Abstract
The lungworm Aelurostrongylus abstrusus is the most important respiratory parasite of domestic cats. Pulmonary aelurostrongylosis has been reported in wild felids, though unequivocally evidence of wildlife infection by A. abstrusus is scant. Recently, Troglostrongylus brevior, a lungworm usually infecting wild felids, has been described in domestic cats from Mediterranean areas. The present work evaluates the sequence variation of an informative region within the gene encoding the mitochondrial cytochrome c oxidase subunit 1 of A. abstrusus and T. brevior, in order to provide novel information on the genetic make-up of these lungworms. Parasitic stages of A. abstrusus and T. brevior were collected from domestic and wild hosts (i.e., domestic cat, European wildcat, caracal, serval, and lion) from Italy, Greece, and South Africa. Five (HI-HV) and four (HI-HIV) haplotypes were recorded for A. abstrusus and T. brevior, respectively, mostly shared between domestic and wild felids in different geographical areas. The phylogenetic analysis showed that all haplotypes of A. abstrusus and T. brevior clustered as monophyletic groups with a strong nodal support, indicating that all haplotypes identified were distinct from each other. All sequence types represent two distinct species, A. abstrusus and T. brevior, and these genetic convergences are also detected within and among populations of these nematodes, irrespective of their hosts and geographical origin. The occurrence of A. abstrusus and T. brevior haplotypes in different hosts from the same regions and between different countries indicates that the same lungworm populations circulate in domestic and wild hosts under the same routes of transmission.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The cat lungworm Aelurostrongylus abstrusus (Nematoda, Metastrongyloidea) has been considered for a long time the only metastrongyloid nematode affecting the respiratory tract of the domestic cat (Felis silvestris catus) (Di Cesare et al. 2015a). This parasite is transmitted by intermediate hosts represented by terrestrial mollusks and causes subclinical to severe respiratory signs in infected cats (Traversa and Di Cesare 2016). Although the clinical scenario may be relevant, life-threatening cases of cat aelurostrongylosis are very few (Traversa and Di Cesare 2013). The international literature reports several cases of A. abstrusus or Aelurostrongylus spp. in felid species other than the domestic cat (Traversa 2014; Di Cesare et al. 2016; Giannelli et al. 2016). Nonetheless, there is few morphological and/or molecular evidence unequivocally showing that A. abstrusus infects a broader range of hosts (Traversa 2014). For instance, the ability of A. abstrusus to cause pulmonary damages in the European wildcat (Felis silvestris silvestris) has been ultimately shown only recently (Veronesi et al. 2016). Also, a definitive evidence that A. abstrusus may infect lions (Panthera leo) has been published only in 2016, along with the first report of aelurostrongylosis in servals (Leptailurus serval) and caracals (Caracal caracal) (Di Cesare et al. 2016).
In the past few years, another lungworm, i.e., Troglostrongylus brevior (Nematoda, Metastrongyloidea), has been unexpectedly recorded from domestic cats living in Mediterranean countries, i.e., Spain, Italy, and Greece (rev. in Di Cesare et al. 2015a). These records of T. brevior in domestic cats were surprising because nematodes of the genus Troglostrongylus usually infect wild felids (Anderson 2000; Brianti et al. 2014). This nematode, also transmitted by slugs and snails, has been firstly described from two species of wild felids (Gerichter 1949). After a local report of few years later in an European wildcat (F. s. silvestris) and in a cat defined as “feral” and of unknown origin from central Italy (Paggi 1959), T. brevior was not described again until few years ago, when single records in domestic cats were published from Ibiza (Jefferies et al. 2010a) and Sicily (Brianti et al. 2012). Since then, T. brevior has been described in F. s. catus from Apennine areas of Italy, Greece, and European islands (Di Cesare et al. 2015b, c, d; Tamponi et al. 2014; Traversa et al. 2014, 2015; Diakou et al. 2014, 2015). Indeed, clinical importance of troglostrongylosis in domestic cats is high, because T. brevior is potentially deadly, especially in kittens and young animals (Brianti et al. 2012; Diakou et al. 2014; Di Cesare et al. 2014a, 2015b; Traversa et al. 2014; Crisi et al. 2015). At the moment, there is uncertainty on the factual origin of this “novel” lungworm in domestic cats, because it is unknown whether T. brevior usually infect cats but it has been most often mistaken for A. abstrusus, or if T. brevior is currently changing its host affiliation from wild felids to domestic hosts (Brianti et al. 2012; Di Cesare et al. 2015a). Though there are pathological, epidemiological, and clinical evidences suggesting that T. brevior is presently spreading in populations of domestic cats as a result of a spill-over from wild felids (Traversa and Di Cesare 2013; Di Cesare et al. 2015a, d), no molecular data have been thus far generated to support this hypothesis. At the same time, further information is necessary to investigate the origin of A. abstrusus and routes of transmission in felids other than the domestic cat. Given the merit in improving knowledge on the genetic features of lungworm populations infecting felid species, the present work evaluated the sequence variation of an informative region within the gene encoding the mitochondrial cytochrome c oxidase subunit 1 (cox1) of A. abstrusus and T. brevior, isolated from domestic and wild felids from different areas.
Materials and methods
First stage larvae (L1) and single adult stages of A. abstrusus (n. 29 samples) and T. brevior (n. 24 samples) were obtained from naturally infected domestic cats and wild felids during previous studies carried out in different regions of Italy, Greece, and South Africa (Table 1).
DNA extracted from adult parasites using the QIAamp Tissue Kit (QIAGEN GmbH, Germany) after disruption with liquid nitrogen, and from larval samples using the QIAampDNA stool Mini Kit (QIAGEN GmbH, Germany) after three freeze (−80 °C)/thaw (100 °C) cycles, was subjected to a PCR specific for an internal region of the cox1 gene of metastrongylids as previously described (Brianti et al. 2012). A negative-control sample containing all of the reaction reagents and sterile distilled water to substitute for the template was added to each PCR run. All amplicons obtained were purified using a QIAquick gel extraction kit (QIAGEN, GmbH, Hilden, Germany) and sequenced directly using BigDye Terminator v.3.1 chemistry (Applied Biosystems, USA). The sequences were compared with those of the DNA of other nematodes available in the GenBank™ using the nucleotide-nucleotide “Basic Local Alignment Search Tool” (BLAST), and analyzed using Data Analysis in Molecular Biology and Evolution version 4.5.55 (DAMBE) and Mega Evolutionary Genetic Analysis version 7.0.20 (MEGA7) software.
The evolutionary relationships of generated sequences were analyzed using the neighbor-joining (NJ) method (Saitou and Nei 1987) using the Tajima-Nei model (Tajima and Nei 1984). The evolutionary distances were computed by MEGA7 (Kumar et al. 2016). The bootstrap consensus trees inferred from over 8000 replicates were taken to represent the evolutionary history of the taxa analyzed (Felsenstein 1985). Toxocara cati (GenBank Accession number NC_010773.1) was chosen as out-group.
Results
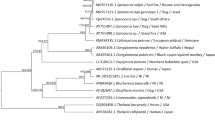
All DNA extracts produced amplicons of ~300 bp, with no intraspecific or interspecific variations for either species. Amplicons of all A. abstrusus and T. brevior isolates from different hosts and geographical areas were successfully sequenced and no nucleotide variations were found in the reverse and forward sequences for each isolate. The alignment of all sequences resulted in a total of 272 analyzed characters including 212 conserved and 60 variable sites, of which 54 were parsimony-informative and 6 were singletons, with an overall pairwise distance of 0.091. The nucleotide frequencies were 22.39% (A), 43.99% (T), 11.92% (C), and 21.71% (G), with an overall transitions/transversion bias of 0.043. Phylogenetic analyses revealed well-defined clades for both A. abstrusus and T. brevior, including all haplotypes identified, that were distinct from each other. The phylograms showed that the five haplotypes of A. abstrusus clustered with a strong nodal support in a monophyletic group, as well as the four haplotypes of T. brevior (Fig. 1). These features indicate that all sequence types represented two distinct species, i.e., A. abstrusus and T. brevior, and these genetic convergences were also detected within and among populations of these nematodes (i.e., haplotypes), irrespective of their hosts and geographical origin (Fig. 1).
Phylogenetic tree based on the neighbor-joining analysis of partial cox1 sequence data for Aelurostrongylus abstrusus (haplotypes HI-HV) and Troglostrongylus brevior (haplotypes HI-HIV). Number of aligned sequences: 54. Number of sites for each sequences: 277. The tree is rooted by outgroup Toxocara cati GenBank accession number NC_010773.1
Five (HI-HV) and four (HI-HIV) haplotypes were recorded for A. abstrusus and T. brevior, respectively (Table 2). The percent identity matrix among A. abstrusus and T. brevior haplotypes ranged from 92.78% (HII vs HV) to 99.64% (HI vs HIII) and from 97.11% (HIV vs HIII and HI) to 98.92% (HII vs HIII and HI), respectively. The most prevalent A. abstrusus and T. brevior haplotypes were HIII (n = 15/29; 51.7%) and HI (n = 7/29; 24.1%), and HII (n = 10/24; 41.7%) and HI (n = 8/24; 33.3%), respectively.
The most diffused haplotypes HI and HIII of A. abstrusus were common in both domestic and wild cats from the same regions of Italy and, moreover, HIII was recorded in domestic cats of Greece and in two wild felids of South Africa (Table 3). HIV and HV were found only in wild felids from South Africa. The most common haplotypes of T. brevior were HI, HII, and HIII. The HI of T. brevior was found in domestic and wild cats from Central Italy, while HII and HIII were present in domestic and wild cats from Central Italy, in domestic cats from Mykonos (Greece) (HII) and in domestic and wild cats from the North of Italy (HIII). HIV was found only in domestic cats from Greece (Table 3).
Discussion
The present results show that the same haplotypes of A. abstrusus (HI-HIII) infecting domestic cats in Italy occur in wildcats living in the same regions (Veronesi et al. 2016). Interestingly, the most common haplotype of A. abstrusus (i.e., HIII) was also here found in domestic cats from Greece and in two wild felids from South Africa, a Country where two further haplotypes (HIV and HV) were described in lions and in a caracal (Table 3). The results here presented support the recent hypothesis that high prevalence of cat aelurostrongylosis in some areas may broaden the host range of A. abstrusus (Di Cesare et al. 2015a; Veronesi et al. 2016). In fact, it should be taken into account that the presence of A. abstrusus in wildcats is uncommon, as shown by most recent studies carried out in other regions of Italy (Beraldo et al. 2014; Falsone et al. 2014) and Germany (Krone et al. 2008). Therefore, as A. abstrusus haplotypes here found in F. s. silvestris are widely spread in domestic cats living in the same regions (Tables 2 and 3), a spill-over may occur from domestic hosts to wildlife in territories with high prevalence of cat aelurostrongylosis (Di Cesare et al. 2015d).
Also, it is here shown that the same populations of T. brevior co-infect F. s. catus and F. s. silvestris in the same study areas of Europe. No haplotypes of this nematode resulted confined in either hosts with a single exception represented by one haplotype (HIV) recorded in two regions of Greece, i.e., Crete island and Attica (Athens) (Table 3). It is not known why T. brevior has been diagnosed in domestic cats only in the past few years. It is undoubted that the increase in the number of troglostrongylosis cases in domestic cats could be due to an increased awareness of this parasitosis (Brianti et al. 2014). However, several evidence have indicated that biological and epizootiological drivers may potentially change the host affiliation of lungworms and have indicated that T. brevior occurs in domestic cats living in areas that offer suitable routes of development and transmission from the wild reservoirs (Di Cesare et al. 2015a). The most important epizootiological indication is that in the vast majority of the cases T. brevior has been up to now isolated from domestic cats living in regions where populations of F. s. silvestris live in sympatry and are infected by this lungworm with high percentage (Di Cesare et al. 2015a). The occurrence of the same haplotypes of T. brevior in domestic and wild cats indicates that this lungworm finds its way to parasitize F. s. catus under certain circumstances and that a spill-over of T. brevior from wild to domestic hosts occurs in some regions of Mediterranean areas. The possible reasons for such epizootiological modifications have been thoroughly described in a recent article (Di Cesare et al. 2015a) and will not be discussed further.
An interesting exception is represented by T. brevior HIV that has been here found only in domestic cats from two Greek regions (Table 3). The European wildcat is present in Crete (Diakou et al. 2014) but, unfortunately, the absence of any information on the occurrence of T. brevior in wildlife of this island prevents from any further analysis or considerations. A future availability and analysis of T. brevior isolates from wildcats of Crete would allow to perform detailed comparisons on the genetic features of these populations. Importantly, F. s. silvestris is not recorded in Mykonos island, where T. brevior HIV was found in domestic cats (Diakou et al. 2015). These results need to be interpreted with caution because few specimens were examined from these areas and it is plausible that also the other haplotypes of this lungworm occur in domestic cats from the same regions. For instance, domestic cats from Mykonos island might harbor T. brevior HII, that has also been recorded in wildcats and F. s. catus from continental Europe (Tables 2 and 3). As wildcats are not present in Mykonos (Diakou et al. 2015), these isolates may originate from movements of pets and/or introduction of paratenic hosts, as previously discussed (Diakou et al. 2015). Although it is possible that T. brevior HIV occurs where the other sub-populations are spread, at the same time its apparent confinement due to geographical barriers, i.e., a major cause for parasite segregation and confined evolution of isolated genetic populations (Traversa et al. 2008), should be taken into account. Other than the obvious natural barriers of the Island of Crete, one should consider that the region of Attica, where T. brevior was recorded, is separated from the North by four mountains, ranging from 468 to 1.413 m altitude. Because the presence of the sea at West, East, and South of Attica, this area is somehow isolated from the rest of continental Greece. Furthermore, there is a large area extending to the North of Attica where no wildcats have ever been recorded (Yamaguchi et al. 2015), and this could represent a barrier for the flow of wildcat haplotypes to domestic hosts. At the same time, this could be a possible explanation for the segregation of T. brevior HIV only in domestic hosts since a long time.
The occurrence of A. abstrusus HIII in wildlife from South Africa clearly shows that this haplotype is able to circulate among several species of felids, even from countries far from each other. Conversely, the absence of A. abstrusus HIV and HV in Europe could be due to a geographical confinement of these populations in the southern hemisphere and/or only in large wild felids. Unfortunately, the absence of information on the genetic make-up of lungworms in domestic cats from the same African territories prevents any further comment on the potential circulation of the same parasite populations in wild and domestic hosts, as here found for two study countries of Europe.
The here reported evidence for bridging infections by lungworms from and to domestic and wild cats due recent changes in the biology and phenology of intermediate and definitive hosts (Di Cesare et al. 2015a) is not surprising if one considers that other recent studies have provided similar evidence for other cardio-respiratory nematodes. In fact, the same haplotypic sub-populations of the respiratory trichuroid Capillaria aerophila (syn. Eucoleus aerophilus) have recently been found to be shared between wild animals (e.g., foxes, beech martens) and companion cats and dogs, thus indicating the existence of common patterns of transmission for extra-intestinal nematodes (Di Cesare et al. 2014b). Analogously, molecular analyses using mitochondrial and nuclear DNA markers confirmed that Angiostrongylus vasorum genotypes are well mixed between dogs and foxes in Europe supporting the hypothesis that transmission occurs between wild and domestic canids (Jefferies et al. 2010b). Along with the increasing records of A. vasorum in dogs and foxes, genetically identical populations of this nematode have been found in wolves from Italy (Eleni et al. 2013).
Another similar overflow between domestic and wild felids has been recently proposed to explain the presence of Ancylostoma tubaeforme in the Iberian lynx from Spain, as the transmission of monoxenous parasites is related to host density and remaining lynx populations in the study region are very small (Millán and Blasco-Costa 2012). Analogously, sympatric domestic cat populations have been indicated as a potential reservoir for A. tubaeforme infection for the lynx based on the existence of shared hookworm haplotypes (Millán and Blasco-Costa 2012).
In conclusion, as previously seen for other extra-intestinal and intestinal parasites of companion animals, the present information illustrates that some sub-populations of the felid lungworms A. abstrusus and T. brevior are shared between common hosts and other closely-related animals. Recent works have suggested a spreading of felid lungworms and a change in host affiliation of T. brevior, at least in some Mediterranean areas (Di Cesare et al. 2014a; Diakou et al. 2015; Traversa et al. 2014, 2015). The present study support the existence of common patterns of transmission for haplotypes shared among different felid hosts. Further studies would be warranted to evaluate these epizootiological affiliations and modifications in broader geographic areas, for instance implementing knowledge on the current occurrence of A. abstrusus and T. brevior in large felids. Moreover, appropriate diagnostic approach and specific control measures in the domestic cat populations living into or near the areas were T. brevior is spreading are encouraged in order to prevent further transmission of this harmful parasite in domestic felid populations. Simultaneously, the control of the infection by A. abstrusus in domestic cats is warranted to avoid the spreading of the infection also in wild hosts, where it can cause pulmonary lesions and damage (Veronesi et al. 2016).
References
Anderson RC (2000) Nematode parasites of vertebrates their development and transmission, 2nd edn. CABI International, Wallingford
Beraldo P, Massimo M, Pascotto E (2014) Analysis of the Helminthofauna of European wild cat in Friuli Venezia Giulia. In: Proceedings of XXVIII Congresso Nazionale Società Italiana di Parassitologia. Rome, Italy, 24–27 June, pp. 225.
Brianti E, Gaglio G, Giannetto S, Annoscia G, Latrofa MS, Dantas-Torres F, Traversa D, Otranto D (2012) Troglostrongylus brevior and Troglostrongylus subcrenatus (Strongylida: Crenosomatidae) as agents of broncho-pulmonary infestation in domestic cats. Parasit Vectors 5:178
Brianti E, Giannetto S, Dantas-Torres F, Otranto D (2014) Lungworms of the genus Troglostrongylus (Strongylida: Crenosomatidae): neglected parasites for domestic cats. Vet Parasitol 202(3–4):104–112
Crisi PE, Traversa D, Di Cesare A, Luciani A, Civitella C, Santori D, Boari A (2015) Irreversible pulmonary hypertension associated with Troglostrongylus brevior infection in a kitten. Res Vet Sci 102:223–227
Diakou A, Di Cesare A, Aeriniotaki T, Traversa D (2014) First report of Troglostrongylus brevior in a kitten in Greece. Parasitol Res 113(10):3895–3898
Diakou A, Di Cesare A, Barros LA, Morelli S, Halos L, Beugnet F, Traversa D (2015) Occurrence of Aelurostrongylus abstrusus and Troglostrongylus brevior in domestic cats in Greece. Parasit Vectors 8:590
Di Cesare A, Frangipane di Regalbono A, Tessarin C, Seghetti M, Iorio R, Simonato G, Traversa D (2014a) Mixed infection by Aelurostrongylus abstrusus and Troglostrongylus brevior in kittens from the same litter in Italy. Parasitol Res 113(2):613–618
Di Cesare A, Otranto D, Latrofa MS, Veronesi F, Perrucci S, Lalosevic D, Gherman CM, Traversa D (2014b) Genetic variability of Eucoleus aerophilus from domestic and wild hosts. Res Vet Sci 96(3):512–515
Di Cesare A, Veronesi F, Traversa D (2015a) Felid lungworms and heartworms in Italy: more questions than answers? Trends Parasitol 31(12):665–675
Di Cesare A, Iorio R, Crisi P, Paoletti B, Di Costanzo R, Dimitri CF, Traversa D (2015b) Treatment of Troglostrongylus brevior (Metastrongyloidea, Crenosomatidae) in mixed lungworm infections using spot-on emodepside. J Feline Med Surg 17(2):181–185
Di Cesare A, Di Francesco G, Frangipane di Regalbono A, Eleni C, De Liberato C, Marruchella G, Iorio R, Malatesta D, Romanucci MR, Bongiovanni L, Cassini R, Traversa D (2015c) Retrospective study on the occurrence of the feline lungworms Aelurostrongylus abstrusus and Troglostrongylus spp. in endemic areas of Italy. Vet J 203(2):233–238
Di Cesare A, Veronesi F, Grillotti E, Manzocchi S, Perrucci S, Beraldo P, Cazzin S, De Liberato C, Barros LA, Simonato G, Traversa D (2015d) Respiratory nematodes in cat populations of Italy. Parasitol Res 114(12):4463–4469
Di Cesare A, Laiacona F, Iorio R, Marangi M, Menegotto A (2016) Aelurostrongylus abstrusus in wild felids of South Africa. Parasitol Res 115(10):3731–3735
Eleni C, De Liberato C, Azam D, Morgan ER, Traversa D (2013) Angiostrongylus vasorum in wolves in Italy. Int J Parasitol Parasites Wildl 3:12–14
Falsone L, Brianti E, Gaglio G, Napoli E, Anile S, Malias E, Giannelli A, Poglayen G, Giannetto S, Otranto D (2014) The European wildcats (Felis silvestris silvestris) as reservoir hosts of Troglostrongylus brevior (Strongylida: Crenosomatidae) lungworms. Vet Parasitol 205:193–198
Felsenstein J (1985) Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39:783–791
Gerichter CB (1949) Studies on the nematodes parasitic in the lungs of Felidae in Palestine. Parasitology 39(3–4):251–262
Giannelli A, Kirkova Z, Abramo F, Latrofa MS, Campbell B, Zizzo N, Cantacessi C, Dantas-Torres F, Otranto D (2016) Angiostrongylus chabaudi in felids: new findings and a review of the literature. Vet Parasitol 228:188–192
Jefferies R, Vrhovec MG, Wallner N, Catalan DR (2010a) Aelurostrongylus abstrusus and Troglostrongylus sp. (Nematoda: Metastrongyloidea) infections in cats inhabiting Ibiza, Spain. Vet Parasitol 173:344–348
Jefferies R, Shaw SE, Willesen J, Viney ME, Morgan ER (2010b) Elucidating the spread of the emerging canid nematode Angiostrongylus vasorum between Palearctic and Nearctic ecozones. Infect Genet Evol 10:561–568
Krone O, Guminsky O, Meinig H, Herrmann M, Trinzen M, Wibbelt G (2008) Endoparasites spectrum of wild cats (Felis silvestris Schreber, 1777) and domestic cats (Felis catus) from the Eifel, Pfalz region and Saarland, Germany. Eur J Wildl Res 54:95–100
Kumar S, Stecher G, Tamura K (2016) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33(7):1870–1874
Millán J, Blasco-Costa I (2012) Molecular evidence of shared hookworm Ancylostoma tubaeforme haplotypes between the critically endangered Iberian lynx and sympatric domestic cats. Vet Parasitol 186:518–522
Paggi L (1959) Segnalazione, in Italia Centrale di Troglostrongylus sp. parassita dei polmoni di felidi. Parassitologia 1:80–81
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4(4):406–425
Tajima F, Nei M (1984) Estimation of evolutionary distance between nucleotide sequences. Mol Biol Evol 1:269–285
Tamponi C, Varcasia A, Brianti E, Pipia AP, Frau V, Pinna Parpaglia ML, Sanna G, Garippa G, Otranto D, Scala A (2014) New insights on metastrongyloid lungworms infecting cats of Sardinia, Italy. Vet Parasitol 203(1–2):222–226
Traversa D (2014) Response to Otranto et al.: lungworms in domestic and wild felids: dilemmas still persisting. Trends Parasitol 30(2):53–54
Traversa D, Di Cesare A (2013) Feline lungworms: what a dilemma. Trends Parasitol 29(9):423–430
Traversa D, Di Cesare A (2016) Diagnosis and management of lungworm infections in cats: cornerstones, dilemmas and new avenues. J Feline Med Surg 18(1):7–20
Traversa D, Kuzmina T, Kharchenko VA, Iorio R, Klei TR, Otranto D (2008) Haplotypic variability within the mitochondrial gene encoding for the cytochrome c oxidase 1(cox1) of Cylicocyclus nassatus (Nematoda, Strongylida): evidence for an affiliation between parasitic populations and domestic and wild equid hosts. Vet Parasitol 156(3–4):241–247
Traversa D, Romanucci M, Di Cesare A, Malatesta D, Cassini R, Iorio R, Seghetti M, Della Salda L (2014) Gross and histopathological changes associated with Aelurostrongylus abstrusus and Troglostrongylus brevior in a kitten. Vet Parasitol 201(1–2):158–162
Traversa D, Lepri E, Veronesi F, Paoletti B, Simonato G, Diaferia M, Di Cesare A (2015) Metastrongyloid infection by Aelurostrongylus abstrusus, Troglostrongylus brevior and Angiostrongylus chabaudi in a domestic cat. Int J Parasitol 45(11):685–690
Veronesi F, Traversa D, Lepri E, Morganti G, Vercillo F, Grelli D, Cassini R, Marangi M, Iorio R, Ragni B, Di Cesare A (2016) Occurrence of lungworms in European wildcats (Felis silvestris silvestris) of central Italy. J Wildl Dis 52(2):270–278
Yamaguchi N, Kitchener A, Driscoll C, Nussberger B (2015) Felis silvestris. The IUCN Red List of Threatened Species 2015: e.T60354712A50652361. http://dx.doi.org/10.2305/IUCN.UK.2015-2.RLTS.T60354712A50652361.en. Downloaded on 27 November 2016
Acknowledgements
The authors are grateful to Dr. Alessia Menegotto (Conservation Global Agency for environmental gain npc, South Africa) and Francesca Laiacona (Faculty of Veterinary Medicine of Teramo, Italy) for collaborating in collecting samples from wild felids from South Africa (specimens from Caracal caracal, Leptailurus serval, and Panthera leo; Table 1) and to Dr. Paola Beraldo (University of Padua, Italy) to provide sample 18 of T. brevior from Wild Cat n. 11 (Table 1).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statement
Procedures applied to collect parasitic stages analyzed in the present study are not within the context of EU legislation for animal experimentations. Samples were collected by veterinarians and caused no suffering to the animals. All applicable international, national, and/or institutional guidelines for the care of animals were followed. This article does not contain any studies with human participants performed by any of the authors.
Rights and permissions
About this article
Cite this article
Traversa, D., Veronesi, F., Diakou, A. et al. Mitochondrial haplotypes of Aelurostrongylus abstrusus and Troglostrongylus brevior (Nematoda, Metastrongyloidea) from domestic and wild felids. Parasitol Res 116, 1227–1235 (2017). https://doi.org/10.1007/s00436-017-5399-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-017-5399-9