Abstract
A new species of acanthocephalan infecting marine and brackish water fishes from the south-west coast of India is described. The parasite belongs to the genus Tenuiproboscis, and the fish hosts include Lutjanus argentimaculatus, L. ehrenbergii, Siganus javus, Epinephelus malabaricus, E. coioides, Scatophagus argus, Parascolopsis aspinosa, Caranx ignobilis, Gerres filamentosus and Lates calcarifer. The parasite inhabits mid- and hindgut regions and is characterised by an elongated, cylindrical, bulbous and posteriorly tapering metasoma and a claviform proboscis having 14–15 rows of 14–15 hooks each. Females larger than males, measured 3898.16–10,318.00 μm (6430.00 ± 1417.30) in length and 458.93–1435.68 μm (929.81 ± 250.39) in width. Males measured 3234.89–8644.20 μm (5729.50 ± 1176.60) in length and 388.30–1584.61 μm (795.88 ± 184.12) in width. Parasites recovered from different host species showed morphological/morphometric variations. However, principal component analysis (PCA) revealed significant overlapping of characters indicating their similarities. Proboscis profiling based on variations in size and position of hooks also yielded similar results. Further, in molecular phylogenetic analysis, parasites from different fish hosts formed a monophyletic clade with strong bootstrap support, again indicating their conspecific nature. These morphological/morphometric variations can be ascribed to differences in host species. Morphology and morphometrics in combination with PCA, proboscis profiling and molecular analysis suggest the present acanthocephalan parasite is different from other described species of Tenuiproboscis. Hence, it is considered as a new species and named T. keralensis n. sp. Prevalence, intensity and abundance of the parasite in different hosts are also discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acanthocephalans are endoparasitic helminths infecting the digestive system of vertebrates. They have complex lifecycles involving arthropods as intermediate hosts and vertebrates as definitive/paratenic hosts (Nickol 2006). Many species are known to cause serious pathological manifestations in fish hosts, including irreversible damage to intestinal tissues leading to reduced digestive and absorptive functions and even occlusion of the gut in heavy infections (Sanil et al. 2010). Acanthocephalan parasites are recognised by their relatively large size and morphology of the trunk which is a hollow structure filled with pseudocoelomic fluid and contains the reproductive and nervous systems (Martins et al. 2001; Maghami et al. 2008). Other unique morphological features include a spiny proboscis at the anterior end, a proboscis receptacle and one or more lemnisci that extend from the neck into the trunk (Santos et al. 2008).
Yamaguti (1935) erected Genus Tenuiproboscis with T. misgurni as type species. Presently, this genus is represented by seven species viz. T. misgurni (Yamaguti 1935), T. guptai (Gupta and Sinha 1989), T. clupei (Gupta and Sinha 1991), T. bilqeesae (Gupta and Naqvi 1992), T. ernakulensis (Gupta and Naqvi 1992), T. edmondi (Gupta and Naqvi 1992) and T. meyeri (Saxena and Gupta 2007). T. sergenti (Choquette and Gayot 1952) found infecting the intestine of dogs was reassigned as Longicollum sergenti by Golvan (1969). Later, Amin et al. (1991) redescribed L. sergenti as Paralongicollum sergenti.
Many acanthocephalans, especially members of the family Pomphorhychidae, are known for high morphological variability and plasticity. Intraspecific variations can have a genetic basis, with independent genetic lineages carrying alternate morphotypes. For endoparasites, host-body serves as the microenvironment, directly affecting them while exterior environment of the host may influence them indirectly. Divergent strains or races originate as an adaptation to altered ecological conditions or host species (Steinauer et al. 2007). Differences in microenvironment (host species, immune system, host-parasite interactions, crowding, atypical sites, etc.) can also lead to phenotypic plasticity (Stunkard 1957; Mouhaid et al. 1997; Poulin 2007; Nolan and Cribb 2005).
Earlier studies have revealed heavy infestation with Tenuiproboscis sp. in various fish species inhabiting the coastal and estuarine waters of Kerala and the pathological manifestations of this parasite in Lutjanus argentimaculatus was described in detail by Sanil et al. (2010). The aims of the present study are to characterise the morphological and molecular aspects and to resolve the species status of morphological variants in relation to different hosts. This will help to understand whether the morphological variations are caused by genetic changes or are due to epigenetic factors.
Materials and methods
Sampling and necropsy
One hundred and seventy-one fishes belonging to 10 species (L. argentimaculatus, L. ehrenbergii, Siganus javus, Epinephelus malabaricus, E. coioides, Scatophagus argus, Parascolopsis aspinosa, Caranx ignobilis, Gerres filamentosus and Lates calcarifer) coming under eight families were collected from Kozhikode (Moorad estuary) and Ernakulam (Vembanad lake) during September 2015 to April 2017 (Table 1). The fish were euthanized by standard necropsy methods, dissected and intestines removed and placed in physiological saline in petri dishes. The entire intestine was dissected longitudinally and examined for acanthocephalan parasites with a Nikon SMZ100 stereozoom microscope (Nikon, Japan). The intestine along with parasites was refrigerated for 24 h for dislodging the attached parasites and extending and everting their proboscis.
Morphology and morphometry
For microscopic studies, the worms were fixed in 70% alcohol-formalin-acetic acid solution (AFA) and stained with Mayer’s acid carmine, destained in 4% hydrochloric acid in 70% ethanol, dehydrated in ascending concentrations of ethanol (70, 80, 90 and 100%), cleared in 100% methyl benzoate and mounted in DPX. Measurements were taken using a Nikon eclipse 80i research microscope and NIS elements BR software (Nikon, Japan) and expressed in micrometres (μm) with mean ± SD followed by range in parenthesis. For scanning electron microscopy (SEM), specimens were fixed in 2% buffered glutaraldehyde for 3 h followed by 1% osmium tetroxide for 1 h, dehydrated in 30, 50, 70, 95 and 100% acetone for at least 30 min each, critical point dried (Hitachi HCP-2) and sputter coated with gold (Quorum SC7620) (Lee 1992). Coated samples were observed and photographed with a TESCAN VEGA3 scanning electron microscope (TESCAN, Brno, Czech Republic). Prevalence of infection, mean intensity, abundance and exponent of crowding were calculated using Quantitative Parasitology 3.0 software (QP3.0) (Bush et al. 1997; Rózsa et al. 2000; Reiczigel and Rózsa 2005). For morphometric studies, 171 parasites (84 males and 87 females) from 10 different fish hosts were examined.
Principal component analysis
Morphometric variations in body characters of parasites from different hosts were studied using principal component analysis (Bell and Sommerville 2002; Agustí et al. 2005; Pinacho-Pinacho et al. 2012; Ortega-Olivares et al. 2013). PCA analysis was performed with 24 and 22 morphometric variables (excluding hook size) for males and females respectively (Tables 4 and 5), using the statistics package PAST v. 3.00 (Hammer et al. 2001) with 95% confidence ellipses (n = 84 males and 87 females) to assess the inter- and intraspecific morphological variations.
Proboscis profiling
Proboscis profiler was used to analyse the morphological heterogeneity based on multivariate statistical analysis of proboscis hook dimensions (Wayland 2010, http://acanthocephala.sourceforge.net). Hook measurements were taken from 89 specimens and measurements of blade and base of each hook in one longitudinal row were taken. Dendrogram was constructed from hierarchical clustering based on principal component scores for hook length and blade base variables with hook measurements of Echinorhynchus leidyi as out-group (Wayland 2013).
Molecular analysis
Genomic DNA was isolated from parasites collected from different hosts following the method described by Aljanabi and Martinez (1997). Parasites were homogenised in 400 μl homogenising buffer (0.4 M NaCl 10 mM Tris–HCl pH 8.0 and 2 mM EDTA pH 8.0) for 10–15 s and 40 μl of 20% SDS (2% final concentration) and 8 μl of 20 mg/ml proteinase K (400 μg/ml final concentration) were added and mixed well. Samples were incubated at 55-65 °C for at least 1 h, after which 300 μl of 6 M NaCl was added to each sample. Samples were vortexed for 30 s, and tubes spun down for 30 min at 10,000 rpm. The supernatant was transferred to fresh tubes, an equal volume of isopropanol was added to each sample, mixed well, and incubated at room temperature for 20 min. Samples were then centrifuged at 8944×g for 20 min at 4 °C. Pellets were washed with 70% ethanol, dried and finally resuspended in 10–50 μl nuclease free H2O.
The ITS1 and ITS2 of the ribosomal RNA gene of the parasite was amplified by PCR using the primers BD1–5’GTCGTAACAAGGTTTCCGTA3’ and BD2–5’TATGCTTAAATTCAGCGGGT3’ (Kral’ova-Hromadova et al. 2003) with some modifications. This included 18S ribosomal RNA, internal transcribed spacer 1, 5.8S ribosomal RNA and partial internal transcribed spacer 2 regions. PCR reaction was performed in a final volume of 25 μl containing DNA template and oligonucleotide primers in Emerald Amp GT PCR master Mix. PCR was carried out in a ProFlex PCR system (Applied Biosystems, USA). Thermal cycling programme included an initial denaturation of 96.0 °C for 3 min, followed by 35 cycles of 95.0 °C for 27 s, 50 °C for 30 s, 72 °C for 40 s and a final elongation of 72 °C for 10 min. PCR products were resolved on 1.5% agarose gel stained with ethidium bromide. A total of 10 positive DNA bands corresponding to parasites isolated from 10 host species were selected, excised from the gel, purified and directly sequenced in both directions through a commercial firm (Table 1).
Phylogenetic analysis
Contigs containing partial 18S and 5.8S rDNA were generated from the sequence information, in BioEdit V 7.25 (Hall 1999) and submitted to NCBI GenBank (Table 1). The sequences were analysed by NCBI BLAST. BLAST hits were analysed manually for redundant sequences and nine closely related sequences were selected. Selected sequences were aligned by Clustal W with the sequence of Echinorhynchus gadi (accession no. EF107646) as out-group. The best fitting evolutionary model for phylogenetic analysis was determined using MEGA v.7 (Kumar et al. 2016). Based on the Bayesian and Akaike Information Criteria, Tamura 3-parameter was considered as the best fitting model and phylogenetic trees were constructed using neighbour joining (NJ) and maximum likelihood (ML) methods with 10,000 resamplings each. Percentage identity and divergence were analysed using Megalign v5.01 (DNASTAR). Nucleotide similarity and divergence analyses were performed using the same dataset.
Abbreviations used in text and figures: Lutjanus argentimaculatus (LA), L. ehrenbergii (LE), Siganus javus (SI), Epinephelus malabaricus (EM), E. coioides (EC), Scatophagus argus (SA), Parascolopsis aspinosa (PA), Caranx ignobilis (CI), Gerres filamentosus (GF), Lates calcarifer (LC).
Results
During the present study, parasites were recovered from 171 marine and brackish water fishes belonging to 10 species under eight families. Parasites were observed primarily infecting the hindgut region as seen in S. javus, E. malabaricus, P. aspinosa, E. coicoides, L. ehrenbergii, L. argentimaculatus and C. ignobilis but in heavy infections, midgut region was also infected (Fig. 1a). Extra-intestinal infections were observed in G. filamentosus with parasites restricted to peritoneal cavity while in L. calcarifer and S. argus infections were observed in both hindgut and peritoneal cavities (Fig. 1b). Parasites recovered from the peritoneal cavity of G. filamentosus were clumped together and/or attached to mesenteries. They were smaller in size, pale in colour, reproductively underdeveloped and were without any eggs. At the site of attachment, presoma of the parasite was found to pierce and damage the mucosal epithelium of the intestine as evidenced by SEM studies (Fig.5b).
Prevalence of infection in different host species varied, with L. ehrenbergii, S. javus and E. malabaricus (100% each) followed by L. calcarifer (80.00%), L. argentimaculatus (79.48%), S. argus (77.77%), G. filamentosus (72.00%), E. coioides (66.66%), P. aspinosa (56.52%) and C. ignobilis (50.00%). The overall prevalence in 10 different host fishes (N = 171) was 71.60% (95% confidence limits 63.21–79.09), intensity 5.11 (bootstrap 95% confidence limits 4.45–5.74) and abundance 3.66 (bootstrap 95% confidence limits 3.10–4.31) parasite per fish. Mean crowding was calculated to be 7.16 (95% confidence limits 6.74–7.62). Host-wise variation in prevalence, intensity and abundance is shown in Fig. 2.
Taxonomic summary
Class
Palaeacanthocephala Meyer 1931.
Order
Echinorhynchida Southwell and Macfie 1925.
Family
Pomphorhynchidae Yamaguti 1939.
Genus
Tenuiproboscis Yamaguti 1935
Host species
L. argentimaculatus (Forsskål 1775), L. ehrenbergii (Peters 1869), S. javus (Linnaeus 1766), E. malabaricus (Bloch & Schneider 1801), E. coioides (Hamilton 1822), S. argus (Linnaeus 1766), P. aspinosa (Rao & Rao 1981), C. ignobilis (Forsskål 1775), G. filamentosus (Cuvier 1829) and L. calcarifer (Bloch 1790).
Locality
Kozhikode (Moorad estuary) and Ernakulam (Vembanad lake).
Site of infection
Hindgut and midgut region of intestine, peritoneal cavity.
Period of collection
September 2015 to April 2017.
Prevalence
71.6%.
Intensity
2–108 worms/host
Etymology
Species name derived from ‘Kerala’, the geographical location from where the type specimen was collected.
Type material
Voucher specimens deposited in the parasite collections of Marine Biodiversity Museum, ICAR-Central Marine Fisheries Research Institute, Kochi (Accession No. CG.1.1.1). Partial sequence of ITS rDNA gene of the parasite deposited in NCBI GenBank (Accession numbers KU726597 (C. ignobilis), KU726598 (E. coioides), KU726599 (L. argentimaculatus), KU726600 (L. ehrenbergii), KU726601 (S. argus), KU726602 (G. filamentosus), KU726603 (L. calcarifer), KU726604 (P. aspinosa), KU726605 (S. javus), KU936060 (E. malabaricus)).
Morphological description
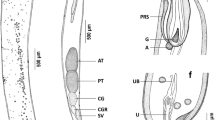
Metasoma elongate, cylindrical, bulbous anteriorly and gradually tapering towards posterior and characterised by yellow to orange colouration (Fig. 1). Proboscis long, cylindrical, claviform and covered with numerous hooks, arranged longitudinally in 14–15 rows, each row equipped with 14–15 hooks (Figs. 3, 4 and 5). Two specimens with 15 rows of 15 hooks each were recovered from S. argus. Hooks curved in shape and variable in size; roots of hooks 1 to 3 rod-shaped, 4 to 5 with slightly bifid posterior tips, 6 to 13 with pointed posterior tips and 14 with a bifurcated posterior tip. Size of hooks decreases progressively from second to fourth row, remains almost uniform from 5th to 13th rows, while hooks on 14th row appeared straight and large (Fig. 4). Measurements of hooks in first, second, third and fourth rows are 36.14–52.14 × 8.01–13.47 (47.61 × 11.79) μm, 32.44–57.17 × 8.16–15.90 (47.00 × 13.75) μm, 26.87–50.21 × 9.69–19.70 (37.29 × 16.27) μm and 20.23–46.03 × 6.98–20.72 (33.35 × 13.69) μm respectively, those from fifth to thirteenth rows measured 24.69–44.95 × 5.98–15.54 (36.01 × 11.24) μm while that of the fourteenth row measured 35.98–66.04 × 10.18–10.41 (51.62 × 12.96) μm. Proboscis receptacle long, bulbous and double walled. Lemnisci two, equal, digitiform and equal to proboscis receptacle in length (Fig. 3a). Proboscis of males measured 385.50–868.54 μm (604.35 ± 102.01) in length and 128.66–336.25 μm (209.33 ± 42.02) in width at its widest point. Proboscis is followed by a long neck, devoid of hooks. Body of male measured 3234.89–8644.20 μm (5729.50 ± 1176.60) in length and 388.30–1584.61 μm (795.88 ± 184.12) in width. Testes oval in shape, tandem, pre-equatorial; anterior testis measured 153.92–699.61 (316 ± 118.70) × 69.53–433.30 (154.00 ± 69.34) μm while the posterior one measured 162.21–669.15 (315.20 ± 105.90) × 65.92–320.08 (159.80 ± 58.79) μm in size (Fig. 3a). Cement glands six in number, pyriform, bunched together in the posterior half of trunk. Saefftigen’s pouch is located below the cement glands, pyriform in shape (Fig. 3a, c). Copulatory bursa ventral, well defined and possessed numerous papillae on its rim as evidenced by SEM studies (Fig. 5c, d). Body of female larger than male, measured 3898.16–10,345.00 μm (6430 ± 1417.3) in length and 458.95–1435.68 μm (929.81 ± 250.39) in width. Proboscis measured 405.06–940.74 μm (670.52 ± 113.05) in length and 173.03–305.03 μm (237.90 ± 44.59) in width. Ovarian balls numerous, round to elliptical in shape (Fig. 3b). Uterine bell elongated, broad when filled with ovarian balls and eggs. Uterus conical in shape, followed by vagina with a well-developed sphincter (Fig. 3b, d). Female genital pore terminal, circular (Figs. 3b and 5e). Mature eggs spindle-shaped, possessed inner, middle and outer shells with the middle shell having polar prolongations (Figs. 3e and 6c), have smooth surface at the centre while reticulations/striations were observed towards the polar ends (Fig. 5f). Eggs measured 21.46–49.8 μm (34.12 ± 6.79) in length and 4.55–9.63 μm (6.30 ± 1.11) in width. Immature eggs at various stages of development and enclosed an embryonic nuclear mass (Fig. 6) while fully mature eggs harboured acanthor larvae (Figs. 3e and 6d). Morphometric variations observed in parasites (male and female) recovered from different fish hosts are given in Tables 4 and 5, respectively.
Line drawings of T. keralensis n. sp. a mature male, b mature female, c posterior end of male, d posterior end of female and e mature egg. (at anterior testis; pt. posterior testis; cg cement glands; sp. Saefftigen’s pouch; cb copulatory bursa; l lemnisci; pr proboscis receptacle; ob ovarine balls; e eggs; gp gonopore; u uterus; vs vaginal sphincter; v vagina; a acanthor; em embryonic mass; is inner shell; ms middle shell; os outer shell)
SEM images of T. keralensis n. sp. a proboscis showing the arrangement of hooks, b damaged surface of intestine (asterisks indicates parasite), c posterior end of male showing the everted bursa, d copulatory bursa showing papillae on its rim (arrow heads), e posterior end of female showing gonopore (arrow) and f mature egg showings surface striations (arrows)
Principal component analysis (PCA)
PCA analysis based on 24 and 22 morphological characters of males and females respectively from different fish hosts indicate very high overlapping. Parasites recovered from G. filamentosus showed least variations while those from S. argus showed maximum variations in size (Fig.7a, b).
Principle component analysis (PCA). a PCA of 84 male specimens of T. keralensis n. sp. b PCA of 87 female specimens of T. keralensis n. sp. Host species denoted by following colours—L. calcarifer (aqua), S. argus (blue), S. javus (brown), E. malabaricus (grey), P. aspinosa (pink), E. coioides (gold), L. ehrenbergii (black), L. argentimaculatus (green), G. filamentosus (blue) and C. ignobilis (orange)
Proboscis profiling
Blade length and base width of proboscis hooks from 89 samples were plotted against a standardised position and proboscis profiles were generated with a moving average segment of 9. In the dendrogram, parasites from different hosts formed a distinct clade with E. leidyi as out-group. Tenuiproboscis clade was further sub-divided into numerous clusters and sub-clusters (Fig. 8). Blade length and blade base profile plots of hooks did not show wide variations in hook size and positioning (Figs. 9a, b and 10a, b). Though parasites with 15 rows of hooks showed positional variation on the proboscis, length-wise and base width-wise they did not show any variation from those with 14 rows of hooks (Fig. 9a,b).
Molecular analysis
In BLAST analysis, the sequences showed 97 to 100% identity among themselves and 99% identity with other two Tenuiproboscis sp. sequences (accession no. JF694277 from L. argentimaculatus and JF694274 from E. malabaricus). The next closest sequence was that of Pomphorhynchus laevis which showed 81 to 82% identity. Percentage identity and divergence analysis using Megalign v5.01 showed 0.2 to 1.1 divergence and 98.3 to 100% identity, respectively (Table 6).
Phylogenetic analyses
Using concatenated ITS1 and ITS2 sequences, NJ and ML trees gave similar topologies. However, the position of some sequences varied within the Tenuiproboscis clade. Sequences of T. keralensis n. sp. from multiple hosts clustered together with high bootstrap values along with other two Tenuiproboscis sp. sequences (JF694277 and JF694274) obtained from GenBank, forming a monophyletic group. The other closest sequences in BLAST analysis were that of Pomphorhynchus which stands out as a distinct cluster in the present analysis (Figs. 11 and 12).
Discussion
The present study describes a new species of Tenuiproboscis infecting marine and brackish water fishes inhabiting the south-west coast of India. Prevalence of parasites in different hosts varied from 50 to 100% throughout the study period (Fig. 2). Sanil et al. (2010) have reported prevalence varying from 57 to 100% in L. argentimaculatus for this parasite. Sakthivel et al. (2016) observed a prevalence of 63.84% for an acanthocephalan species in C. ignobilis from Nagapattinam coast while Martins et al. (2001) recorded 83.30% prevalence for Neoechinorhynchus curemai in Prochilodus lineatus . Taraschewski (2005) reported prevalence varying from 15 to 84% for Acanthocephalus anguillae in different fish hosts and observed that prevalence was independent of seasons. The intensity of infection in the present study varied from 2 to 108 worms per host while Sanil et al. (2010) observed it to be 4 to 268 worms per host for the same parasite in L. argentimaculatus suggesting over dispersion which is quite common in acanthocephalans (Kennedy 2006). The high overall prevalence of 71.6% shown by T. keralensis n. sp. is an indication of low host specificity, easy availability of intermediate hosts and high transmission potential coupled with conducive ecological conditions prevailing in the habitats (Sanil et al. 2010; Wayland 2013). Variations in intensity, abundance and mean crowding can be attributed to the availability of infected intermediate hosts, spatial aggregation of infective stages and susceptibility of hosts (Stunkard 1957). Sanil et al. (2010) have described the pathology caused by Tenuiproboscis sp. in L. argentimaculatus from the south-west coast of India in detail. Though the authors have discussed the prevalence and intensity of infections, the taxonomic status of the parasite was not studied.
Taxonomy and species delimitation in acanthocephalans have been largely based on morphology and morphometry. However, there have been many instances where morphology alone cannot resolve taxonomic ambiguities, especially when dealing with phenotypic plasticity and cryptic species (Nolan and Cribb 2005). Statistical tools like Proboscis profiler and PCA are often used to study the degree of plasticity and analyse inter- and intraspecific differences (Wayland 2010). Molecular phylogeny approach provides a better insight in understanding host-induced morphological changes and cryptic forms (Herlyn et al. 2003; Verweyen et al. 2011; Abdel-Ghaffar et al. 2014). Hence, a multipronged approach where morphology, morphometry and molecular systematics are used in tandem is always preferable (Miller and Cribb 2013).
Yamaguti (1935) erected the Genus Tenuiproboscis with T. misgurni infecting Misgurnus fossilis as type species. Members of the genus are characterised by filiform to claviform proboscis with several longitudinal rows of hooks, long neck without bulbous swelling, 4–6 cement glands and eggs with polar prolongations. Presently, this genus includes seven valid species, T. misgurni (Yamaguti 1935), T. guptai (Gupta and Sinha 1989), T. clupei (Gupta and Sinha 1991), T. bilqueesae (Gupta and Naqvi 1992), T. ernakulensis (Gupta and Naqvi 1992), T. edmondi (Gupta and Naqvi 1992) and T. meyeri (Saxena and Gupta 2007). Golvan (1969) reassigned T. sergenti (Choquette and Gayot 1952) as L. sergenti and Amin et al. (1991) further created a new genus Paralongicollum and redescribed it as P. sergenti. Gupta and Naqvi (1992) reported T. sergenti from the marine fish, Pristipoma gouraka, overlooking the fact that T. sergenti is no longer included under genus Tenuiproboscis. Morphological characters place the present acanthocephalan under the genus Tenuiproboscis. But, it differs significantly from other members of the genus in the number, shape and arrangement of hooks, in the number of cement glands and in morphometrics. In the number and arrangement of hooks, the present species (14–15 rows with 14–15 hooks) differs from T. misgurni (9 rows with 18–19 hooks), T. guptai (16–17 rows), T. clupei (14–16 rows with 10 hooks), T. bilqueesae (11–12 rows with 13–14 hooks), T. ernakulensis (13–14 rows with 15–16 hooks), T. edmondi (18 rows with 17 hooks) and T. meyeri (12–14 rows with 14–15 hooks). It also differs from all other species except T. guptai in the size of hooks. Though T. misgurni, T. meyeri and T. clupei have six cement glands each, the present form can be differentiated from them based on the size and shape of the glands. Further, it differs from all the above species in morphometrics (Tables 2 and 3).
BLAST analysis of nucleotide sequences showed 97 to 100% identity among themselves and 99% identity with other two Tenuiproboscis sp. sequences (accession nos. JF694277 and JF694274) indicating their closeness while sequences of P. laevis, the next closest sequence, showed only 81 to 82% identity. Submissions were not available for other previously reported species of Tenuiproboscis in GenBank. Molecular analysis revealed that isolates from all the 10 fish hosts showed only 78.1 to 79.2% molecular identity and 19.2 to 19.6 diversity with the closest reference sequences of P. laevis, strongly suggesting a separate species status. A difference of 14 nucleotides was observed among the 10 ITS rDNA sequences generated for T. keralensis n. sp. Further, in phylogenetic trees (NJ and ML), sequences of the present species formed a well separated, monophyletic clade, with high bootstrap value (91 and 99 for NJ and ML, respectively), supporting the creation of a new species. Based on the morphology, morphometry and molecular analysis, the present parasite is distinctly different from all previously described forms, hence treated as a new species and named Tenuiproboscis keralensis n. sp.
Morphological/morphometric variations were observed in T. keralensis n. sp. recovered from various fish hosts. But, in spite of these differences, evidence from PCA based on morphological characters indicates significant overlapping among parasites from different hosts indicating their conspecificity. T. keralensis n. sp. recovered from G. filamentosus was the smallest, with underdeveloped reproductive system and exhibited least variations in PCA. The worms are confined to the peritoneal cavity which appears to be an abnormal site and the reason for this is not known. In normal conditions, the worms obtain nutrition from digested food in the gut lumen while in an abnormal site like peritoneal cavity, limited nutrients in the peritoneal fluid may help the parasite to survive but may not be sufficient for its growth and reproductive development as indicated by the absence of eggs. Further, immune responses in the peritoneal cavity and absence of host-derived, growth promoting factors if any, may also have contributed to the suppressed growth (Escobedo et al. 2005). Pale colouration of the parasites inhabiting the peritoneal cavity further reflects the non-availability of carotenoids which otherwise imparts strong yellow to orange colours in worms inhabiting the lumen. Proboscis profiling of hooks indicates that T. keralensis n. sp. from different hosts formed a distinct clade with several sub-clusters, in spite of the apparent morphometric differences (Fig. 9a, b).The blade length and blade base profile plots (Fig. 10a, b) also did not show variations in hook size and positioning, pointing to the conspecific nature of parasites. Though two worms with 15 rows of 15 hooks each, showed positional variation, their blade length and blade base profiles were similar to that of others with 14 hooks, indicating morphological plasticity. These parasites with 15 hooks were recovered only from S. argus and surprisingly, the parasites from this host showed maximum variations in morphometry (Tables 4 and 5). In spite of the morphometric variations, molecular analysis and phylogeny revealed that isolates of T. keralensis n. sp. from all the 10 fish hosts showed high identity (98.6–100%) and low divergence (0.2–1.2) indicating their conspecific nature (Table 6).
Both parasites and their hosts in the present study share common habitats, favouring cross infections and hence, chances for genetic differences between the parasites should be low. This indicates that morphometric variations could be host-induced and many authors have stressed that age, sex, host species and geographical location can alter morphological characters in acanthocephalans (Amin and Redlin 1980; Shostak et al. 1986). Poulin (2007) observed that differences in microenvironment (i.e. host species, immune system, host-parasite interactions) can induce phenotypic plasticity in the form of differences in body size or fecundity. Such phenotypic effects are often considered species-specific and may result in misidentifications and even taxonomic chaos in some groups (Nolan and Cribb 2005). Stunkard (1957) suggested that intensity of infection or ‘crowding effect’ could induce phenotypic variations while Mouhaid et al. (1997), observed development in atypical hosts could induce morphological variations in parasitic helminths. Steinauer et al. (2007) have opined that some species are broad generalists that can exploit a variety of environments or hosts and variations are due to different ecological or physiological environments. Hildebrand et al. (2015) during their studies on the echinostome, Isthmiophora melis in various host species opined that morphological traits are highly variable and host-dependent and stressed the importance of molecular analysis while describing new species or genera. In the present study though morphological/morphometric variations exist, PCA, proboscis profiling and molecular analysis clearly indicate that T. keralensis n. sp. recovered from various fish hosts are conspecific.
References
Abdel-Ghaffar F, Morsy K, Abdel-Gaber R, Mehlhorn H, Al Quraishy S, Mohammed S (2014) Prevalence, morphology and molecular analysis of Serrasentis sagittifer (Acanthocephala: Palaeacanthocephala: Rhadinorhynchidae), a parasite of the gilthead Sea bream Sparus aurata (Sparidae). Parasitol Res 113(7):2445–2454
Agustí C, Aznar FJ, Raga JA (2005) Tetraphyllidean plerocercoids from western Mediterranean cetaceans and other marine mammals around the world: a comprehensive morphological analysis. J Parasitol 91:83–92
Aljanabi SM, Martinez I (1997) Universal and rapid salt-extraction of high quality genomic DNA for PCR-based techniques. Nucleic Acids Res 25(22):4692–4693
Amin OM, Redlin MJ (1980) The effect of host species on growth and variability of Echinorhynchus salmonis Müller, 1784 (Acanthocephala: Echinorhynchidae), with special reference to the status of the genus. Syst Parasitol 2:9–20
Amin OM, Bauer ON, Sidorovt EG (1991) The description of Paralongicollum nemacheili n. gen., n. sp. (Acanthocephala: Pomphorhynchidae) from freshwater fishes in Kazakh S.S.R. J Parasitol 77(1):26–31
Bell AS, Sommerville C (2002) Molecular evidence for the synonymy of two species of Apatemon szidat, 1928, A. gracilis (Rudolphi, 1819) and A. annuligerum (von Nordmann, 1832) (Digenea: Strigeidae) parasitic as metacercariae in British fishes. J Helminthol 76:193–198
Bush AO, Lafferty KD, Lotz JM, Shostak AW (1997) Parasitology meets ecology on its own terms: Margolis et al. revisited. J Parasitol 83:575–583
Choquette LPE, Gayot G (1952) Tenuiproboscis sergenti nov. sp. Acanthocephala trouve chez le chien a. Alger Arch Inst Pasteur Algeria 30:51–54
Escobedo G, Roberts CW, Carrero JC, Morales-Montor J (2005) Parasite regulation by host hormones: an old mechanism of host exploitation? Trends Parasitol 21(12):588–593
Golvan YJ (1969) Systematique des Acanthocephales (Acanthocephala Rudolphi, 1801), L’ordre des Palaeacanthocephala Meyer, 1931, La superfamille des Echinorhynchidea (Cobbold, 1876) Golvan et Houin 1963. Mém Mus Nat Hist 47:1–373
Gupta SP, Naqvi M (1992) On four species of the genus Tenuiproboscis Yamaguti, 1935 (Acanthocephala: Pomphorhynchidae) from marine fishes of Kerala. Indian J Helminthol 44(1):17–26
Gupta V, Sinha G (1989) On a new Acanthocephala Tenuiprobocis guptai sp. nov. (Pomphorhynchidae, Yamaguti, 1939) from a marine fish Gerres setifer Ham. From Calcutta, West Bengal. Indian J Helminthol 41(2):104–107
Gupta V, Sinha G (1991) On some acanthocephalan parasites from marine fishes of Bay of Bengal, Puri coast, Orissa. Indian J Helminthol 43(2):108–118
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl Acids Symp Ser 41:95–98
Hammer O, Harper DAT, Ryan PD (2001) PAST: Paleontological Statistics software package for education and data analysis. Palaeontol Electronica 4, 9 pp.
Herlyn H, Piskurek O, Schmitz J, Ehlers U, Zischler H (2003) The syndermatan phylogeny and the evolution of acanthocephalan endoparasitism as inferred from 18S rDNA sequences. Mol Phyl Evol 26:155–164
Hildebrand J, Adamczyk M, Laskowski Z, Zaleśny G (2015) Host-dependent morphology of Isthmiophora melis (Schrank, 1788) Luhe, 1909 (Digenea, Echinostomatinae)—morphological variation vs. molecular stability. Parasit Vectors 8:481
Kennedy CR (2006) Ecology of the Acanthocephala. Cambridge University Press, Cambridge, pp 75–124
Kral’ova-Hromadova I, Tietz DF, Shinn AP, Spakulova M (2003) ITS rDNA sequences of Pomphorhynchus laevis (Zoega in Muller, 1776) and P. lucyi Williams and Rogers, 1984 (Acanthocephala: Palaeacanthocephala). Syst Parasitol 56:141–145
Kumar S, Stecher G, Tamura K (2016) MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol Biol Evol 33(7):1870–1874
Lee RE (1992) Scanning electron microscopy and X-ray microanalysis. Prentice Hall, Englewood Cliffs, 458 pp
Maghami SSG, Khanmohammadi M, Kerdeghari M (2008) Serrasentis sagittifer (Acanthocephala: Rhadinorhynchidae) from the Japanese thread fin bream, Nemipterus japonicus in Bushehr waters of Persian Gulf. J Anim Vet Adv 7(11):1430–1433
Martins ML, Moraes FR, Fujimoto RY, Onaka EM, Quintana CIF (2001) Prevalence and histopathology of Neoechinorhynchus curemai Noronha, 1973 (Acanthocephala: Neoechinorhynchidae) in Prochilodus lineatus Valenciennes, 1836 from Volta Grande Reservoir MG Brazil. Braz J Biol 61(3):517–522
Miller TL, Cribb TH (2013) Dramatic phenotypic plasticity within species of Siphomutabilus n. g. (Digenea: Cryptogonimidae) from Indo-Pacific caesionines (Perciformes: Lutjanidae). Syst Parasitol 86:101–112
Mouhaid G, Casanova JC, Moné H (1997) Plasticidad fenotípica y determinacion sistemática de parásitos: el caso de Echinoparyphium elegans. Acta Parasitol Portuguesa 4:127
Nickol BB (2006) Phylum Acanthocephala. In: Woo PTK (ed) Fish diseases and disorders, Second Edition, Protozoan and Metazoan Infections, CAB International, vol I, Wallingford, pp 444–465
Nolan MJ, Cribb TH (2005) The use and implications of ribosomal DNA sequencing for the discrimination of digenean species. Adv Parasitol 60:102–163
Ortega-Olivares MP, Rosas-Valdez R, García-Varela M (2013) First description of adults of the type species of the genus Glossocercus Chandler, 1935 (Cestoda: Gryporhynchidae). Folia Parasitol 60(1):35–42
Pinacho-Pinacho CD, Pérez-ponce Deleón G, Garcíavarela M (2012) Description of a new species of Neoechinorhynchus (Acanthocephala: Neoechinorhynchidae) a parasite of Dormitator latifrons from southwestern Mexico based on morphological and molecular characters. Parasitol Int 6:634–644
Poulin R (2007) Evolutionary ecology of parasites. Princeton University Press, Princeton
Reiczigel J, Rózsa L (2005) Quantitative parasitology 3.0. Budapest (http://www.zoologia.hu/qp/qp)
Rózsa L, Reiczigel J, Majoros G (2000) Quantifying parasites in samples of hosts. J Parasitol 86:228–232
Sakthivel A, Selvakumar P, Gopalkrishnan A (2016) Acanthocephala (Acanthocephalus lucii) infection on Caranx ignobilis from Nagapattinam, South east coast of India. Indian J Geo-Mar Sci 45(3):448–452
Sanil NK, Asokan PK, John L, Vijayan KK (2010) Pathological manifestations of the acanthocephalan parasite, Tenuiproboscis sp. in the mangrove red snapper (Lutjanus argentimaculatus) (Forsskål, 1775), a candidate species for aquaculture from Southern India. Aqua 310:259–266
Santos CP, Gibson DI, Tavares LER, Luque JL (2008) Checklist of Acanthocephala associated with the fishes of Brazil. Zootaxa 1938:1–22
Saxena AM, Gupta R (2007) On a new species of the genus Tenuiproboscis meyeri from a marine fish Scatophagus argus (Cuv. & Val.) at Deegha, West Bengal. Indian J Helminthol 25:35–40
Shostak AW, Dick TA, Szalai AJ, Bernier LMJ (1986) Morphological variability in Echinorhynchus gadi, Echinorhynchus leidyi and Echinorhynchus salmonis (Acanthocephala: Echinorhynchidae) from fishes in northern Canadian waters. Can J Zool 64(4):985–995
Steinauer ML, Nickol BB, Ortí G (2007) Cryptic speciation and patterns of phenotypic variation of variable acanthocephalan parasite. Mol Eco 16:4097–4109
Stunkard HW (1957) Intraspecific variation in parasitic flatworms. Syst Zool 6:7–18
Taraschewski H (2005) Acanthocephala (thorny spiny-headed worms). In: Rhode K (ed) Marine parasitology. CSIRO Publishing, Collingwood, pp 116–121
Verweyen L, Klimpel S, Palm HW (2011) Molecular phylogeny of the Acanthocephala (Class Palaeacanthocephala) with a paraphyletic assemblage of the orders Polymorphida and Echinorhynchida. PLoS One 6(12):e28285
Wayland MT (2010) Proboscis profiler: a tool for detecting acanthocephalan morphotypes. Syst Parasitol 76(3):159–167
Wayland MT (2013) Morphological variation in Echinorhynchus truttae Schrank, 1788 and the Echinorhynchus bothniensis Zdzitowiecki & Valtonen, 1987 species complex from freshwater fishes of northern Europe. Biodivers Data J 1:e975. https://doi.org/10.3897/bdj.1.e975
Yamaguti S (1935) Studies on the Helminth fauna of Japan Acanthocephala. Jap J Zool 6:1–247
Acknowledgements
The authors are thankful to Madhya Pradesh Council of Science and Technology for FTYS fellowship to one of the authors (PK) and to The Director, ICAR-Central Marine Fisheries Research Institute, Kochi for providing facilities to carry out the present work. The authors thank Dr. T.V. Sathianandan for statistical analysis and Mr. David for line drawings. We also thank the anonymous reviewers who have contributed to the improvement of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kaur, P., Shamal, P., Chandran, A. et al. Morphometric and molecular characterisation of Tenuiproboscis keralensis n. sp. infecting marine and brackish water fishes from the south-west coast of India with a note on morphological plasticity. Parasitol Res 116, 3131–3149 (2017). https://doi.org/10.1007/s00436-017-5628-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-017-5628-2