Abstract
Zygocercous (aggregating) cercarial larvae were recently discovered emerging from a physid snail during a molecular survey of cercariae from molluscs in lakes in central Alberta, Canada. This manuscript delves into the characterization of these cercariae through morphological and molecular techniques and provides the first genetic information for a zygocercous larval trematode. Analyses of cytochrome c oxidase I of mitochondrial DNA and two partial regions of nuclear ribosomal DNA sequences revealed the zygocercous cercariae to belong to the genus Australapatemon Sudarikov, 1959. Further analyses of sequences of Australapatemon burti (Miller, 1923), from cercariae and adults collected from across North America, indicate a complex of nine genetically-distinct lineages within this species, a surprising level of diversity. The zygocercous cercariae, along with adult worms collected from ducks in Manitoba, Canada, and from Mexico, represent one of these lineages, and are herein described as Australapatemon mclaughlini n. sp. Seven lineages cannot yet be identified, but one is tentatively identified as Australapatemon burti.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Reports of digenetic trematodes with zygocercous cercariae, often referred to as “Rat King”, “Rattenkönig” or “Aggregacercaria”, are rare. In the past century (since 1888), there have been only 11 descriptions of aggregating cercariae. Aggregation is a unique behavioural adaptation that results in the joining together of several to hundreds of individual cercariae into bundles or nets (Beuret and Pearson 1994; Cable 1956, 1963; Cable and McLean 1943; Dronen 1973; Hendrickson and Kingston 1974; Komiya 1941; Martin 1968; Martin and Gregory 1951; Miller 1929, 1930; Pintner 1891; Ward 1916; Wardle 1988). Zygocercous cercariae join by the tails to form rosettes, like the “Rat King” phenomenon from which they were originally described, or pine cone-like structures (reviewed in Suppl. Table 1). This adaptation is believed to assist cercariae in being consumed by their next host, thought to be a fish, based on a few experimental studies (Dronen 1973). Little information regarding life cycle progression or host use is available for zygocercous specimens described to date. Moreover, there are no available genetic resources for any of these cercariae, resulting in limited understanding of their phylogenetic affinity, beyond tentative identifications at the family level.
The present study began with a morphological and molecular characterization of zygocercous cercariae emerging from the snail, Physella gyrina (Say, 1821), collected from a lake in central Alberta. The morphology of these cercarial larvae was compared to that of other zygocercous larval trematodes in the literature, and to close relatives identified through mitochondrial cytochrome c oxidase 1 (cox1) DNA sequences. Sequence comparisons suggested the zygocercous cercariae belonged to Australapatemon burti (Miller, 1923), based on an adult trematode sequenced by Hernández-Mena et al. (2014), although the zygocercous forms we collected displayed morphological differences to cercariae of this species (Miller 1923, 1926; Cort and Brooks 1928; Stunkard et al. 1941). An expanded sampling effort provided additional cox1 sequences from cercariae collected during a large-scale survey of digeneans in central Alberta (Gordy et al. 2016) and from cercariae and adult worms collected across North America. These results placed the zygocercous cercariae in one of nine genetically distinct lineages of Australapatemon, matching sequence from adult A. burti from ducks sampled by Hernández-Mena et al. (2014) in Mexico. The morphological differences between the zygocercous and non-zygocercous cercariae, along with morphology and host-use of adults in Manitoba and Mexico, further corroborated the distinctions among lineages identified in cox1 comparisons. These findings indicate hidden species diversity within Australapatemon. Among the nine lineages distinguished herein, one is tentatively identified as A. burti (Miller, 1923), and one is a new species of Australapatemon.
Methods
Specimen collection
Most data reported here are based on cercariae from snails collected as part of a parasitological survey of several lakes in central Alberta (see methods within Gordy et al. 2016), from June 2013 to September 2015. On July 13th and August 10th, 2015, collections from Rochon Sands Provincial Park at Buffalo Lake (52.4638361 N, −112.8843833 W) yielded two P. gyrina snails infected with a trematode with zygocercous type cercariae. These snails were placed in small plastic containers with artificial spring water (ASW) (Ulmer 1970), fed red leaf lettuce ad libitum, and monitored over several days to count and capture emerging cercariae. One individual cercaria of an aggregate was separated in a dilute solution of tricaine mesylate, used to relax the aggregate, before using fine forceps to pull it apart. The aggregates were otherwise impossible to separate. The individual cercaria was wet mounted and photographed using the Zeiss Axio Imager.A2 compound microscope and mounted Zeiss AxioCam MRc camera. The number of zygocercous aggregates per day was recorded, and finally, both cercariae and snail were preserved in 100% ethanol for later analyses. A permanent mount was prepared for the zygocercous cercariae aggregates using Grenacher’s Borax-Carmine stain and mounted in Canada balsam. Drawings were made from photographs of wet-mounted specimens.
Additional material was obtained from gastropod and avian hosts elsewhere in North America. The latter included cercariae from planorbid snails at two localities in California (Santa Clara area, sampled in August 2009 and Pleasanton area, June 2009) and cercariae from Helisoma campanulatum (Say, 1821) from a lake in Cape Breton, May 2012. In addition, we included data from adult worms from Anatidae (Anserinae: Anas acuta (Linnaeus, 1758) (n = 2), Aythya collaris (Donovan, 1809) (n = 1), Bucephala albeola (Linnaeus, 1758) (n = 1), and Anatinae: Oxyura jamaicensis (Gmelin, 1789) (n = 1)) collected from the southern end of Lake Manitoba, Manitoba, Canada in 2008 and 2009. In the latter collections, live adults and cercariae from freshly killed hosts were placed directly into 70–95% ethanol.
Voucher samples of permanent mount slides for the zygocercous cercariae and for several representative adult worms, including type material, were donated to the Royal Alberta Museum, in Edmonton, Alberta, Canada.
Molecular analyses
Cercariae collected in Alberta were initially identified by partial sequencing of the mitochondrial cox1 gene, using primers Dice1F and Dice11R for amplification, and a shortened version of these primers for Sanger sequencing (Van Steenkiste et al. 2014), as previously described (Gordy et al. 2016). Additionally, partial large subunit (28S) and internal transcribed spacer regions (ITS1–5.8S–ITS2) of ribosomal DNA (rDNA) sequences were generated for select specimens to include in phylogenetic analyses. Universal primers BD1 and BD2 were used to amplify ITS1–5.8S–ITS2 sequences as previously described for Clonorchis sinensis (Looss, 1907) (Tatonova et al. 2012). Sequences for 28S rDNA were amplified as previously described for other trematodes (Gordy et al. 2016). Cox1 fragments from cercariae and adults obtained outside Alberta were amplified and sequenced as described by Moszczynska et al. (2009). Sequences of rDNA from non-Albertan specimens were generated using primers and protocols in Littlewood and Olson (2001) (for 28S) and Galazzo et al. (2002) for ITS1–5.8S–ITS2. All newly generated sequences were submitted to GenBank (accession numbers: HM385485–HM385486, HM385534–HM385538, KY207548–KY207628, KY570946–KY570948, KY587394–KY587403, KY587405–KY587406, MF124269–MF124270).
Our molecular analysis built on a recent phylogeny of Australapatemon Sudarikov, 1959 and Apatemon Szidat, 1928 (Blasco-Costa et al. 2016). The cox1, 28S, and ITS strigeid sequences used and generated by Blasco-Costa et al. (2016) were separately aligned with newly obtained sequences using MUltiple Sequence Comparison by Log-Expectation (MUSCLE) (Edgar 2004) in Geneious v.10.0.5 (http://www.geneious.com, Kearse et al. 2012). Alignments were trimmed to the shortest available sequence prior to phylogenetic analyses (cox1: 408 nt; 28S: 809 nt; ITS: 525 nt). MEGA7 was used for model testing, initial maximum likelihood (ML) analyses, and p distance calculations (Kumar et al. 2016). Model selection for each dataset was based on ML fits of 24 different nucleotide substitution models, with the best model of evolution being that with the lowest BIC score (Bayesian information criterion). The models of nucleotide evolution used for ML trees in MEGA7 were HKY + G + I (cox1) and K2 + G (28S and ITS) and tree nodes were assessed with 1000 bootstrap replicates. All ML analyses in MEGA7 employed four discrete gamma categories, used complete deletion if there were gaps/missing data and inferred trees using nearest-neighbour-interchange as the heuristic method, while initial trees were generated automatically with the neighbour-joining method. Tree options in Geneious were slightly different; thus, the second-best models were used, namely HKY85 + G (cox1) and JC69 + G (28S and ITS). The PhyML plugin (Guindon et al. 2010) in Geneious was used to test the robustness of initial ML trees generated in MEGA7. The following options were selected to run these analyses: bootstrap branch support with 1000 replicates, transition/transversion ratio estimated, proportion of invariable sites estimated, number of substitution categories was four, gamma distribution parameter estimated, optimized for topology/length/rate, and using an SPR topology search. The MrBayes plugin v. 3.2.6 (Huelsenbeck and Ronquist 2001) in Geneious was used for Bayesian inference (BI) analyses. All BI trees were constructed from two independent MCMC runs of four chains (temp 0.2) for 107 generations, sub-sample frequencies of 104 generations, and burn-in of 105 generations, per the standard deviation of split frequency values (<0.01). A consensus topology and nodal support, estimated as posterior probability values, were generated from trees remaining beyond the burn-in period (Huelsenbeck and Ronquist 2001). The rate variation among sites was modelled with a gamma distribution (shape parameter = 0.652, as estimated by PhyML).
The web app for Automatic Barcode Gap Discovery (ABGD; wwwabi.snv.jussieu.fr/public/abgd/abgdweb.html) was used to test hypotheses of lineage separation (Puillandre et al. 2012), coupled with a priori assumptions of species differentiation, using a 5% cut-off value for cox1 (Vilas et al. 2005). Default values were applied to a p distance matrix input into the ABGD program. The ABGD method has been used previously as a supportive tool for delimiting species among trematodes of the families Clinostomidae (Locke et al. 2015b; de León Pérez-Ponce et al. 2016), Diplostomidae (Locke et al. 2015a) and Opecoelidae (López et al. 2015; Oliva et al. 2015).
Morphological analyses of cercariae
The zygocercous cercariae and other cercariae (representative samples from each following intermediate host: Stagnicola elodes (Say, 1821), Helisoma trivolvis (previously named Planorbella trivolvis (Say, 1817)) and Pysella gyrina) with high nucleotide identity (>95%) to A. burti sequences, matched through BLAST (tblastn), were selected for morphological analyses. Samples of cercariae stored in 95–100% ethanol were imaged using a scanning electron microscope (SEM) (Model XL30 by FEI Company North America NanoPort, 5350 NE Dawson Creek Drive Hillsboro, Oregon 97,124 USA). Samples were prepared by first transferring to a 0.2-μm GTTP membrane atop a 25-mm swinnex filter holder with o-ring (Millipore). While the membrane was still wet, the top of the filter holder was tightened into place. Then, using a 1-ml luer lock syringe, the samples were taken to 100% hexamethyldisilazane (HMDS) through the following series: 1 ml of 100% ethanol (twice), 1 ml of 75% ethanol:25% HMDS, 1 ml of 50% ethanol:50% HMDS, 1 ml of 25% ethanol:75% HMDS, and 1 ml of 100% HMDS (twice). After the fluid was run through the syringe, there was a five-minute waiting period before the next fluid was run through. After the samples were in 100% HMDS, all remaining fluid was pushed through by filling the syringe with air and pressing through until no further liquid came out the other end. Finally, the membranes were dried completely for several hours before being cut and placed onto the SEM stud for sputtering and subsequent imaging.
Measurements were taken from SEM images using Scandium 5.0 (Olympus Soft Imaging Systems) to capture the following major external morphological features for comparison between specimens and those in the literature: cercarial body length and width, tail stem length and width, furcal length and width, oral sucker to ventral sucker length, ventral sucker to tail stem length, ventral sucker length and width, length and width of spines found on the body, tail stem and furcae. Additionally, for the zygocercous cercariae, measurements were taken for the papules found on the tail stem. Because the dehydration step during the SEM processing appeared to cause some collapsing of the tissues, creating wrinkles, measurements were also taken from a small number of cercariae preserved for permanent mounting, to test for artefacts or distortion due to different methods of specimen preparation. Because resolution of the permanent-mount material was not as great as in that prepared for SEM, the only measurements taken were of body length and width and tail stem length and width. Light microscope images were taken using a compound Zeiss Axio Scope.A1 and PictureFrame v. 2.3 (Optronics) software, and measurements were made using ZEN (Zeiss) software. Sizes of morphological features were compared using an independent samples Kruskal-Wallis analysis of variance, and post hoc multiple comparisons test, with Bonferroni correction for multiple tests, and an alpha level of 0.05, using IBM SPSS Statistics v. 24.0.
Morphological analyses of adults
In the lineages genetically distinguished herein for which adult specimens were available, DNA was often extracted from a subsample of a worm, and the remainder was stained in acetocarmine and mounted laterally on a slide in Canada balsam (i.e. as hologenophores, sensu Pleijel et al. 2008). In one lineage (LIN8), DNA was extracted from the entire worm, and the voucher is an intact worm that appeared indistinguishable from the sequenced specimen when it was taken from the same host (i.e. a paragenophore, sensu Pleijel et al. 2008). The adult vouchers were compared to published descriptions, with emphasis on accounts originating geographically close or from the same host as in the original description. Measurements were made with both an ocular micrometre and using imaging software, and one specimen was drawn using a camera lucida. Measurements were taken from uncollapsed, laterally oriented eggs along the entire length of the uterus, unless eggs were clearly different in size or shape due to differences in maturation. Unless otherwise stated, measurements (in μm) are reported as a range followed in parentheses by mean ± standard deviation, and number of specimens in which the structure was measured.
Results
Molecular phylogenetics
The cox1 sequence derived from the zygocercous cercariae showed a 99.56% nucleotide similarity to A. burti (JX977725) from Anas americana in Mexico (isolate 180 from Hernández-Mena et al. 2014). Other matches to other A. burti isolates from Hernández-Mena et al. (2014) were attained with cox1 sequences from non-zygocercous cercariae derived from a previous study (Gordy et al. 2016). Therefore, nucleotide alignments and phylogenetic analyses of these combined specimens were used to assess the relationships among these samples to clarify these extreme differences in cercarial behaviour.
Confirming initial tblastn results, both ML and BI trees in all three data sets (cox1, 28S, ITS) strongly supported the placement of the zygocercous cercaria (MGC1935) in Australapatemon, nested among other A. burti samples from Mexico, and separate from Australapatemon niewiadomski Blasco-Costa, Poulin, and Presswell, 2016 and, in separate clades, other strigeid genera (Blasco-Costa et al. 2016) (Fig. 1 and Suppl. Fig. 1). Both 28S and ITS markers, though generally supportive, and confirming genus-level monophyly seen in Blasco-Costa et al. (2016), were less informative for discriminating between species than cox1, possibly because sequences were available from fewer samples and had relatively low intrageneric divergence (mean intrageneric divergence across the Strigeidae: 28S ≤1.2%, ITS ≤6%) (Suppl. Table 2). Thus, initial species-level delineation among samples within the genus Australapatemon was achieved by analysis of cox1.
a Bayesian inference phylogram generated from partial cox1 gene sequences with posterior probability values >0.50 reported. GenBank (GB) accession numbers are associated with samples derived from the database, while all other sample names represent new sequences. Sample names correspond to new GB accession numbers provided in Suppl. Table 5. Sequences from adult worms are indicated by a black star. Adults collected in Mexico and studied by Hernández-Mena et al. (2014) are labelled A. burti. Scale bar denotes number of substitutions per site. Lineages are identified by differently coloured rounded rectangles that correspond to same colour-shaded rectangles directly to the right, b indicating first intermediate and definitive host use for each lineage. Singletons are not indicated on the tree and are denoted by unshaded rectangles. Each lineage is labelled at the far right of the rectangles as LIN1–LIN9. Question marks denote missing host information. c Examples of cercarial morphologies from LIN1, LIN6 and LIN7, in SEM. Coloured outlines correspond to lineage colours, and lines indicate placement within each lineage. First intermediate host use is indicated on each image: Pg Physella gyrina, Se Stagnicola elodes and Ht Helisoma trivolvis
The cox1 phylogeny showed nine lineages (LIN1–LIN9) within A. burti. Members within each lineage differed by less than 6.8% and by at least 6.7% from those in other lineages. The lineages corresponded to identical clusters of sequences in ABGD (prior maximal distance P = 2.15e-02, MinSlope = 1.5) (Puillandre et al. 2012) (evolutionary divergences as p distances given in Suppl. Table 2). Lineages 2, 3 and 5 were each represented by a single sequence. In the other six lineages, maximum intraspecific divergence ranged from 0.5–6.8% (LIN1 (A. burti): 6.5%, LIN4: 5.0%, LIN6: 3.3%, LIN7 (Australapatemon mclaughlini n. sp.): 0.5%, LIN8: 1.0%, LIN9: 6.8%), and mean intraspecific divergence from 0.2–3.5% (LIN1 = 1.8%, LIN4 = 3.5%, LIN6 = 2.2%, LIN7 = 0.2%, LIN8 = 0.3%, and LIN9 = 3.0%). Between lineages, the greatest interspecific divergence value was found between LIN5 and LIN8 (14.4%), and the smallest interspecific difference between LIN6 and LIN7 (6.7%). Overall, the range of genetic divergence by gene region among each new lineage and congeneric species was 6.7–14.4% (cox1), 0.0–1.2% (28S), and 0.4–1.9% (ITS).
Sequences of cox1 from the zygocercous cercariae grouped phylogenetically with those from six adult worms from North America to form a distinct lineage, LIN7 (A. mclaughlini n. sp.). Five of the adult worms were collected from Northern Pintail (A. acuta) in Lake Manitoba, Manitoba, Canada, and one adult worm was from an American widgeon, Anas americana (Gmelin, 1789) collected from Baja California Sur, Mexico, i.e., isolate 180, identified as A. burti by Hernández-Mena et al. (2014) (JX977725.1). LIN7 was positioned within a monophyletic clade also composed of lineages 4 (LIN4), 5 (LIN5), and 6 (LIN6). With the exception of LIN5, all members of this clade utilize P. gyrina as an intermediate host and anatid birds as their definitive host. A single cercarial specimen, representing LIN5, was found emerging from the snail S. elodes (Fig. 1).
Host use and parasite morphology
The genetic distinction between clades was supported by differences in intermediate and definitive-host use in several lineages that were sampled more than once (LIN6 and LIN8 in P. gyrina, LIN9 in S. elodes; LIN7 in P. gyrina and Anas spp.; LIN8 in O. jamaicensis). However, because host specificity may correlate with sampling effort, the support that host-specific distributions imply for putative species is also likely related to sampling effort. For example, 68/80 sequenced cercarial isolates were from S. elodes (Lymnaeidae), and consequently a lineage in our samples might be associated only with S. elodes by chance, even if it naturally occurs in other hosts. On the other hand, only 7/80 sequenced cercarial samples were obtained from P. gyrina (Physidae), such that lineages found only in this host are more likely truly specific to it. To address this, we did not assess host specificity of three lineages recovered from single individual hosts (LIN2, LIN3, LIN5). In other lineages, we randomized the 80 lineage-snail-host associations 10,000 times. The observed host distribution of LIN9 (nine isolates all from in S. elodes) was not different from random (P = 0.351), but the likelihoods of recovering LIN6, LIN7 and LIN8 only from P. gyrina were very small (P ≤ 0.0099) if these lineages were equally capable of infecting other snail species sampled. The mixed snail-host associations of LIN1 mirror those of the data as a whole (60/66 LIN1 isolates in S. elodes; P = 0.827). Neither the wide snail-host spectrum of LIN1, nor the narrow host ranges of the other lineages seem to be purely an artefact of sampling effort, because the number of cercarial isolates sequenced was not related to the number of snail-host species in each lineage (Spearman’s rho = 0.616, P = 0.11, n = 8). Permuting the smaller database of adult-parasite avian-host associations (consisting of seven lineages, LIN1, 2, 4, 6, 7, 8, 9, from nine individual birds in seven host species) 10,000 times showed the probability of two LIN8 occurring only in O. jamaicensis (one in Manitoba, one in Durango, Mexico (Hernández-Mena et al. 2014)) to be 0.0278. The only other potential host specificity among the adult worms is in LIN7, which was only recovered from Anas spp. The probability of two LIN7 samples occurring in Anas was 0.275. Thus, three cox1 lineages (LIN6, LIN7 and LIN8) are supported by host-specific distributions that are unlikely to be an artefact of sampling effort.
Cercarial morphometrics (14/16) were significantly different across lineages (Suppl. Table 3). There was no significant difference between measurements in SEM and those of permanently mounted, stained samples, nor from wet-mounts. The only lineage within which there were significant morphometric differences among cercariae was LIN1 (Suppl. Table 4). Notably, there were no differences between genetically divergent samples within LIN9 (P > 0.05 for all comparisons between MGC1376 and MGC1360).
Within LIN1, cercariae varied morphometrically. For instance, samples MGC1557 (S. elodes) and MGC1179 (H. trivolvis) had significantly different body and furcal spine dimensions, as well as different furcal lengths (4/16 morphometrics in Table 1; Suppl. Table 4). This lineage was recovered from five different pulmonate species in Western and Eastern Canada and the Southwestern USA (Fig. 1 and Suppl. Table 5). Despite this broad distribution, the only link to an adult was with the isolate from Anas diazi (Ridgway, 1886) in Mexico (sample 138 of Hernández-Mena et al. 2014).
Varying degrees of morphological distinction were observed among adults of LIN2, LIN4, LIN7, LIN8 and LIN9 (Tables 2 and 3). Adults of most lineages could be distinguished mainly by their total length, ratio of hindbody to forebody length and egg size. Dense vitelline follicles prevented visualization of the ovary in all specimens, and the genital cone characteristic of Australapatemon was observed in two specimens of LIN7, as well as in the single specimen of LIN9. No substantial difference was seen among the adults of LIN4 and LIN8, and cercariae of both lineages emerged from P. gyrina.
Because these parasites mature in migratory waterfowl, and some species have been recorded in diverse birds from distant localities (e.g. McDonald 1981; Drago et al. 2007; Drago and Lunaschi 2010), we compared the morphology of the adult vouchers of the nine lineages with all species of Australapatemon (Table 2; Suppl. Table 6), although we focus on species known in North America. One lineage is newly described herein (LIN7 = A. mclaughlini n. sp.), and most others could not be assigned to described species, nor described.
Description
Australapatemon mclaughlini n. sp.
Family Strigeidae Railliet, 1919
Subfamily Strigeinae Railliet, 1919
Genus Australapatemon Sudarikov, 1959
Description of adult (Fig. 2a, b; Table 2)
[Measurements from 7 specimens (4 subsampled hologenophores, 2 paragenophores, and holotype), ex Anas acuta L. measurements in micrometres; widths dorso-ventral]
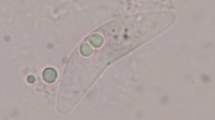
Australapatemon mclaughlini n. sp. a Adult (holotype); scale bar = 500 μm. b Silhouettes of paratypes (paragenophores and hologenophores, based on photographs taken prior to subsampling and DNA extraction); scale bar = 500 μm. c Outline of cercarial zygocercous aggregate; scale bar = 200 μm. d Individual cercaria, ventral view; scale bar = 50 μm
Total length 1484–1851 (1671 ± 143, 5); body distinctly bipartite. Maximum width of forebody at level of ventral sucker. Forebody cup-shaped, 347–473 (386 ± 55, 5) long, 475–630 (527 ± 64, 5) wide. Hindbody arcuate, curved dorsally, widest at level of anterior testis, 1137–1378 (1298 ± 92, 6) long, 535–662 (616 ± 48, 6) wide. Ratio of forebody to hindbody length 1:2.9–3.7 (3.4 ± 0.3, 5). Oral sucker terminal, 78–117 (102 ± 14, 5) × 88–113 (102 ± 9, 5). Ventral sucker in median dorsal wall of forebody, 125–160 (142 ± 15, 4) × 173–185 (177 ± 15, 4). Holdfast organ bilobed; proteolytic gland not observed. Pharynx small, difficult to observe, 39–58 (52 ± 11, 3) long. Testes tandem, large; anterior testis asymmetrical, bilobed; anterior margin at 17–27 (23 ± 4, 4) % of hindbody; 341 × 390 (1). Posterior testis asymmetrical, bilobed; posterior margin at 72–79 (77 ± 3, 4) % of hindbody; 293(1) × 240–341 (291 ± 72, 3). Seminal vesicle convoluted in dorsal post-testicular region, often distending adjacent tegument. Ovary not observed. Vitellaria follicular, confined to hindbody, densely distributed, extending posteriorly in two ventro-lateral fields to level of copulatory bursa. Vitelline reservoir intertesticular; median. Eggs 115–131 (120 ± 5, 6) × 83–88 (85 ± 2, 6). Copulatory bursa large with dorsally oriented terminal opening. Genital cone delimited from surrounding parenchyma, one eighth to one sixth of hindbody length; 192–218 (202 ± 14, 3) × 166–188 (177 ± 16, 2). Hermaphroditic duct within genital cone lined with internal rugae.
Description of cercaria (Fig. 2c, d; Table 4)
[Measurements in micrometres, based on three aggregates preserved in 100% ethanol and imaged with SEM. Body and tail lengths/widths are composite measurements of SEM and permanent mount cercariae from a total of six aggregates.] Freshwater, apharyngeate, distomate and zygocercous furcocercaria. Body elliptical, 78.69–149.50 (115.56 ± 18.71, 69) long, 29.82–49.00 (39.08 ± 5.07, 71) wide. Tegument spines dense at oral sucker and becoming sparser towards posterior extremity of body. Body spines 1.41–2.53 (2.00 ± 0.36, 12) long, 0.25–0.55 (0.45 ± 0.09, 12) wide. No apparent eye-spots. Ventral sucker post-equatorial, 4.83–7.70 (6.46 ± 1.27, 6) long, 7.36–13.59 (9.44 ± 2.25, 6) wide, and containing 4–5 rows of large spines 1.38–2.26 (1.75 ± 0.46, 3) long, 0.30–0.45 (0.38 ± 0.07, 3) wide. Tail stem thinner or of similar width to body at junction but widens further towards furcae, 101.24–247.40 (179.29 ± 27.37, 50) long, 18.66–82.70 (47.28 ± 11.39, 43) wide, aspinose, and covered in protruding papules 2.36–3.92 (3.0 ± 0.44, 14) long, 1.22–3.03 (1.50 ± 0.19, 14) wide. Furcae 47.33–98.90 (80.30 ± 28.70, 3) long (from base of tail stem to bundle with other furcae), 12.17–34.19 (21.70 ± 8.40, 11) wide, and narrowing to a blunt end. Furcal spines 1.06–1.59 (1.40 ± 0.23, 5) long, 0.62–1.06 (0.80 ± 0.17, 8) wide. Excretory bladder bilobed and roughly triangular in shape, in posterior body region near base of tail stem. Cercarial aggregation by attachment of distal portion of furcae and specialized furcal muscles, in masses of 4–44 individuals. Aggregates non-swimming, resting on substrate in spherical mass, emerging from snail at rate of 20 per day (based on two captive snails observed over 3 days), occurring free and between mantle tissue and shell of snail, suggesting aggregation immediately post-emergence.
Details of type material
Type-host: Anas acuta (definitive host)
First intermediate host: Physella gyrina Say
Site of infection: intestine (definitive host); ovo-testes and digestive gland (first intermediate host).
Prevalence: in 1 out of 2 birds (Lake Manitoba); in 2 of 127 snails collected (Buffalo Lake)
Type-locality: Delta Marsh, Lake Manitoba, Manitoba, Canada (50.183° N, −98.383° W) (definitive host); Rochon Sands Provincial Park, Buffalo Lake, Alberta, Canada (52.464° N, −112.884° W) (first intermediate host)
Other localities: Guerrero Negro, Baja California Sur (27.959° N, −114.056° W) (definitive host: Anas americana JX977725, sample 180 (Hernández-Mena et al. 2014)).
Type-material: holotype (adult worm); holotype (cercariae). Deposited in the Royal Alberta Museum
Representative DNA sequences: cox1: KY207615, KY587395, KY587402, KY587403, KY587405, KY587406, JX977725; 28S rDNA: KY207627; ITS1–5.8S–ITS2: KY207628
Etymology: This species is named after J. Daniel Mclaughlin, who collected and generously provided adult worms studied herein, and who has made numerous, important contributions to the systematics and ecology of helminths in aquatic systems in North America.
Remarks
Adults of Australapatemon mclaughlini n. sp. possess characters typical of Australapatemon Sudarikov, 1959, namely, a muscular genital cone delimited from surrounding parenchyma that is traversed by a rugose hermaphroditic duct. The length of the hindbody relative to the forebody (see Fig. 2b) is greater in adults of A. mclaughlini n. sp. compared to all species of Australapatemon, as well as the lineages characterized genetically herein, but not Australapatemon anseris (Dubois, 1967). Australapatemon mclaughlini n. sp. is also distinguished from most species and lineages (other than Australapatemon canadensis (Dubois and Rausch, 1950), Australapatemon fuhrmanni (Dubois, 1937), Australapatemon minor (Yamaguti, 1933), and LIN2 and LIN9, by having larger eggs (Suppl. Table 6). Several features distinguish A. mclaughlini n. sp. from species of Australapatemon reported in North America. Compared with A. anseris (a predominantly European species reported in North America by McDonald 1981), A. mclaughlini n. sp. is characterized by a smaller total length, oral sucker, pharynx, ventral sucker, posterior testis and genital cone, and greater egg length. Australapatemon mclaughlini n. sp. is distinguished from A. burti (as described by Stunkard et al. 1941) and A. canadensis by having a long hindbody relative to its forebody, and eggs are longer in A. mclaughlini n. sp. than in A. burti. Australapatemon mclaughlini n. sp. has smaller total length and is more robust (wider relative to length in both fore- and hindbody) than A. canadensis. The aggregating habit of cercariae of A. mclaughlini n. sp. differs from the behaviour of cercariae in Australapatemon bdellocystis (Lutz, 1921), A. burti, Australapatemon intermedius (Johnston, 1904), Australapatemon magnacetabulum (Dubois, 1988) and A. minor (Dubois 1968; Davies and Ostrowski de Núñez, 2012) and other genetic lineages herein. The cercariae of A. mclaughlini n. sp. are also distinct from cercariae of other lineages genetically characterized herein, as well as from Cercaria burti Miller, 1923, in body, tail, and tegumental spine sizes (Tables 1 and 4; Fig. 3). In addition, spines on the body of cercariae of A. burti extend only to the ventral sucker (Miller 1923, 1926) while those of A. mclaughlini n. sp. span the length of the cercarial body (Fig. 3b). The presence of tegumental spines on cercariae of A. mclaughlini n. sp. distinguishes it from Cercaria laramiensis, which lacks spines (Hendrickson and Kingston 1974) (Table 4, Suppl. Table 1 and Fig. 3). The only other described aggregating cercariae possibly belonging to the Diplostomoidea, Cercaria absurda Miller, 1927, forms chain-like aggregates (Miller 1930) rather than the rosette formation seen in A. mclaughlini n. sp.
Scanning electron micrographs (SEM) of zygocercous (LIN7, MGC1935: a–d) Australapatemon mclaughlini n. sp. and non-zygocercous (LIN1, MGC1179: e–h) Australapatemon burti cercariae. a Zygocercous aggregate; scale bar = 100 μm. b Ventral view of three individual zygocercous bodies. White arrows indicate tegumental spination; scale bar = 20 μm. c Ventral sucker of zygocercous cercaria. White arrows indicate sucker spines; scale bar = 5 μm. d Zoomed-in view of cercaria tail among the aggregate tail bundle. White arrow indicates papules on tail, black arrow indicates narrowing of furcal muscles, specialized for holding on to others; scale bar = 50 μm. e Four individual cercariae; scale bar = 100 μm. f Ventral view of individual cercaria body. White arrow indicates body spination; scale bar = 20 μm. g Zoomed-in view of ventral sucker. White arrows indicate ventral sucker spines; scale bar = 5 μm. h Zoomed-in view of cercaria furcae. White arrows indicate tail and furcal spines used for measurements; scale bar = 20 μm
The other four genetically distinguished lineages of Australapatemon in which adults were recovered (LIN2, LIN4, LIN8, LIN9) also displayed varying degrees of morphological distinction from species already described in this genus (Suppl. Table 3, Suppl. Table 6). The single adult specimen of LIN2 had larger oral and ventral suckers and eggs than A. burti, and was smaller in total length, but otherwise similar, to A. canadensis. The two adult specimens in LIN4 were smaller in total length and oral sucker width, but with a larger oral sucker, and were otherwise similar to A. burti. Compared with A. canadensis, adults of LIN4 were smaller in total length and egg size, and had a more spherical forebody. The adult of LIN8 was smaller in total length and oral sucker width, and had a larger pharynx and ventral sucker than A. burti, but was otherwise similar to A. burti. In comparison to A. canadensis, the forebody of LIN8 was more spherical, and the hindbody wider relative to its length. The adult of LIN9 also resembled A. burti, although it was smaller in total length, with longer hindbody relative to forebody. It differed from A. canadensis in its smaller total length, ventral sucker, genital cone and in its flattened, ovoid forebody. However, because most adults from these four lineages were incomplete specimens (subsampled for DNA extraction), and only 1–2 adults were obtained per lineage (Table 2), assessment of morphological variation was not possible and key features for many comparisons were not observed. For example, adults of LIN4 and LIN8 resemble both A. burti and Australapatemon congolensis (Dubois and Fain, 1956), two species usually discriminated by dimensions of the genital cone (Dubois 1968), a structure obscured by vitelline fields in vouchers of these lineages. Moreover, high divergence in cox1 within one lineage (LIN9) may indicate the presence of additional species (see discussion). For these reasons, lineages 2, 4, 8 and 9 were not identified or described as new species based on this material.
Discussion
Historically, the status of A. burti (Miller, 1923) has been in a state of flux. When still referred to as Cercaria burti, it was believed that these cercariae may be the larval form of Apatemon gracilis (Rudolphi, 1819), a worm commonly found in palmate birds across Europe, Japan and North America. It was determined, however, that because C. burti had a longer metacercarial developmental period and utilized pulmonate snails in North America, it was likely a different species from A. gracilis, known to infect branchiate snails (Stunkard et al. 1941). However, since then, A. burti has been one of the most common cercariae found in Europe among pulmonate snails, despite no reports of adult A. burti in this region (Faltýnková et al. 2007; Blasco-Costa et al. 2016 and references within).
The distinction between Apatemon and Australapatemon has also been questioned, with the latter considered a subgenus of Apatemon by Dubois (1968). Recent authorities, however, consider Australapatemon a valid genus, distinguished from Apatemon by a well-defined genital cone in the adult (Niewiadomska 2002). This was supported by the phylogenetic analyses of Blasco-Costa et al. (2016) based on two species of Australapatemon and several species of Apatemon. Blasco-Costa et al. (2016) noted high variation in cox1 (6.3–13.1%) within the four isolates of A. burti from Mexico (Hernández-Mena et al. 2014) studied here and predicted that further analyses may reveal cryptic species among this group, and among other strigeid genera. Our results support this prediction, revealing nine strongly supported lineages nested within A. burti from Mexico, and separate from A. niewiadomski from New Zealand, the only two species from which molecular data were available. With data linking larvae to adults across North America, it is apparent that these nine lineages are supported by statistically significant distinctions in host use, morphological characters that do not overlap with other known North American Australapatemon species, and by high cox1 sequence divergence between monophyletic clades. The molecular, morphological and host-use data gathered here suggests four adults identified as A. burti (Hernández-Mena et al. 2014) belong to four separate species with ranges extending into the USA and Canada. The prior collections in Mexico include A. diazi (JX977727.1–LIN1), Anas cyanoptera (Vieillot, 1816) (JX977726.1–LIN6), A. americana (JX977725.1–LIN7, A. mclaughlini n. sp.) and O. jamaicensis (JX977728.1–LIN8) (Hernández-Mena et al. 2014).
Among the samples considered here, LIN1 appears the most likely to contain A. burti as described by Miller (1923, 1926) and others (Cort and Brooks 1928; Stunkard et al. 1941). The wide geographic distribution and diversity of snails infected by LIN1 are consistent with this identification. Australapatemon (Cercaria) burti was described from Helisoma (Planorbis) trivolvis in Burt Lake, Michigan (Miller 1923, 1926), and found in Lymnaea humilis (modicella) (Say, 1822) in the type locality soon after (Cort and Brooks 1928), suggesting early on that this species is a generalist. Similarly, cercariae of LIN1, the only first-intermediate-host generalist in this report, were found emerging from H. trivolvis (MGC1179 in Tables 1 and 4 and Figs. 1 and 3), H. campanulatum, Lymnaea stagnalis (Linnaeus, 1758), S. elodes (MGC1557 in Tables 1 and 4 and Fig. 1), and P. gyrina (Fig. 1), from localities in Nova Scotia, Alberta and California, which together encompass the type locality. All other lineages reported here infect a single snail species, none of which belong to Helisoma or Lymnaea, the hosts associated with A. burti in the type locality. This line of evidence also suggests members of LIN4, 8 and 9 are not A. burti, despite morphological resemblance of adults to those described by Stunkard et al. (1941). Unfortunately, we encountered no adults from LIN1, but the samples from A. diazi studied by Hernández-Mena et al. (2014) were also evidently similar to A. burti. Anas diazi is limited to the lower Southwestern USA and Mexico (Lepage et al. 2014), implying that another definitive host species must be transmitting LIN1 to snails in Alberta, where this bird has never been reported (Committee ABR 2015). Previous studies have reported A. burti from at least ten other anatid species (see Blasco-Costa et al. 2016 and reference within). Further sampling is needed to better understand definitive host specificity. However, if LIN1 does truly represent A. burti, then it would not be surprising to find a wide variety of anatids can be infected with members of this lineage.
However, several factors suggest an alternative hypothesis for the high genetic diversity within LIN1, namely, that it is comprised of multiple, recently derived species. For example, within our molecular phylogeny, the cercariae that utilize Helisoma species display greater nucleotide substitutions within cox1 than those from other snail hosts. Also, while the mean intraspecific cox1 divergence in LIN1 is under the 5% cut-off, the range extends to 6.5%, suggesting more than one species within this lineage by this measure alone, even if no partition was made in ABGD barcode gap analysis. There is also significant variation in cercarial morphometrics within LIN1 (Table 1 and Suppl. Table 4). Different snail hosts could account for this phenotypic variability in LIN1, but it is also possible that multiple, closely related, host-specific species lie within it.
Isolates of LIN9 also display high intra-clade cox1 divergence (1.0–6.8%) that exceeds the 5% cut-off hypothesis for species delimitation, but again, no further distinction was indicated by ABGD. Sample MGC1376 is 6.5–6.8% different from all other representatives in LIN9, yet there was no significant morphometric difference between this and other isolates in LIN9 (MGC1360). In both LIN1 and LIN9, we predict that further sampling, more detailed morphological analysis, and data from other molecular markers may support separate species within these lineages.
The aggregating habit of cercariae of A. mclaughlini n. sp. highlights the importance of pairing classical approaches with molecular analyses. By taking such a combined approach, our results can be shown to support the hypothesis of Cable and McLean (1943) that aggregation has evolved independently as a secondary specialization, in this case, within a member of the genus Australapatemon. This has implications for the life cycle of A. mclaughlini n. sp. and the taxonomy of the genus Australapatemon. One of the characters distinguishing Australapatemon from Apatemon is that metacercariae of members of the former genus are found in leeches, rather than fish (Blair 1976; Blasco-Costa et al. 2016; Dubois 1968; Johnston and Angel 1951; McCarthy 1990; Negm-Eldin and Davies 2002; Niewiadomska 2002; Stunkard et al. 1941; Vojtek 1964). The spherical structure and loss of the ability to swim en masse of zygocercariae is generally thought to imply ingestion by a fish host, but only Dronen (1973) has successfully infected fish with (echinostomatid) zygocercous cercariae. Notably, Hendrickson and Kingston (1974) were unable to infect fish with zygocercous cercariae closely resembling the isolates we collected. This raises several possibilities for the zygocercous phenotype of A. mclaughlini n. sp. and the potential mechanism for infecting its next host, namely, (1) external penetration of leech tegument, a counter-intuitive strategy for aggregating cercariae, (2) ingestion by leeches, which generally lack fine-scale visually acuity (Harley et al. 2011, 2013), (3) ingestion by a fish, rather than a hirudinid second intermediate host or (4) loss of the second intermediate host altogether, with ingestion of the aggregate by the definitive host. The latter is possible because both bird hosts of A. mclaughlini n. sp., A. acuta and A. americana, are dabbling ducks that mainly eat bits of vegetation in water, and other small items like invertebrates. Davies and Ostrowski de Núñez (2012) noted a similar incongruence in the life cycle of A. magnacetabulum, in which infection of leeches was verified experimentally, but which was described from birds (Rupornis magnirostris (Gmelin, 1788), Strix rufipes (King, 1828)) (Dubois 1988) not known to feed on leeches.
The encounter of multiple lineages in a strigeid morphotype may not seem surprising, considering the diversity that has emerged in the sister family Diplostomatidae (Locke et al. 2015a) and the intraspecific plasticity and genetic diversity among the Strigeidae (Blasco-Costa et al. 2016 and references within). However, the diversity encountered here is nonetheless remarkable; we are unaware of a molecular survey revealing nine candidate species within one nominal digenetic trematode. Most other studies report much less cryptic diversity, and the number of cryptic species encountered is driven by sampling effort, both in the Diplostomoidea (Blasco-Costa and Locke 2017) and other parasitic and free-living taxa (Poulin and de León Pérez-Ponce 2016; de León Pérez-Ponce and Poulin 2017). Interestingly, in the only study of digenetic trematodes reporting a comparable number of lineages (8) within a single morphotype, sampling effort was much larger than herein (324 isolates sequenced by Miura et al. 2005, versus 97 sequenced herein). Our material was collected mainly for the purposes of molecular analysis, and one disappointing consequence is that most of the lineages could not be described or identified. However, morphological and ecological distinctions were nonetheless observed among vouchers of these lineages, several of which were considered a single species by researchers with deep expertise in molecular phylogenetics and morphological taxonomy (Hernández-Mena et al. 2014). Further identifications or taxonomic descriptions will require additional collections, but the general finding of (at least) nine species of Australapatemon in North America has implications for diversity and distribution of species in this genus. For one, the two-to-three species of Australapatemon known in North America (A. burti, A. canadensis and possibly A. anseris) (Dubois 1968; McDonald 1981) clearly do not represent the true diversity of the genus in the Nearctic. Although the migratory ranges of anatids and other definitive hosts make wide distributions plausible in all species in Australapatemon, most are known from a single biogeographic region (Dubois 1968; McDonald 1981; Davies and Ostrowski de Núñez 2012). Moreover, few digeneans that mature in birds have been confirmed from more than one biogeographic region with DNA sequences. There are two noteworthy exceptions: a recent molecular study of the diplostomid, Austrodiplostomum ostrowskiae, infecting double-crested cormorants, revealed its range extends to both the Nearctic and Neotropics (García-Varela et al. 2016); and perhaps the most suggestive exception is Trichobilharzia querquedulae (McLeod, 1937), a schistosome parasite of anatids that Ebbs et al. (2016) found to be globally distributed. Despite these exceptions, it is more common to find putatively cosmopolitan avian parasites to be made up of geographically isolated, genetically distinct lineages (Caffara et al. 2011; Locke et al. 2015a). This suggests that the lineages reported here likely include undescribed species, rather than new North American records of existing species of Australapatemon, and that molecular verification is desirable for species described in North America and reported in South America (Drago et al. 2007; Drago and Lunaschi 2010). Taken together, these results add to our understanding of the life cycles of these parasites—linking larvae to adults and other larval stages—and extend our knowledge of regional trematode distributions and local biodiversity.
To characterize diversity within the A. burti group, and the genus Australapatemon, molecular data are needed from the other seven species in this genus: Australapatemon minor (Yamaguti,1933), A. bdellocystis (Lutz, 1921), A. fuhrmanni (Dubois, 1937), A. canadensis (Dubois and Rausch, 1950), A. congolensis (Dubois et Fain, 1956), A. anseris (Dubois, 1967), and A. magnacetabulum (Dubois, 1988). Further studies may reveal even greater species diversity within the genus Australapatemon, and likely within other genera of the Strigeidae.
References
Beuret J, Pearson JC (1994) Description of a new zygocercous cercaria (Opisthorchioidea: Heterophyidae) from prosobranch gastropods collected at Heron Island (Great Barrier Reef, Australia) and a review of zygocercariae. Syst Parasitol 27:105–125. doi:10.1007/BF00012269
Blair D (1976) Observations on the life-cycle of the strigeoid trematode, Apatemon (Apatemon) gracilis (Rudolphi, 1819) Szidat, 1928. J Helminthol 50:125–131. doi:10.1017/S0022149X00027607
Blasco-Costa I, Locke SA (2017) Life history, systematics and evolution of the Diplostomoidea Poirier, 1886: progress, promises and challenges emerging from molecular studies. Adv Parasitol. doi:10.1016/bs.apar.2017.05.001
Blasco-Costa I, Poulin R, Presswell B (2016) Species of Apatemon Szidat, 1928 and Australapatemon Sudarikov, 1959 (Trematoda: Strigeidae) from New Zealand: linking and characterising life cycle stages with morphology and molecules. Parasitol res 115:271–289. doi:10.1007/s00436-015-4744-0
Cable RM (1956) Marine cercariae of Puerto Rico. In: scientific survey of Porto Rico and Virgin Islands. Vol XVI-Part 4. pp 491–577
Cable RM (1963) Marine cercariae from curacao and Jamaica. Zeitschrift für Parasitenkd 23:429–469
Cable RM, McLean RA (1943) The occurrence of Cercaria clausii Monticelli, a marine Rattenkönig larval trematode, on the west coast of Florida. Not Naturae 129:1–7
Caffara M, Locke SA, Gustinelli A et al (2011) Morphological and molecular differentiation of Clinostomum complanatum and Clinostomum marginatum (Digenea: Clinostomidae) metacercariae and adults. J Parasitol 97:884–891. doi:10.1645/GE-2781.1
Committee ABR (2015) The Official List of Birds of Alberta. http://www.royalalbertamuseum.ca/research/lifeSciences/ornithology/birdlist/taxonomy.cfm
Cort WW, Brooks ST (1928) Studies on the Holostome cercariae from Douglas Lake, Michigan. Trans am Microsc Soc 47:179. doi:10.2307/3222175
Davies D, Ostrowski de Núñez M (2012) The life cycle of Australapatemon magnacetabulum (Digenea: Strigeidae) from northwestern Argentina. J Parasitol 98:778–783
Drago FB, Lunaschi LI (2010) Digenea, Strigeidae, Australapatemon canadensis Dubois and Rausch, 1950: first record in South America and a new host record. Check List 6:382. doi:10.15560/6.3.382
Drago F, Lunaschi L, Hinojosa-Saez A, González-Acuña D (2007) First record of Australapatemon burti and Paramonostomum pseudalveatum (Digenea) from Anas georgica (Aves, Anseriformes) in Chile. Acta Parasitol 52:201–205. doi:10.2478/s11686-007-0040-1
Dronen NO Jr (1973) Studies on macrocercous cercariae of the Douglas Lake, Michigan area. Trans am Microsc Soc 92:641–648
Dubois G (1968) Synopsis des Strigeidae et des Diplostomatidae (Trematoda). Mém Soc Sci Nat Neuchatel 10:1–258
Dubois G (1988) Quelques Strigeoidea (Trematoda) récoltés au Paraguay par les expéditions du Muséum d’Histoire naturelle de Genève, au cours des années 1979, 1982 et 1985. Rev Suisse Zool 95:521–532. doi:10.5962/bhl.part.79670
Ebbs ET, Loker ES, Davis NE et al (2016) Schistosomes with wings: how host phylogeny and ecology shape the global distribution of Trichobilharzia querquedulae (Schistosomatidae). Int J Parasitol 46:669–677. doi:10.1016/j.ijpara.2016.04.009
Edgar RC (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids res 32:1792–1797. doi:10.1093/nar/gkh340
Faltýnková A, Niewiadomska K, Santos MJ, Valtonen ET (2007) Furcocercous cercariae (Trematoda) from freshwater snails in Central Finland. Acta Parasitol 52:310–317. doi:10.2478/s11686-007-0050-z
Galazzo DE, Dayanandan S, Marcogliese DJ, McLaughlin JD (2002) Molecular systematics of some North American species of Diplostomum (Digenea) based on rDNA-sequence data and comparisons with European congeners. Can J Zool 80:2207–2217. doi:10.1139/z02-198
García-Varela M, Sereno-Uribe AL, Pinacho-Pinacho CD et al (2016) Molecular and morphological characterization of Austrodiplostomum ostrowskiae Dronen, 2009 (Digenea: Diplostomatidae), a parasite of cormorants in the Americas. J Helminthol 90:174–185. doi:10.1017/S0022149X1500005X
Gordy MA, Kish L, Tarrabain M, Hanington PC (2016) A comprehensive survey of larval digenean trematodes and their snail hosts in central Alberta, Canada. Parasitol Res 115:3867–3880. doi:10.1007/s00436-016-5152-9
Guindon S, Dufayard JF, Lefort V et al (2010) New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol 59:307–321. doi:10.1093/sysbio/syq010
Harley CM, Cienfuegos J, Wagenaar DA (2011) Developmentally regulated multisensory integration for prey localization in the medicinal leech. J Exp Biol 214:3801–3807
Harley CM, Rossi M, Cienfuegos J, Wagenaar D (2013) Discontinuous locomotion and prey sensing in the leech. J Exp Biol 216:1890–1897
Hendrickson GL, Kingston N (1974) Cercaria laramiensis sp. n., a freshwater zygocercous cercaria from Physa gyrina say, with a discussion of cercarial aggregation. J Parasitol 60:777–781
Hernández-Mena DI, García-Prieto L, García-Varela M (2014) Morphological and molecular differentiation of Parastrigea (Trematoda: Strigeidae) from Mexico, with the description of a new species. Parasitol Int 63:315–323. doi:10.1016/j.parint.2013.11.012
Huelsenbeck JP, Ronquist F (2001) MrBayes: Bayesian inference of phylogeny. Bioinformatics 17:754–755. doi:10.1093/bioinformatics/17.8.754
Johnston TH, Angel LM (1951) The morphology and life cycle of the trematode Apatemon intermedius from the black swan. Trans R Soc South Aust 74:66–78
Kearse M, Moir R, Wilson A et al (2012) Geneious basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28:1647–1649. doi:10.1093/bioinformatics/bts199
Komiya Y (1941) A new “zygocercaria”, Cercaria radiata, and its excretory system (cercaria from Chinese fresh waters no. 3). J Shanghai Sci Inst [ns] 1:229–232
Kumar S, Stecher G, Tamura K (2016) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33:msw054. doi:10.1093/molbev/msw054
de León Pérez-Ponce G, Poulin R (2017) An updated look at the uneven distribution of cryptic diversity among parasitic helminths. J Helminthol:1–6. doi:10.1017/S0022149X17000189
de León Pérez-Ponce G, Garcia-Varela M, Pinacho-Pinacho CD et al (2016) Species delimitation in trematodes using DNA sequences: middle-American Clinostomum as a case study. Parasitology 143:1773–1789. doi:10.1017/S0031182016001517
Lepage D, Vaidya G, Guralnick R (2014) Avibase–a database system for managing and organizing taxonomic concepts. Zookeys 420:117–135. doi:10.3897/zookeys.420.7089
Littlewood DTJ, Olson PD (2001) Small subunit rDNA and the Platyhelminthes: signal, noise, conflict and compromise. In: Littlewood DTJ, Bray RA (eds) Interrelationships of the Platyhelminthes. Taylor & Francis, London, pp 262–278
Locke SA, Al-Nasiri FS, Caffara M et al (2015a) Diversity, specificity and speciation in larval Diplostomidae (Platyhelminthes: Digenea) in the eyes of freshwater fish, as revealed by DNA barcodes. Int J Parasitol 45:841–855. doi:10.1016/j.ijpara.2015.07.001
Locke SA, Caffara M, Marcogliese DJ, Fioravanti ML (2015b) A large-scale molecular survey of Clinostomum (Digenea, Clinostomidae). Zool Scr 44:203–217. doi:10.1111/zsc.12096
López Z, Cárdenas L, Runil F, González MT (2015) Contrasting definitive hosts as determinants of the genetic structure in a parasite with complex life cycle along the south-eastern Pacific. Mol Ecol 24:1060–1073. doi:10.1111/mec.13080
Martin WE (1968) Cercaria gorgoncephala Ward, 1916, a zygocercous species in northwestern United States. Trans am Microsc Soc 87:472–476
Martin WE, Gregory VL (1951) Cercaria buchanani n.sp., an aggregating marine trematode. Trans am Microsc Soc 70:359–362
McCarthy AM (1990) Experimental observations on the specificity of Apatemon (Australapatemon) minor (Yamaguti 1933) (Digenea: Strigeidae) toward leech (Hirudinea) second intermediate hosts. J Helminthol 64:161–167. doi:10.1017/S0022149X00012074
McDonald ME (1981) Key to trematodes reported in waterfowl. United States Department of the Interior. Fish and Wildlife Service, Fort Collins, p 156
Miller HM (1923) Notes on some furcocercous larval trematodes. J Parasitol 10:35–46
Miller HM (1926) Comparative studies on furcocercous cercariae. University of Illinois biological monographs 10:1–112
Miller HM (1929) Continuation of study on behavior and reactions of marine cercariae from Tortugas. Carnegie Institute of Washington Yearbook No.28 (for 1928–29) pp 292–294
Miller HM (1930) Formations and behaviour of aggregations of cercariae. J Parasitol 17:111–112
Miura O, Kuris A, Torchin M (2005) Molecular-genetic analyses reveal cryptic species of trematodes in the intertidal gastropod, Batillaria cumingi (Crosse). Int J Parasitol 35:793–801
Moszczynska A, Locke SA, McLaughlin JD et al (2009) Development of primers for the mitochondrial cytochrome c oxidase I gene in digenetic trematodes (Platyhelminthes) illustrates the challenge of barcoding parasitic helminths. Mol Ecol Resour 9(Suppl 1):75–82. doi:10.1111/j.1755-0998.2009.02634.x
Negm-Eldin M, Davies RW (2002) Morphology and life cycle of Apatemon hypseleotris species novum from Australia including metacercariae viability and excystment. Dtsch Tierarztl Wochenschr 109:314
Niewiadomska K (2002) Family Strigeidae Railliet, 1919. In: Gibson DI, Jones A, Bray RA (eds) Keys to the Trematoda. Wallingford: CABI publishing and the Natural History Museum, London, pp 231–241
Oliva ME, Valdivia IM, Chavez RA et al (2015) Molecular and morphological evidence demonstrating two species of Helicometrina Linton 1910 (Digenea: Opecoelidae) in northern Chile. J Parasitol 101:694–700. doi:10.1645/14-523
Pintner T (1891) Ueber Cercaria clausii Monticelli. Arb Aus Dem Zool Insituten der Univ Wein 9:285–294
Pleijel F, Jondelius U, Norlinder E et al (2008) Phylogenies without roots? A plea for the use of vouchers in molecular phylogenetic studies. Mol Phylogenet Evol 48:369–371. doi:10.1016/j.ympev.2008.03.024
Poulin R, de León Pérez-Ponce G (2016) Data from: global analysis reveals that cryptic diversity is linked with habitat but not mode of life. J Evol Biol 30(3):1420–9101. doi:10.5061/DRYAD.1HJ46
Puillandre N, Lambert A, Brouillet S, Achaz G (2012) ABGD, automatic barcode gap discovery for primary species delimitation. Mol Ecol 21:1864–1877. doi:10.1111/j.1365-294X.2011.05239.x
Stunkard HW, Willey CH, Rabinowitz Y (1941) Cercaria burti Miller, 1923, a larval stage of Apatemon gracilis (Rudolphi, 1819) Szidat, 1928. Trans am Microsc Soc 60:485–497. doi:10.2307/3222768
Tatonova YV, Chelomina GN, Besprosvannykh VV (2012) Genetic diversity of nuclear ITS1-5.8S-ITS2 rDNA sequence in Clonorchis sinensis Cobbold, 1875 (Trematoda: Opisthorchidae) from the Russian far east. Parasitol Int 61:664–674. doi:10.1016/j.parint.2012.07.005
Ulmer MJ (1970) Notes on rearing of snails in the laboratory. In: Experiments and techniques in parasitology. W. H. Freeman and Co. San Francisco, pp 143–144
Van Steenkiste N, Locke SA, Castelin M et al (2014) New primers for DNA barcoding of digeneans and cestodes (Platyhelminthes). Mol Ecol Resour 15:945–952. doi:10.1111/1755-0998.12358
Vilas R, Criscione CD, Blouin MS (2005) A comparison between mitochondrial DNA and the ribosomal internal transcribed regions in prospecting for cryptic species of platyhelminth parasites. Parasitology 131:839–846. doi:10.1017/S0031182005008437
Vojtek J (1964) Zur Kenntnis des Entwicklungszyklus von Apatemon cobitidis (Linstow, 1890). Zeitschrift für Parasitenkd 24:578–599. doi:10.1007/BF00328726
Ward HB (1916) Notes on two free-living larval trematodes from North America. J Parasitol 3:10–20
Wardle WJ (1988) A Bucephalid larva, Cercaria pleuromerae n.Sp. (Trematoda:Digenea), parasitizing a deepwater bivalve from the Gulf of Mexico. J Parasitol 74:692–694
Acknowledgements
We thank Arlene Oatway of the Advanced Microscopy Facility at the University of Alberta for her assistance with SEM processing and imaging. We also thank Lisa Kish, Valerie K. Phillips, and Mahmoud Tarrabain for assistance with field collections and sample processing in Alberta, where work was funded by Alberta Innovates Energy and Environment Solutions grant 2078 and 2332, and National Sciences and Engineering Research Council (NSERC) grant 418540 (PCH), and in Manitoba by NSERC grant A6979 to J. Daniel Mclaughlin. MAG was partly supported by an NSERC CREATE Host-Parasite Interactions student scholarship. SAL was supported by the Puerto Rico Science, Technology and Research Trust. We also thank Pieter Johnson (University of Colorado), for providing samples from California, and David J. Marcogliese (Environment and Climate Change Canada) for encouraging early phases of this study, which was also funded by the Canadian Federal Government’s Genomics Research Development Initiative, NSERC Discovery Grant (A6979), and by Paul D. N. Hebert at the Center for Biodiversity Genomics, University of Guelph, Canada through funding from NSERC, Genome Canada, the Ontario Genomics Institute and the International Barcode of Life initiative. We acknowledge two anonymous reviewers for their constructive comments on an earlier version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Suppl. Fig. 1
Bayesian inference phylograms of the Strigeidae and outgroups (Diplostomidae) derived from a) partial 28S, and b) partial ITS1–5.8S rDNA gene sequences with posterior probability values followed by bootstrap proportions given above branches. Values less than 0.5 are not reported. Scale bars denote number of substitutions per site. (PDF 3077 kb).
Suppl. Table 1
Review of zygocercous and aggregating cercariae. (XLSX 14 kb).
Suppl. Table 2
Estimates of evolutionary divergence over sequence pairs between and within groups among three genetic markers (cox1, 28S, ITS1–5.8S–ITS2). Each marker is represented by a matrix. Below the diagonal is the number of base pair differences per site from averaging over all sequence pairs between groups (interspecific divergence values), otherwise referred to as p distance values. Values pertaining specifically to the genus Australapatemon are within the grey box. Minimum and maximum mean interspecific divergence values among A. burti lineages are in bold and underlined. Standard error estimates are based on 1000 bootstrap replicates and above the diagonal and are coloured blue. The number of base pair differences per site from averaging over all sequence pairs within each group (intraspecific divergence values) is shown along the diagonal in red. Missing values, represented by a minus sign, are present for groups that contain singletons, and therefore estimates cannot be made. For cox1, the analysis involved 120 nucleotide sequences with a total of 399 positions in the final dataset. For 28S, the analysis involved 14 nucleotide sequences with a total of 807 positions in the final dataset. Finally, for ITS1–5.8S–ITS2, the analysis involved 26 nucleotide sequences with a total of 482 positions in the final dataset (partial ITS1–5.8 s after final trimming). All positions containing gaps and missing data were eliminated. (XLSX 15 kb).
Suppl. Table 3
Statistics for morphometric comparisons of cercariae of lineages 1, 6, 7, 8, and 9 with those of Australapatemon (Cercaria) burti (Miller, 1923), Cercaria laramiensis (Hendrickson & Kingston, 1974), and Cercaria absurda (Miller, 1927). Statistical tests used were Kruskal-Wallis analysis of variance (H), and post hoc multiple comparisons test, with Bonferroni correction for multiple tests. Statistical significance (α = 0.05) is indicated by bold text and an asterisk. Only significant comparisons between lineages/species are listed. (PDF 27 kb).
Suppl. Table 4
Statistics for significant comparisons between cercaria within LIN1. Statistics reported are from a post hoc multiple comparisons test, with Bonferroni correction for multiple tests. Statistical significance (α = 0.05) is indicated by bold text and an asterisk. (PDF 11 kb).
Suppl. Table 5
Summary data from samples from which DNA sequences were obtained. (XLSX 15.9 kb).
Suppl. Table 6
Comparison of morphology of adults in four genetically distinguished lineages of Australapatemon and A. mclaughlini n. sp., with ten species of Australapatemon. Listed are differences between the lineage and the species (e.g., adults of A. mclaughlini n. sp. are smaller than A. anseris, but have larger eggs). TL=total length; OS=oral sucker; PH=pharynx; VS=ventral sucker; FB=forebody; HB=hindbody; HB:FB=hindbody length/forebody length; AT=anterior testis; PT=posterior testis; GC=genital cone. (XLSX 11 kb).
Rights and permissions
About this article
Cite this article
Gordy, M.A., Locke, S.A., Rawlings, T.A. et al. Molecular and morphological evidence for nine species in North American Australapatemon (Sudarikov, 1959): a phylogeny expansion with description of the zygocercous Australapatemon mclaughlini n. sp.. Parasitol Res 116, 2181–2198 (2017). https://doi.org/10.1007/s00436-017-5523-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-017-5523-x