Abstract
Trematodes of the family Orientocreadiidae are mostly parasites of freshwater fishes. Here, the phylogenetic position of this family is inferred based on the partial 28S rDNA sequence from a representative of the genus Orientocreadium s. str.–О. pseudobagri Yamaguti, 1934. Sequences were analysed by maximum likelihood and Bayesian inference algorithms. Both approaches placed the Orientocreadiidae within a clade corresponding to the superfamily Plagiorchioidea and supported the family Leptophallidae as a sister taxon.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Adult orientocreadiid trematodes typically inhabit the intestine of freshwater Eurasian and African siluriform and perciform fishes, but some species parasitise Indian terrestrial reptiles (Beverley-Burton 1962; Yamaguti 1971; Hafeezullah 1989). The systematic position of this group of parasites has repeatedly been discussed in the literature (e.g. Tubangui 1931; Yamaguti 1958; Skrjabin and Koval 1963; Fischthal and Kuntz 1963; Sirikantayakul 1985). According to the current point of view (Jones and Bray 2008), these trematodes belong to the separate family Orientocreadiidae Yamaguti 1958 within the superfamily Plagiorchioidea. This opinion has developed based on Fischthal and Kuntz (1963) taxonomic analysis of adult orientocreadiids and data on the morphology of Оrientocreadium batrachoides Tubangui, 1931 and Оrientocreadium pseudobagri Yamaguti, 1934 cercariae (Tang and Lin 1973; Besprozvannykh 1984). According to Jones and Bray (2008), the Orientocreadiidae is a monogeneric family with the following list of invalidated generic taxa that are congeneric with its type-genus—Оrientocreadium Tabangui, 1931, Ganada Chatterji, 1933, Neoganada Dayal, 1938, Nizamia Dayal, 1938, Ganadotrema Dayal, 1949, Macrotrema Gupta, 1951 nec Regan, 1912 and Paratormopsolus Bychowsky et Dubinina 1954. A total of 28 nominal species of orientocreadiids have been described (Yamaguti 1971; Agrawal and Sharma 1990; Shimazu 1990; Kim and Rim 1995; Besprozvannykh et al. 2009; Nigam et al. 2015); however, the validity of many of them is questionable (Hafeezullah 1989). Sequences of 28 rDNA have been used successfully as a data source for phylogenetic reconstruction within the superfamily Plagiorchioidea (Tkach et al. 1999, 2000a, b, 2001a, b; Pérez-Ponce de León et al. 2011; Hernández-Mena et al. 2016). In this paper, we investigate the phylogenetic position of the Orientocreadiidae inferred from the same fragment of DNA from one of the representatives of the genus Orientocreadium s. str.–О. pseudobagri.
Materials and methods
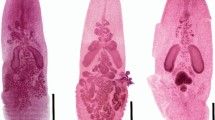
Specimens of О. pseudobagri (Fig. 1) were recovered from the intestine of Perccottus glenii (Dybowski, 1877) (Actinopterygii, Odontobutidae), caught in July 2010 in the water body with the working name “Ozero 1”, Primorsky Kray, Russia (Sokolov 2013). Trematodes were fixed in 70% ethanol and stained with acetocarmine. Some specimens were fixed in 96% ethanol for further molecular analysis. Trematode species were identified with the aid of the publications of Yamaguti (1934), Shimazu (1990, 2014), Kim and Rim (1995), Besprozvannykh et al. (2009) and Shimazu et al. (2011).
In order to obtain 28 rDNA sequence, total DNA was isolated with a ZymoBead Genomic DNA Kit (http://www.zymoresearch.com). Only single trematode specimens were used for each DNA extraction. The DNA fragment of about 1200 bp localised at the 5′ end of 28 rDNA was amplified using the BIO-RAD C1000 Thermal Cycler. PCR were performed in a total volume of 20 μl (11.5 μl) H20, 2.5 μl Taq buffer, 2 μl dNTP at concentration 10 pM, 0.5 μl of each primer at concentration 10 pM, 1 μl of Taq polymerase (“Syntol”) and 1 μl of DNA template.
Trematode-specific forward primer LSU-5 (5′-TAG GTC GAC CCG CTG AAY TTA AGC A-3′) and reverse primer 1500R (5′-GCT ATC CTG AGG GAA ACT TCG-3′) were used. Genbank numbers of sequences used in analysis are provided in the Table 1. Thermal cycle parameters were as follows: initial denaturation at 95 °C (3 min); 35 cycles of 20 s at 95 °C; 20 s at 56 °C; 120 s at 72 °C; 5 min at 72 °C for final extension. Amplicons were purified using Cleanup mini Purification Kit (Eurogene). All amplicons were sequenced directly using the equipment of the Research Park of Saint-Petersburg State University (Centre for Molecular and Cell Technologies). Sequences from both forward and reverse primers were assembled using Chromas Pro 1.7.4.
Obtained sequences were included in the general alignment (Table 1). In total, 133 sequences (in addition to the newly obtained one) were used for alignment. First, sequences were automatically aligned using Muscle algorithm (Edgar 2004), as implemented in SeaView 4.0 (Gouy et al. 2010), followed by manual alignment verification. The phylogenetic analysis was performed using the maximum likelihood method (ML) with GTR + G + I model. In total, about 1100 sites were selected for the analysis. The ML phylogenetic tree was obtained using RaxML program (Stamatakis 2006) at CIPRES Science Gateway (www.phylo.org) (Miller et al. 2010). The stability of clades was assessed using a non-parametric bootstrap with 1000 pseudoreplicates. All model parameters were estimated from the data. Bayesian inference analysis (BI) was performed using MrBayes 3.1.2, GTR model with gamma correction for inter-site rate variation (8 categories) and the covarion model. Trees were run as two separate chains (default heating parameters) for 15 million generations at which point they had ceased converging. The quality of chains was estimated using built-in MrBayes tools and additionally using Tracer 1.6 (Rambaut et al. 2014). Based on the estimates by Tracer, 50,000 generations were discarded for burn-in (relative burn-in parameter was switched off).
Results
The general topology of the trees constructed by ML and BI was almost coincided (Fig. 2). Incongruent branches are labelled with an asterisk (*). In most of the cases, the mismatches of branching in ML and BI are caused by settings of BI analysis (because all trees with tripartitions were excluded).
Bayesian tree of the Orientocreadiidae based on the analysis of 28S rDNA partial sequences. Nodal numbers are indicated: bootstrap value to the left from slash mark and Bayesian statistics to the right; only significant values are shown (above 80% for bootstrap value and 0.9 for BI). Sequence of Echinostoma revolutum is used as outgroup
Phylograms from both ML and BI placed the Orientocreadiidae (=O. pseudobagri) within a major clade corresponding to the superfamily Plagiorchioidea (see Table 1) with the family Leptophallidae supported as the sister taxon (Fig. 2). In turn, the clade of Orientocreadiidae + Leptophallidae has a strongly supported sister relationship with the clade of the Alloglossidiidae. The monophyletic clade uniting orientocreadiids, leptophallids and alloglossidiids grouped into a large weakly supported clade containing members of the families Brachycoeliidae, Choanocotylidae, Glypthelminthidae, Auridistomidae and Macroderoididae. In all cases, the Auridistomidae and Macroderoididae appeared sister groups, as did the Brachycoeliidae and Choanocotylidae + Glypthelminthidae (with strong and weak support, respectively). BI analysis revealed that the group of Auridistomidae + Macroderoididae is aggregated with the Alloglossidiidae + (Orientocreadiidae + Leptophallidae) clade, while the Brachycoeliidae + (Choanocotylidae + Glypthelminthidae) clade occupied a sister position to all the former mentioned trematodes. In ML analysis, Auridistomidae + Macroderoididae appear as a weakly supported sister clade to that formed by the brachycoeliids, choanocotylids and glypthelminthids.
Other plagiorchioid trematodes analysed in the phylogenetic reconstruction are the Haematoloechidae, Omphalometridae, Plagiorchiidae, Telorchiidae, Cephalogonimidae, Reniferidae and the genera Choledocystus Pereira et Cuocolo, 1941, Infidum Travassos, 1916, and Rauschiella Babero, 1951 (as Plagiorchioidea incertae sedis by Hernández-Mena et al. (2016) and Martínez-Salazar et al. (2016)) are basal taxa to orientocreadiids. The families Haematoloechidae, Omphalometridae, Plagiorchiidae, Reniferidae and the clade of Choledocystus + Infidum are resolved inside it as well-supported monophyletic groups. In the same time, intergeneric relationships of the telorchiids and cephalogonimids were poorly resolved with the exception of node Cephalogonimus retusus (Dujardin, 1845)/Opisthioglyphe ranae (Frölich, 1791) in BI analysis. Position of the genus Rauschiella on phylograms that are produced by different methods is unstable.
Discussion
Our molecular data reveal a close phylogenetic relationship between orientocreadiids and both alloglossidiids and leptophallids, which justifies the placement of the Orientocreadiidae in the Plagiorchioidea. Previously, Hernández-Mena et al. (2016) showed molecular evidence of phylogenetic affinity between alloglossidiids and leptophallids. Analysis of the sequences obtained in the present study demonstrated a closer relationship between leptophallids with orientocreadiids than with the alloglossidiids. Dayal (1938) was the first to notice that orientocreadiids (at that time attributable to the genera Ganada, Neoganada and Nizamia) are morphologically close to leptophallids. An indisputable synapomorphy of the Orientocreadiidae + Leptophallidae clade is the presence of an external seminal vesicle. The external seminal vesicle in both families is unipartite, tubular or saccular, without associated gland cells (Tkach et al. 1999; Shimazu 2014). Within the Plagiorchioidea, this organ is characteristic only of representatives of the said clade (Bray 2008). In general, this structure is not unique to plagiorchioids and appears with varying frequency in other superfamilies of trematodes, in particular, in the Opecoeloidea and Lepocreadioidea. The most significant morphological difference between adult leptophallids and orientocreadiids is the presence of a canalicular seminal receptacle in the Leptophallidae. In orientocreadiids, there is a uterine seminal receptacle (Bray 2008).
Leptophallid and orientocreadiid trematodes are parasites of different groups of vertebrates. Adult leptophallids are parasites of the intestine or lungs of snakes (Tkach 2008) and orientocreadiids mainly parasitise the intestine of the ray-finned fishes (Actinopterygii). Only two species of the family have been described from terrestrial lizards (Hafeezullah 1989), one of which—Orientocreadium ottoi Agrawal, 1966—is considered by some authors as conspecific with О. batrachoides, a parasite of the catfishes (Pandey 1970; Hafeezullah 1989).
The first intermediate hosts of the trematode groups in question are pulmonate snails—Lymnaeidae for orientocreadiids (Tang and Lin 1973; Besprozvannykh 1984; Sirikantayakul 1985; Besprozvannykh et al. 2009), and Lymnaeidae or Planorbidae for leptophallids (Grabda-Kazubska 1963; Dobrovolʼski 1969). Xiphidiocercariae of orientocreadiids and leptophallids are similar in general morphology. These larvae have a relatively large bodies (about 0.3 mm in length), anterior organs with a stylet of an “open type” (the small bulb is not covered), and a relatively long prepharynxes (Tkach et al. 1999; Besprozvannykh et al. 2009). The excretory bladder in orientocreadiid and many leptophallid cercariae is Y-shaped, thick-walled, with the terminal mouths of the main collecting ducts (Tkach et al. 1999; Besprozvannykh et al. 2009). Only in representatives of the genus Macrodera Lühe, 1899 do the main collecting ducts open into the arms subterminally (Tkach et al. 1999). The protonephridial formula is 2[(3 + 3 + 3) + (3 + 3 + 3)] = 36 in all known cercariae in both families. Cercariae of leptophallids, however, have 4 or 8 pairs of non-differentiated penetration glands (Tkach et al. 1999), whereas only 3 or 5 pairs of penetration glands have been reported for orientocreadiid cercariae (Tang and Lin 1973; Besprozvannykh et al. 2009).
General topology of orientocreadiid cercarial sensillae demonstrates great similarities with other plagiorchioid trematodes including leptophallids (Besprozvannykh et al. 2009). The presence of numerous sensillae on the anterior end of O. pseudobagri cercariae (well expressed “C”-circles and groups of “St”), as well as AID row (equal to StD3 group in Besprozvannykh et al. 2009) and two S-circles (9S1 and 5S2 according to Besprozvannykh et al. 2009), is consistant with the plagiorchioid type of the chaetotaxy. Nevertheless, the main character, which approximates O. pseudobagri cercaria with other species of plagiorchioid trematodes, is the presence of 2 UD (“U” in Besprozvannykh et al. 2009) sensillae on tail tegument.
Miracidia of representatives of the genus Orientocreadium are very similar to those at other plagiorchioid species including leptophallids. They have the same epithelial formula (3 + 3) and related glandular apparatus—two large penetration glands situated immediately posterior to the terebratorium (Tang and Lin 1973). Unfortunately, nothing is known about organisation of the excretory system and germinative primordium in orietocreadiid miracidia. In addition, even less is known about sporocysts of leptophallids and orientocreadiids other that they have an elongated body with a thick wall. In all studied species, the birth pore is situated terminally (Dobrovolʼski 1969; Tang and Lin 1973; Besprozvannykh et al. 2009).
No morphological synapomorphy is apparent for the clade of Alloglossidiidae + (Orientocreadiidae + Leptophallidae). External phylogenetic connections of this group with other plagiorchioid trematodes cannot yet be adequately identified.
In general, only the Telorchiidae among the nine families of the Plagiorchioidea (represented in our study by more than one species) has not been demonstrated to be monophyletic. The Telorchiidae is represented here by members of two subfamilies—Telorchiinae (Telorchis assula (Dujardin, 1845)) and Opisthioglyphinae (Opisthioglyphe ranae (Frölich, 1791)) (see Font and Lotz 2008). The association of these taxa into one family is supported by data on the cercarial chaetotaxy (Grabda-Kazubska and Lis 1993) and the results of the molecular study by Tkach et al. (2000a). However, the molecular data of a number of subsequent authors testify to the paraphyly of the Telorchiidae (Olson et al. 2003; Bray et al. 2005; Pérez-Ponce de León et al. 2011; Martínez-Salazar et al. 2016).
References
Agrawal SC, Sharma SK (1990) Pseudoorientocreadium tori n. Subgen., n. Sp. (Trematoda, Allocreadiidae) from the intestine of a fresh water fish tor tor (ham) at Jhansi. J Sci Res Benares Hindu Univ 40:9–13
Andrade-Gómez L, Pinacho-Pinacho CD, Hernández-Orts JS, Sereno-Uribe AL, García-Varela M (2017) Morphological and molecular analyses of a new species of Saccocoelioides Szidat, 1954 (Haploporidae Nicoll, 1914) in the fat sleeper Dormitator maculatus (Bloch) (Perciformes: Eleotridae) from the Gulf of Mexico. J Helminthol 91:504–516. https://doi.org/10.1017/S0022149X1600047X
Andres MJ, Pulis EE, Overstreet RM (2014) New genus of opecoelid trematode from Pristipomoides aquilonaris (Perciformes: Lutjanidae) and its phylogenetic affinity within the family Opecoelidae. Folia Parasitol 61:223–230. 10.14411/fp.2014.033
Andres MJ, Pulis EE, Overstreet RM (2016) Description of three species of Isorchis (Digenea: Atractotrematidae) from Australia. Acta Parasitol 61:590–601. https://doi.org/10.1515/ap-2016-0079
Atopkin DM, Besprozvannykh VV, Ngo HD, Van Ha N, Van Tang N, Ermolenko AV, Beloded AY (2017) Morphometric and molecular data of the two digenean species Lasiotocus lizae Liu, 2002 (Monorchiidae) and Paucivitellosus vietnamensis sp. n. (Bivesiculidae) from mullet fish in Tonkin Bay, Vietnam. J Helminthol 91:346–355. https://doi.org/10.1017/S0022149X16000389
Atopkin DM, Shedko MB (2014) Genetic characterization of far eastern species of the genus Crepidostomum (Trematoda: Allocreadiidae) by means of 28S ribosomal DNA sequences. Adv Biosci Biotechnol 5:209–215. https://doi.org/10.4236/abb.2014.53027
Besprozvannykh VV (1984) Life cycles of Orientocreadium pseudobagri Yamaguti, 1934 and Allocreadium baueri Spassky et Roitman, 1960 (Trematoda) from fishes of Khanka lake. In: Mamaev YL (ed) Parasites of animals and plants. Far East Science Centre, Vladivostok, pp 71–76 (In Russian)
Besprozvannykh VV, Atopkin DM, Ngo HD, Beloded AY, Ermolenko AV, Ha NV, Tang NV (2015b) The trematode Skrjabinolecithum spasskii Belous, 1954 (Digenea: Haploporidae), a mullet parasite (Mugilidae) from Peter the great bay of the sea of Japan and from Vietnamese waters of the Gulf of Tonkin: morphology and molecular data. Rus J Mar Biol 41:286–294. https://doi.org/10.1134/S1063074015040021
Besprozvannykh VV, Ermolenko AV, Atopkin DM (2012) The life cycle of Asymphylodora perccotti sp. n. (Trematoda: Lissorchiidae) in the Russian southern far east. Parasitol Int 61:235–241. https://doi.org/10.1016/j.parint.2011.10.001
Besprozvannykh VV, Ermolenko AV, Deveney MR (2009) Orientocreadium elegans n. Sp. and Orientocreadium pseudobagri Yamaguti (Digenea: Orientocreadiidae), from freshwater fish of the Primorsky region (southern far east, Russia) with a description of their life cycles. Zootaxa 2176:22–32. https://doi.org/10.5281/zenodo.189374
Besprozvannykh VV, Atopkin DM, Ermolenko AV, Kharitonova AV, Khamatova AY (2015a) Life-cycle and genetic characterization of Astiotrema odhneri Bhalerao, 1936 sensu Cho & Seo 1977 from the Primorsky region (Russian far east). Parasitol Int 64:533–539. https://doi.org/10.1016/j.parint.2015.07.008
Beverley-Burton M (1962) Some trematodes from Clarias spp. in the Rhodesias, including Allocreadium mazoensis n. Sp. and Eumasenia bangweulensis n. Sp., and comments on the species of the genus Orientocreadium Tubangui, 1931. Proc Helm Soc Wash 29:103–115
Blasco-Costa I, Balbuena JA, Kostadinova A, Olson PD (2009) Interrelationships of the Haploporinae (Digenea: Haploporidae): a molecular test of the taxonomic framework based on morphology. Parasitol Int 58:263–269. https://doi.org/10.1016/j.parint.2009.03.006
Bray RA (2008) Superfamily Plagiorchioidea Lühe, 1901. In: Bray RA, Gibson D, Jones A (eds) Keys to the Trematoda Vol 3. CABI Publishing and The Natural History Museum, Wallingford, pp 291–294
Bray RA, Cribb TH (2012) Reorganization of the superfamily Lepocreadioidea Odhner, 1905 based on an inferred molecular phylogeny. Syst Parasitol 83:169–177. https://doi.org/10.1007/s11230-012-9386-3
Bray RA, Cribb TH, Littlewood DTJ, Waeschenbach A (2016) The molecular phylogeny of the digenean family Opecoelidae Ozaki, 1925 and the value of morphological characters, with the erection of a new subfamily. Folia Parasitol 63:013. 10.14411/fp.2016.013
Bray RA, Waeschenbach A, Cribb TH, Weedall GD, Dyal P, Littlewood DTJ (2009) The phylogeny of the Lepocreadioidea (Platyhelminthes, Digenea) inferred from nuclear and mitochondrial genes: implications for their systematics and evolution. Acta Parasitol 54:310–329. https://doi.org/10.2478/s11686-009-0045-z
Bray RA, Waeschenbach A, Dyal P, Littlewood DTJ, Morand S (2014) New digeneans (Opecoelidae) from hydrothermal vent fishes in the south eastern Pacific Ocean, including one new genus and five new species. Zootaxa 3768:73–87. 10.11646/zootaxa.3768.1.5
Bray RA, Webster BL, Bartoli P, Littlewood DTJ (2005) Relationships within the Acanthocolpidae Lühe, 1906 and their place among the Digenea. Acta Parasitol 50:281–291
Choudhury A, Rosas Valdez R, Johnson RC, Hoffmann B, Pérez-Ponce de León G (2007) The phylogenetic position of Allocreadiidae (Trematoda: Digenea) from partial sequences of the 18S and 28S ribosomal RNA genes. J Parasitol 93:192–196. https://doi.org/10.1645/GE-966R.1
Curran SS, Tkach VV, Overstreet RM (2006) A review of Polylekithum Arnold, 1934 and its familial affinities using morphological and molecular data, with description of Polylekithum catahoulensis sp. nov. Acta Parasitol 51:238–248. https://doi.org/10.2478/s11686-006-0037-1
Curran SS, Tkach VV, Overstreet RM (2011) Phylogenetic affinities of Auriculostoma (Digenea: Allocreadiidae), with descriptions of two new species from Peru. J Parasitol 97:661–670. https://doi.org/10.1645/GE-2641.1
Cutmore SC, Bennett MB, Cribb TH (2010) Staphylorchis cymatodes (Gorgoderidae: Anaporrhutinae) from carcharhiniform, orectolobiform and myliobatiform elasmobranchs of Australasia: low host specificity, wide distribution and morphological plasticity. Parasitol Int 59:579–586. https://doi.org/10.1016/j.parint.2010.08.003
Cutmore SC, Miller TL, Bray RA, Cribb TH (2014) A new species of Plectognathotrema layman, 1930 (Trematoda: Zoogonidae) from an Australian monacanthid, with a molecular assessment of the phylogenetic position of the genus. Syst Parasitol 89:237–246. https://doi.org/10.1007/s11230-014-9523-2
Cutmore SC, Miller TL, Curran SS, Bennett MB, Cribb TH (2013) Phylogenetic relationships of the Gorgoderidae (Platyhelminthes: Trematoda), including the proposal of a new subfamily (Degeneriinae n. Subfam.) Parasitol Res 112:3063–3074. https://doi.org/10.1007/s00436-013-3481-5
Dayal J (1938) Studies on the trematode parasites of fishes. A new trematode Nizamia hyderabadi, n. Gen., n. Sp., from the intestine of a fresh-water fish, Ophiocephalus punctatus. Proc Nat Acad Sci India 8:53–58
Dobrovol'ski AA (1969) The life cycle of Paralepoderma cloacicola (Liihe, 1909) Dollfus, 1950 (Trematoda, Plagiorchiidae). Vestn Leningr Univ 9:28–38 (in Russian)
Edgar RC (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32:1792–1797. https://doi.org/10.1093/nar/gkh340
Fischthal JH, Kuntz RE (1963) Trematode parasites of fishes from Egypt. Part VII. Orientocreadium batrachoides Tubangui, 1931 (Plagiorchioidea) from Clarias lazera, with a review of the genus and related forms. J Parasitol 49:425–436. https://doi.org/10.2307/3275816
Font WF, Lotz JM (2008) Family Telorchiidae Looss, 1899. In: Bray RA, Gibson D, Jones A (eds) Keys to the Trematoda Vol 3. CABI Publishing and The Natural History Museum, Wallingford, pp 545–602
Galactionov K, Blasco-Costa I, Olson PD (2012) Life cycles, molecular phylogeny and historical biogeography of the ‘pygmaeus’ microphallids (Digenea: Microphallidae): widespread parasites of marine and coastal birds in the Holarctic. Parasitology 139:1346–1360. https://doi.org/10.1017/S0031182012000583
Georgieva S, Faltynkova A, Brown R, Blasco-Costa I, Soldanova M, Sitko J, Scholz T, Kostadinova A (2014) Echinostoma revolutum (Digenea: Echinostomatidae) species complex revisited: species delimitation based on novel molecular and morphological data gathered in Europe. Parasit Vectors 7:520. https://doi.org/10.1186/s13071-014-0520-8
Gouy M, Guindon S, Gascuel O (2010) SeaView version 4: a multiplatform graphical user interface for sequence alignment and phylogenetic tree building. Mol Biol Evol 27:221–224. https://doi.org/10.1093/molbev/msp259
Grabda-Kazubska B (1963) The life-cycle of Metaleptophallus gracillimus (Lühe, 1909) and some observation on the biology and morphology of developmental stages of Leptophallus nigrovenosus (Bellingham, 1844). Acta Parasitol Polon 11:349–370
Grabda-Kazubska B, Lis A (1993) Chaetotaxy of the cercaria of Telorchis assula (Dujardin, 1845) (Trematoda, Telorchiidae). Acta Parasitol 38:96–98
Hafeezullah M (1989) Digenetic trematodes of vertebrates. In: Jairajpuri MS (ed) Fauna of Orissa Pt II. Zoological Survey of India, Calcutta, pp 225–252
Heneberg P, Literák I (2013) Molecular phylogenetic characterization of Collyriclum faba with reference to its three host-specific ecotypes. Parasitol Int 62:262–267. https://doi.org/10.1016/j.parint.2013.01.002
Hernández-Mena DI, Mendoza-Garfias B, Ornelas-García CP, Pérez-Ponce de León G (2016) Phylogenetic position of Magnivitellinum Kloss, 1966 and Perezitrema Baruš & Moravec, 1967 (Trematoda: Plagiorchioidea: Macroderoididae) inferred from partial 28S rDNA sequences, with the establishment of Alloglossidiidae n. Fam. Syst Parasitol 93:525–538. https://doi.org/10.1007/s11230-016-9645-9
Hildebrand J, Sitko J, Zaleśny G, Jeżewski W, Laskowski Z (2016) Molecular characteristics of representatives of the genus Brachylecithum Shtrom, 1940 (Digenea, Dicrocoeliidae) with comments on life cycle and host specificity. Parasitol Res 115:1417–1425. https://doi.org/10.1007/s00436-015-4875-3
Jones A, Bray RA (2008) Family Orientocreadiidae Yamaguti, 1958. In: Bray RA, Gibson D, Jones A (eds) Keys to the Trematoda Vol 3. CABI Publishing and The Natural History Museum, Wallingford, pp 545–602
Kanarek G, Zaleśny G, Czujkowska A, Sitko J, Harris PD (2015) On the systematic position of Collyricloides massanae Vaucher, 1969 (Platyhelminthes: Digenea) with notes on distribution of this trematode species. Parasitology Res 114:1495–1501. https://doi.org/10.1007/s00436-015-4333-2
Kanarek G, Zaleśny G, Sitko J, Tkach VV (2014) Phylogenetic relationships and systematic position of the families Cortrematidae and Phaneropsolidae (Platyhelminthes: Digenea). Folia Parasitol 61:523–528. 10.14411/fp.2014.057
Kasl EL, Fayton TJ, Font WF, Criscione CD (2014) Alloglossidium floridense n. Sp. (Digenea: Macroderoididae) from a spring run in north Central Florida. J Parasitol 100:121–126. https://doi.org/10.1645/13-251.1
Kim K-H, Rim H-J (1995) Two korean digenetic trematodes: Orientocreadium koreanum sp. nov. and O. pseudobagri Yamaguti, 1934 (Orientocreadiidae) from freshwater fishes. J Fish Pathol 8:81–90
Kudlai O, Stunžėnas V, Tkach V (2015) The taxonomic identity and phylogenetic relationships of Cercaria pugnax and C. helvetica XII (Digenea: Lecithodendriidae) based on morphological and molecular data. Folia Parasitol 62:003. 10.14411/fp.2015.003
Littlewood DTJ, Bray RA, Waeschenbach A (2015) Phylogenetic patterns of diversity in cestodes and trematodes. In: Morand S, Krasnov B, Littlewood DTJ (eds) Parasite diversity and diversification: evolutionary ecology meets Phylogenetics. Cambridge University Press, Cambridge, pp 304–319
Lockyer AE, Olson PD, Littlewood DTJ (2003) Utility of complete large and small subunit rRNA genes in resolving the phylogeny of the Neodermata (Platyhelminthes): implications and a review of the cercomer theory. Biol J Linn Soc 78:155–171. https://doi.org/10.1046/j.1095-8312.2003.00141.x
Martínez-Salazar EA, Rosas-Valdez R, Gregory TR, Violante-González J (2016) Molecular phylogenetic analysis of Infidum similis, including morphological data and estimation of its genome size. J Parasitol 102:468–475. https://doi.org/10.1645/15-915
Miller MA, Pfeiffer W, Schwartz T (2010) Creating the CIPRES science gateway for inference of large phylogenetic trees. In: Proceedings of the gateway computing environments workshop (GCE). IEEE, New Orleans, pp 1–8. https://doi.org/10.1109/GCE.2010.5676129
Nigam A, Chandra S, Johri S, Saxena AM (2015) A new digenean trematode of genus Orientocreadium Tubangui, 1931 parasitizing fresh water fishes of Uttar Pradesh (India). Helix 2:648–650
Olson PD, Cribb TH, Tkach VV, Bray RA, Littlewood DTJ (2003) Phylogeny and classification of Digenea (Platyhelminthes: Trematoda). Int J Parasitol 33:733–755. https://doi.org/10.1016/S0020-7519(03)00049-3
Overstreet RM, Curran SS (2005) Family Haploporidae Nicoll, 1914. In: Jones A, Bray RA, Gibson DI (eds) Keys to the Trematoda Vol. 2. CABI Publishing and the Natural History Museum, Wallingford, pp 129–165
Pandey KC (1970) Studies on trematode parasite of fishes of Lucknow (India). 1. Indian J Zoot 11:145–148
Patitucci KF, Kudlai O, Tkach VV (2015) Nephromonorcha varitestis n. Sp. (Digenea: Renicolidae) from the American white pelican, Pelecanus erythrorhynchos in North Dakota, U.S.a. Comp Parasitol 82:254–261. https://doi.org/10.1654/4775.1
Pérez-Ponce de León G, Mendoza-Garfias B, Razo-Mendivil U, Parra-Olea G (2011) A new genus and species of Brachycoeliidae (Digenea) from Chiropterotriton sp. (Caudata: Plethodontidae) in Mexico and its phylogenetic position within the Plagiorchiida based on partial sequences of the 28S ribosomal RNA gene. J Parasitol 97:128–134. https://doi.org/10.1645/GE-2346.1
Pérez-Ponce de León G, Pinacho-Pinacho CD, Mendoza-Garfias B, Choudhury A, García-Varela M (2016) Phylogenetic analysis using the 28S rRNA gene reveals that the genus Paracreptotrema (Digenea: Allocreadiidae) is not monophyletic; description of two new genera and one new species. J Parasitol 102:131–142. https://doi.org/10.1645/15-815
Petkevičiūtė R, Stunžėnas V, Stanevičiūtė G, Sokolov SG (2010) Comparison of the developmental stages of some European allocreadiid trematode species and a clarification of their life-cycles based on ITS2 and 28S sequences. Syst Parasitol 76:169–178. https://doi.org/10.1007/s11230-010-9249-8
Petkevičiūtė R, Stunžėnas V, Stanevičiūtė G, Zhokhov AE (2015) European Phyllodistomum (Digenea, Gorgoderidae) and phylogenetic affinities of Cercaria duplicata based on rDNA and karyotypes. Zool Scr 44:191–202. https://doi.org/10.1111/zsc.12080
Pulis EE, Tkach VV, Newman RA (2011) Helminth parasites of the wood frog, Lithobates sylvaticus, in prairie pothole wetlands of the northern Great Plains. Wetlands 31:675–685. https://doi.org/10.1007/s13157-011-0183-6
Rambaut A, Suchard MA, Xie D, Drummond AJ (2014). Tracer v1.6, Available from http://beast.bio.ed.ac.uk/Tracer
Razo-Mendivil UJ, León-Reògagnon V, Pérez-Ponce de León G (2006) Monophyly and systematic position of Glypthelmins (Digenea), based on partial lsrDNA sequences and morphological evidence. Org Divers Evol 6:308–320. https://doi.org/10.1016/j.ode.2005.12.005
Razo-Mendivil U, Mendoza-Garfias B, Pérez-Ponce de León G, Rubio-Godoy M (2014a) A new species of Auriculostoma (Digenea: Allocreadiidae) in the Mexican tetra Astyanax mexicanus (Actinopterygii: Characidae) from Central Veracruz, Mexico, described with the use of morphological and molecular data. J Parasitol 100:331–337
Razo-Mendivil U, Pérez-Ponce de León G (2011) Testing the evolutionary and biogeographical history of Glypthelmins (Digenea: Plagiorchiida), a parasite of anurans, through a simultaneous analysis of molecular and morphological data. Mol Phylogenet Evol 59:331–341. https://doi.org/10.1016/j.ympev.2011.02.018
Razo-Mendivil U, Pérez-Ponce de León G, Rubio-Godoy M (2014b) Testing the systematic position and relationships of Paracreptotrema heterandriae within the Allocreadiidae through partial 28s rRNA gene sequences. J Parasitol 100:537–541. https://doi.org/10.1645/13-421.1
Santoro M, Tkach VV, Mattiucci S, Kinsella JM, Nascetti G (2011) Renifer aniarum (Digenea: Reniferidae), an introduced north American parasite in grass snakes Natrix natrix in Calabria, southern Italy. Dis Aquat Org 95:233–240. https://doi.org/10.3354/dao02365
Searle EL, Cutmore SC, Cribb TH (2014) Monorchiid trematodes of the painted sweetlips, Diagramma labiosum (Perciformes: Haemulidae), from the southern great barrier reef, including a new genus and three new species. Syst Parasitol 88:195–211. https://doi.org/10.1007/s11230-014-9499-y
Shedko MB, Sokolov SG, Atopkin DM (2015) The first record of Dimerosaccus oncorhynchi (Trematoda: Opecoelidae) in fishes from rivers of Primorsky territory, Russia, with a discussion on its taxonomic position using morphological and molecular data. Parazitologiya 49:171–189
Shimazu T (1990) Trematodes of the genus Orientocreadium (Digenea: Orientocreadiidae) from freshwater fishes of Japan. Zool Sci 7:933–938
Shimazu T (2014) Digeneans parasitic in freshwater fishes (Osteichthyes) of Japan. II. Gorgoderidae and Orientocreadiidae. Bull Natl Mus Nat Sci Ser A 40:53–78
Shimazu T (2016) Digeneans parasitic in freshwater fishes (Osteichthyes) of Japan. IX. Opecoelidae. Bull Natl Mus Nat Sci Ser A 42:163–180
Shimazu T, Urabe M, Grygier MJ (2011) Digeneans (Trematoda) parasitic in freshwater fishes (Osteichthyes) of the Lake Biwa basin in Shiga prefecture, central Honshu, Japan. Natl Mus Nat Sci Monogr 43:1–105
Sirikantayakul S (1985) Observations on the life cycle and egg-shell of Orientocreadium batrachoides Tubangui, 1931 (Trematoda: Allocreadiidae) in Clarias macrocephalus Gunther, 1864. Philipp J Sci 114:183–206
Skrjabin KI, Koval VP (1963) Family Orientocreadiidae Skrjabin et Koval, 1960. In: Skrjabin KI (ed) Trematodes of animals and man. Principles of trematodology, vol 21. Publishing House AN SSSR, Moscow, pp 269–469 (In Russian)
Snyder SD, Tkach VV (2001) Phylogenetic and biogeographical relationships among some holarctic frog lung flukes (Digenea: Haematoloechidae). J Parasitol 87:1433–1440. https://doi.org/10.1645/0022-3395(2001)087[1433:PABRAS]2.0.CO;2
Sokolov SG (2013) New data on the parasite fauna of the Chinese sleeper Perccottus Glenii (Actinoperygii: Odontobutidae) in Primorsky territory with the description of a new myxozoan species from the genus Myxidium (Myxozoa: Myxidiidae). Parazitologiya 47:77–99 (in Russian)
Stamatakis A (2006) RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22:2688–2690. https://doi.org/10.1093/bioinformatics/btl446
Tang CC, Lin S-M (1973) On the life history of Orientocreadium batrachoides Tubangui, with a consideration on the phylogeny of the superfamily Plagiorchioidea. Acta Zool Sin 19:11–25 (In Chinese)
Tkach VV (2008) Family Leptophallidae Dayal, 1938. In: Bray RA, Gibson D, Jones A (eds) Keys to the Trematoda, vol 3. CABI Publishing and The Natural History Museum, Wallingford, pp 367–371
Tkach VV, Grabda-Kazubska B, Pawlowski J, Swiderski Z (1999) Molecular and morphological evidences for close phylogenetic affinities of the genera Macrodera, Leptophallus, Metaleptophallus and Paralepoderma (Digenea, Plagiorchioidea). Acta Parasitol 44:170–179
Tkach V, Grabda-Kazubska B, Swiderski Z (2001a) Systematic position and phylogenetic relationships of the family Omphalometridae (Digenea, Plagiorchiida) inferred from partial lsrDNA sequences. Int J Parasitol 31:81–85. https://doi.org/10.1016/S0020-7519(00)00154-5
Tkach VV, Littlewood DTJ, Olson PD, Kinsella JM, Swiderski Z (2003) Molecular phylogenetic analysis of the Microphalloidea Ward, 1901 (Trematoda: Digenea). Syst Parasit 56:1–15. https://doi.org/10.1023/A:1025546001611
Tkach VV, Pulis EE, Overstreet RM (2010) A new Paramacroderoides species (Digenea: Macroderoididae) from two species of gar in the southeastern United States. J Parasitol 96:1002–1006. https://doi.org/10.1645/GE-2385.1
Tkach VV, Pawlowski J, Mariaux J (2000a) Phylogenetic analysis of the suborder Plagiorchiata (Platyhelminthes, Digenea) based on partial lsrDNA sequences. Int J Parasitol 30:83–93. https://doi.org/10.1016/S0020-7519(99)00163-0
Tkach VV, Pawlowski J, Mariaux J, Swiderski Z (2000b) Molecular phylogeny of the suborder Plagiorchiata and its position in the system of Digenea. In: Littlewood DTJ, Bray RA (eds) Interrelationships of the Platyhelminthes. Taylor & Francis, London, pp 186–193
Tkach VV, Snyder SD (2007) Choanocotyle platti sp. nov. from the northern long-necked turtle, Chelodina rugosa (Pleurodira, Chelidae) in Australia. Acta Parasitol 52:318–324. https://doi.org/10.2478/s11686-007-0057-5
Tkach VV, Snyder SD, Swiderski Z (2001b) On the phylogenetic relationships of some members of Macroderoididae and Ochetosomatidae (Digenea, Plagiorchioidea). Acta Parasitol 46:267–275
Tubangui MA (1931) Trematode parasites of Philippine vertebrates, III: flukes from fish and reptiles. Phil J Sci 44:417–423
Unwin S, Chantrey J, Chatterton J, Aldhoun JA, Littlewood DTJ (2013) Renal trematode infection due to Paratanaisia bragai in zoo housed Columbiformes and a red bird-of-paradise (Paradisaea rubra). Int J Parasitol Parasites Wildl 2:32–41. https://doi.org/10.1016/j.ijppaw.2012.11.001
Yamaguti S (1934) Studies on the helminth fauna of Japan. Part 2. Trematodes of fishes. Jap J Zool 5:249–541
Yamaguti S (1958) Systema helminthum. Vol. I. The digenetic trematodes of vertebrates. Part I. Interscience, New York
Yamaguti S (1971) Synopsis of digenetic trematodes of vertebrates, vol I. Keigaku, Tokyo
Zikmundová J, Georgieva S, Faltýnková A, Soldánová M, Kostadinova A (2014) Species diversity of Plagiorchis Lühe, 1899 (Digenea: Plagiorchiidae) in lymnaeid snails from freshwater ecosystems in central Europe revealed by molecules and morphology. Syst Parasitol 88:37–54. https://doi.org/10.1007/s11230-014-9481-8
Acknowledgements
The work was supported by state order 0221–2014–0004. This research was performed at the Research park of St. Petersburg State University Center for Molecular and Cell Technologies. Special thanks to Andrey A. Dobrovol’ski (St. Petersburg State University).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Sokolov, S.G., Shchenkov, S.V. Phylogenetic position of the family Orientocreadiidae within the superfamily Plagiorchioidea (Trematoda) based on partial 28S rDNA sequence. Parasitol Res 116, 2831–2844 (2017). https://doi.org/10.1007/s00436-017-5594-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-017-5594-8