, all pregnant women attending their first ANC visit for that pregnancy and who had not received any dose of IPTp-SP were invited to participate in the study. After obtaining written informed consent, a finger prick blood sample was obtained. Thick and thin blood smears, as well as dried blood spots (DBS) on filter paper (Whatman® grade 3), were prepared. Molecular genotyping was performed only on microscopy-positive samples (Fig. 1).
Laboratory procedures
DNA extraction, genotyping procedures and analysis of Pfdhfr and Pfdhps genes
Genomic DNA was extracted from DBS filter papers using QIAamp Blood Mini Kit 50 (Qiagen, Krefeld, Germany) according to the manufacturer’s instructions.
P. falciparum parasites were genotyped for mutations in the dhfr and dhps genes by polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP). Mutations were investigated at codons 51, 59, 108 and 164 of Pfdhfr gene and codons 436, 437, 540, 581 and 613 of Pfdhps gene as previously reported (Duraisingh et al. 1998).
Statistical analysis
Data were entered in Excel version 2013 and analysed using STATA v12 (STATA Corporation, College Station, TX, USA). Mixed genotypes were considered as mutants, and the prevalence of each type of allele (wild or mutant) was calculated.
The frequencies of the mutations were compared based on gravidity and parasite density using chi-square test. Mann-Whitney test was used to compare parasite densities. A P value < 0.05 was considered as statistically significant for all tests.
Ethical considerations
The study proposal and informed consent forms were reviewed and approved by two ethics committees: the Cross River Health Research Ethics Committee, Calabar, Nigeria, and the Ethics Board of the Medical Center of LMU, Munich, Germany. Written informed consent was obtained from all participants before sample collection. Participation was voluntary, and information obtained from all subjects was confidential.
Results
Out of 459 women screened for malaria parasitaemia, 32 had a microscopically confirmed malaria infection. Twenty-eight pre-treatment samples were successfully genotyped for Pfdhfr and Pfdhps mutations. Among these 28 women with asymptomatic malaria, the mean age was 27.3 (± 4.3) years. About 46% (13/28) of the women were primigravidae. Ownership of bed nets was 46.4% (13/28), less than half of the women (6/13) slept under a bed net the previous night (Table 1). Women who reportedly slept under a bed net the previous night did not have significantly lower parasite densities compared to those who did not (P = 0.748). Most women (67.9%) had haemoglobin concentration between 8 and 10.9 g/dl. Overall, the mean of haemoglobin was 10.1 ± 1.4 g/dl. The median parasite density (interquartile range) was 768.5 (256–2799) asexual parasite/μl.
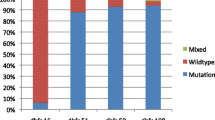
The most frequent Pfdhfr mutation was 108N. Pfdhfr mutations were detected in 92.9% (26/28) of P. falciparum isolates for codons 51 (51I) and 59 (59R). The 164L mutation was not found in any of the pre-treatment samples (Table 2).
Pfdhps mutations were detected in 82.1% (23/28), 96.4% (27/28), 71.4% (20/28) and 71.4% (20/28) of P. falciparum isolates for codons 436 (436A), 437 (437G), 581 (581G) and 613 (613S) respectively. None of the samples carried the Pfdhps mutation K540E (Table 2).
Table 3 shows the prevalence of mutant alleles and haplotypes. A single mutation in the Pfdhfr gene at codon 108 (108N) was detected in only one isolate (3.6%).
A double mutation, made up of single mutations in the Pfdhfr and Pfdhps genes (108N and 437G), was found in one sample (3.6%). Also, the frequency of quadruple (51I/59R/108N + 437G) and quintuple (51I/59R/108N + 436A/437G) Pfdhfr-Pfdhps mutations were both 10.7% (3/28).
The prevalence of Pfdhfr and Pfdhps mutant alleles and haplotypes were not significantly lower in primigravidae compared to secundi- and multi-gravidae women (P = 0.436, P = 0.144, P = 0.11, P = 0.871 for the triple Pfdhfr mutation, quadruple, quintuple and septuple Pfdhfr-Pfdhps mutations respectively).
Similarly, the prevalence of Pfdhfr and Pfdhps mutant alleles and haplotypes did not differ significantly between high- and low-density malaria infections (Table 4).
Discussion
In Calabar, among asymptomatic pregnant women before IPTp-SP, the Pfdhfr mutations 51I, 59R and 108N were almost fixed with all three mutations being present in more than 90% of the isolates. There was no I164L mutation in the study samples, a finding similar to a previous study in Nigeria (Happi et al. 2005). However, the frequency of the triple Pfdhfr mutation was also very high (93%). The Pfdhfr triple mutation is known to confer intense pyrimethamine resistance in vitro (Gregson and Plowe 2005). The Pfdhfr mutations are known to have emerged about a decade or two before the Pfdhps double mutant genotype in Africa (Talisuna et al. 2004) and are now well established across sub-Saharan Africa. The high level of triple Pfdhfr mutations found in this study could be explained in part by the fact that pyrimethamine was previously used as weekly chemoprophylaxis to prevent malaria in pregnancy (Fawole and Onyeaso 2008; Yusuf et al. 2008). Also, cotrimoxazole use has been associated with the emergence, spread and intensification of the A437G and K540E mutations in the Pfdhps gene (Gesase et al. 2009). In Nigeria, there is a high burden of pneumonia and cotrimoxazole is commonly used as prophylaxis or treatment among HIV patients and children with pneumonia (Onyedum and Chukwuka 2011).
In the Pfdhps gene, the frequency of the core mutation, A437G, was over 90%. Although the K540E mutation is very frequently found in association with the A437G mutation (Kublin et al. 2002; Pearce et al. 2009), the K540E mutation was absent from all the P. falciparum isolates in this study. Thus, there was no Pfdhps double mutation, at codon 437 and 540 which is a predictor of post-treatment SP resistance (Kublin et al. 2002; Plowe et al. 2004). Although Pfdhfr/dhps quintuple mutants are rare in West Africa, recent studies have reported emergence of the K540E mutation. This mutation is known to be a reliable marker for parasites carrying the quintuple mutants. Studies from the western part of Nigeria have found an emergence of mutant P. falciparum isolates carrying sulfadoxine resistance associated A437G and K540E mutations in the Pfdhps gene (Happi et al. 2005; Iwalokun et al. 2015; Olasehinde et al. 2014).
However, the occurrence of A437G combined with A581G mutation confers higher levels of SP resistance (Pearce et al. 2009), and this combination of A581G/A437G mutations was present in 20 of 28 isolates. The prevalence of the S436A mutation, which is an additional mutation that follows the emergence of A437G mutation, was over 80%. This additional mutation corresponds to an increase in the degree of resistance to SP.
The combination of A437G mutation with the Pfdhfr triple mutation (51I/59R/108N) is considered to be associated with SP treatment failure (Mockenhaupt et al. 2005) and was detected in over 70% of the isolates in this study.
Conclusion
In this study, Pfdhfr and Pfdhps gene mutations associated with SP resistance were highly prevalent among asymptomatic pregnant women pre-treatment in the study area. The prevalence of the triple dhfr mutation and the A437G/A581G mutations were very high; suggesting that the efficacy of SP as IPTp in Southeast Nigeria may be severely threatened. However, the K540E mutation was absent suggesting that SP may still be efficacious when used as IPTp. Nevertheless, screening for Pfdhps K540E, a predictor of the quintuple mutant, remains a priority in Nigeria and West Africa. Also, the evaluation of alternative preventive treatment options for preventing malaria in pregnancy may be necessary.
Abbreviations
- ANC:
-
antenatal care
- DBS:
-
dried blot spot
- DHFR:
-
dihydrofolate reductase enzyme
- dhfr:
-
dihydrofolate reductase gene
- DHPS:
-
dihydropteroate synthetase enzyme
- dhps:
-
dihydropteroate synthetase gene
- IPTp:
-
intermittent preventive treatment in pregnancy
- ITN:
-
insecticide-treated bed net
- PCR-RFLP:
-
polymerase chain reaction-restriction fragment length polymorphism
- SP:
-
sulfadoxine-pyrimethamine
- WHO:
-
World Health Organization
References
Brabin B (1991) The risks and severity of malaria in pregnant women. World Health Organization, Geneva Applied Field Research in Malaria Reports TDR/FIELD-MAL, 1
Chauvin P et al. (2015) Prevalence of Plasmodium falciparum parasites resistant to sulfadoxine/pyrimethamine in pregnant women in Yaoundé, Cameroon: emergence of highly resistant pfdhfr/pfdhps alleles J Antimicrob Chemother. dkv160
Cottrell G, Moussiliou A, Luty AJF, Cot M, Fievet N, Massougbodji A, Deloron P, Tuikue Ndam N (2015) Submicroscopic Plasmodium falciparum infections are associated with maternal anemia, premature births, and low birth weight. Clin Infect Dis 60(10):1481–1488. https://doi.org/10.1093/cid/civ122
Dellicour S, Tatem AJ, Guerra CA, Snow RW, ter Kuile FO (2010) Quantifying the number of pregnancies at risk of malaria in 2007: a demographic study. PLoS Med 7(1):e1000221. https://doi.org/10.1371/journal.pmed.1000221
Desai M, ter Kuile FO, Nosten F, McGready R, Asamoa K, Brabin B, Newman RD (2007) Epidemiology and burden of malaria in pregnancy. Lancet Infect Dis 7(2):93–104. https://doi.org/10.1016/S1473-3099(07)70021-X
Duraisingh MT, Curtis J, Warhurst DC (1998) Plasmodium falciparum: detection of polymorphisms in the Dihydrofolate Reductase and Dihydropteroate SynthetaseGenes by PCR and restriction digestion. Exp Parasitol 89:1–8
Fawole AO, Onyeaso N (2008) Perception and practice of malaria prophylaxis in pregnancy among primary health care providers in Ibadan, Nigeria. West Afr J Med 27(2):92–96
Gesase S, Gosling RD, Hashim R, Ord R, Naidoo I, Madebe R, Mosha JF, Joho A, Mandia V, Mrema H, Mapunda E, Savael Z, Lemnge M, Mosha FW, Greenwood B, Roper C, Chandramohan D (2009) High resistance of Plasmodium falciparum to sulphadoxine/pyrimethamine in northern Tanzania and the emergence of dhps resistance mutation at Codon 581. PLoS One 4(2):e4569. https://doi.org/10.1371/journal.pone.0004569
Gregson A, Plowe CV (2005) Mechanisms of resistance of malaria parasites to antifolates. Pharmacol Rev 57(1):117–145. https://doi.org/10.1124/pr.57.1.4
Happi C et al (2005) Polymorphisms in Plasmodium falciparum dhfr and dhps genes and age related in vivo sulfadoxine–pyrimethamine resistance in malaria-infected patients from Nigeria. Acta Trop 95(3):183–193. https://doi.org/10.1016/j.actatropica.2005.06.015
Harrington WE, Mutabingwa TK, Kabyemela E, Fried M, Duffy PE (2011) Intermittent treatment to prevent pregnancy malaria does not confer benefit in an area of widespread drug resistance. Clin Infect Dis 53(3):224–230. https://doi.org/10.1093/cid/cir376
Iwalokun BA, Iwalokun SO, Adebodun V, Balogun M (2015) Carriage of mutant Dihydrofolate reductase and Dihydropteroate synthase genes among plasmodium falciparum isolates recovered from pregnant women with asymptomatic infection in Lagos, Nigeria. Med Princ Pract 24(5):436–443. https://doi.org/10.1159/000430987
Kern SE, Tiono AB, Makanga M, Gbadoé A, Premji Z, Gaye O, Sagara I, Ubben D, Cousin M, Oladiran F, Sander O, Ogutu B (2011) Community screening and treatment of asymptomatic carriers of Plasmodium falciparum with artemether-lumefantrine to reduce malaria disease burden: a modelling and simulation analysis. Malar J 10(1):210. https://doi.org/10.1186/1475-2875-10-210
Kublin JG, Dzinjalamala FK, Kamwendo DD, Malkin EM, Cortese JF, Martino LM, Mukadam RAG, Rogerson SJ, Lescano AG, Molyneux ME, Winstanley PA, Chimpeni P, Taylor TE, Plowe CV (2002) Molecular markers for failure of sulfadoxine-pyrimethamine and chlorproguanil-dapsone treatment of Plasmodium falciparum malaria. J Infect Dis 185(3):380–388. https://doi.org/10.1086/338566
McGready R, White NJ, Nosten F (2011) Parasitological efficacy of antimalarials in the treatment and prevention of falciparum malaria in pregnancy 1998 to 2009: a systematic review. BJOG 118(2):123–135. https://doi.org/10.1111/j.1471-0528.2010.02810.x
McGready R, Lee SJ, Wiladphaingern J, Ashley EA, Rijken MJ, Boel M, Simpson JA, Paw MK, Pimanpanarak M, Mu O, Singhasivanon P, White NJ, Nosten FH (2012) Adverse effects of falciparum and vivax malaria and the safety of antimalarial treatment in early pregnancy: a population-based study. Lancet Infect Dis 12(5):388–396. https://doi.org/10.1016/S1473-3099(11)70339-5
Mockenhaupt FP, Teun Bousema J, Eggelte TA, Schreiber J, Ehrhardt S, Wassilew N, Otchwemah RN, Sauerwein RW, Bienzle U (2005) Plasmodium falciparum dhfr but not dhps mutations associated with sulphadoxine-pyrimethamine treatment failure and gametocyte carriage in northern Ghana. Tropical Med Int Health 10(9):901–908. https://doi.org/10.1111/j.1365-3156.2005.01471.x
Naidoo I, Roper C (2013) Mapping ‘partially resistant’, ‘fully resistant’, and ‘super resistant’ malaria. Trends Parasitol 29(10):505–515. https://doi.org/10.1016/j.pt.2013.08.002
National Population Commission (2012) Nigeria malaria indicator survey 2010 Abuja, Nigeria. 1–66
Nigeria Demographic Health Survey (2014) Nigeria demographic health survey (2013). NPC and ICF International, Abuja
Oduwole O et al (2011) Congenital malaria in Calabar, Nigeria: the molecular perspective. Am J Trop Med Hyg 84(3):386–389. https://doi.org/10.4269/ajtmh.2011.10-0253
Olasehinde G, Ojurongbe O, Fagade O, Ruchi S, Egwari L, Ajayi A, Adeyeba AO (2014) Detection of molecular markers of antimalarial drug resistance in Plasmodium falciparum from South-Western Nigeria. Covenant J Phys Life Sci 1:61–75
Onyedum CC, Chukwuka J (2011) Admission profile and management of community acquired pneumonia in Nigeria-5 year experience in a tertiary hospital. Respir Med 105(2):298–302. https://doi.org/10.1016/j.rmed.2010.11.003
Pearce RJ, Pota H, Evehe MSB, Bâ EH, Mombo-Ngoma G, Malisa AL, Ord R, Inojosa W, Matondo A, Diallo DA, Mbacham W, van den Broek IV, Swarthout TD, Getachew A, Dejene S, Grobusch MP, Njie F, Dunyo S, Kweku M, Owusu-Agyei S, Chandramohan D, Bonnet M, Guthmann JP, Clarke S, Barnes KI, Streat E, Katokele ST, Uusiku P, Agboghoroma CO, Elegba OY, Cissé B, A-Elbasit IE, Giha HA, Kachur SP, Lynch C, Rwakimari JB, Chanda P, Hawela M, Sharp B, Naidoo I, Roper C (2009) Multiple origins and regional dispersal of resistant dhps in African plasmodium falciparum malaria. PLoS Med 6(4):e1000055. https://doi.org/10.1371/journal.pmed.1000055
Peterson DS, Milhous WK, Wellems TE (1990) Molecular basis of differential resistance to cycloguanil and pyrimethamine in Plasmodium falciparum malaria. Proc Natl Acad Sci 87(8):3018–3022. https://doi.org/10.1073/pnas.87.8.3018
Plowe CV, Cortese JF, Djimde A, Nwanyanwu OC, Watkins WM, Winstanley PA, Estrada‐Franco JG, Mollinedo RE, Avila JC, Cespedes JL, Carter D, Doumbo OK (1997) Mutations in Plasmodium falciparum dihydrofolate reductase and dihydropteroate synthase and epidemiologic patterns of pyrimethamine-sulfadoxine use and resistance. J Infect Dis 176(6):1590–1596. https://doi.org/10.1086/514159
Plowe CV, Kublin JG, Dzinjalamala FK, Kamwendo DS, Chimpeni P, Molyneux ME, Taylor TE (2004) Sustained clinical efficacy of sulfadoxine-pyrimethamine for uncomplicated falciparum malaria in Malawi after 10 years as first line treatment: five year prospective study. BMJ 328(7439):545–540. https://doi.org/10.1136/bmj.37977.653750.EE
Sutherland CJ, Fifer H, Pearce RJ, bin Reza F, Nicholas M, Haustein T, Njimgye-Tekumafor NE, Doherty JF, Gothard P, Polley SD, Chiodini PL (2009) Novel pfdhps haplotypes among imported cases of Plasmodium falciparum malaria in the United Kingdom. Antimicrob Agents Chemother 53(8):3405–3410. https://doi.org/10.1128/AAC.00024-09
Talisuna AO, Bloland P, d’Alessandro U (2004) History, dynamics, and public health importance of malaria parasite resistance. Clin Microbiol Rev 17(1):235–254. https://doi.org/10.1128/CMR.17.1.235-254.2004
Triglia T, Cowman AF (1994) Primary structure and expression of the dihydropteroate synthetase gene of Plasmodium falciparum. Proc Natl Acad Sci 91(15):7149–7153. https://doi.org/10.1073/pnas.91.15.7149
Vinayak S, Alam MT, Mixson-Hayden T, McCollum AM, Sem R, Shah NK, Lim P, Muth S, Rogers WO, Fandeur T, Barnwell JW, Escalante AA, Wongsrichanalai C, Ariey F, Meshnick SR, Udhayakumar V (2010) Origin and evolution of sulfadoxine resistant Plasmodium falciparum. PLoS Pathog 6(3):e1000830. https://doi.org/10.1371/journal.ppat.1000830
World Health Organization (2004) A strategic framework for malaria prevention and control during pregnancy in the African region Brazzaville: WHO Regional Office for Africa
World Health Organization (2012) Updated WHO policy recommendation: intermittent preventive treatment of malaria in pregnancy using sulfadoxine-pyrimethamine (IPTp-SP) World Health Organization
World Health Organization (2015) World malaria report 2015. WHO, Geneva
Yusuf O, Dada-Adegbola H, Ajayi I, Falade C (2008) Malaria prevention practices among mothers delivering in an urban hospital in southwest Nigeria. J Vector Borne Dis 45(3):217–224
Acknowledgments
We thank the study participants, the staff of the antenatal clinic and management of the General Hospital Calabar where this study took place. Special thanks to Dr. Olabisi Oduwole, Ms.Obiamaka Okafo and Mr. David Agamse at the Calabar Institute of Tropical Diseases Research and Prevention, University of Calabar Teaching Hospital, Calabar, Nigeria, for their invaluable help. We acknowledge the assistance of Prof Dr. Frank Mockenhaupt at the Institute of Tropical Medicine and International Health, Charité-Universitätsmedizin Berlin, with genotyping of molecular markers. We are also grateful to the Center for International Health (CIH), Ludwig-Maximilians University of Munich, DAAD, BMZ and Exceed for supporting the first author for his PhD program.
Author information
Authors and Affiliations
Contributions
EE, MM, NB, MP and TL designed the study. CT and PG were responsible for the laboratory work. EE wrote the first draft of the paper. All authors contributed to the interpretation of the data and the revision of the manuscript.
Corresponding author
Ethics declarations
Consent for publication
Not applicable.
Availability of data and materials
The datasets generated during the current study are available from the corresponding author on reasonable request.
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Esu, E., Tacoli, C., Gai, P. et al. Prevalence of the Pfdhfr and Pfdhps mutations among asymptomatic pregnant women in Southeast Nigeria. Parasitol Res 117, 801–807 (2018). https://doi.org/10.1007/s00436-018-5754-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-018-5754-5