Abstract
The study describes the morphological changes associated with parasitism by the intestinal acanthocephalan Neoechinorhynchus buttnerae in tambaqui juveniles Colossoma macropomum farmed in an excavated nursery, in Manaus (Amazon) in September 2013. After fish biometrics, analysis of macroscopic changes in morphology and counting of parasites, bowel fragments were fixed and submitted to histological and histochemical processing. All fish analyzed had acanthocephalans in the intestine; intestinal loops were milky white in color, with the presence of nodules with heavy parasitism. The changes in tissues that form the intestine varied according to the arrangement of the parasites: either free in the intestinal lumen or fixed by the proboscis on the organ wall. In the first case, the changes found were flaking, abrasion, compression, hypertrophy of goblet cells and disappearance of the villi on the mucosa, leukocytic cell infiltration in the submucosa, and muscle layer thickening. In the second case, in addition to these, other changes were observed as metaplasia in muscle tissue with its replacement by a loose connective tissue with severe leukocytic infiltration, edema in blood vessels, and necrotic foci. The histochemical analysis revealed that positive Alcian Blue mucosal cells (pH 2.5) were more expressive in parasitized intestines than in intestines not parasitized by N. buttnerae.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The production of round fish in fish farm has grown significantly in Brazil in recent years, equaling the production of tilapia in the biennium 2013/2014, and this increase is mainly due to tambaqui, main native species grown in the country. The production of this species in the north is relatively recent, about 10 years, compared to the other states, and most of the producers in these regions do not apply sanitary control measures, which causes increased problems such as the spread of pathogens (CNA 2016).
The tambaqui (Colossoma macropomum) is a fish species native to the Amazon region and is farmed in many regions in Brazil (Ribeiro et al. 2016). It originates from South America, widely distributed in the basins of the Orinoco and Amazon rivers, and is one of the species of greatest importance in the Amazon fish populations. It lives in lakes and flooded marginal areas associated to the rails of the main rivers and can reach over a meter in length and reach 30 kg. It is considered the largest Characiformes in the Amazon Basin (Araujo-Lima and Goulding 1998). In the North and Northeast of Brazil, the production of this species in captivity is increasing, where the intensive culture system is most commonly used. This system, characterized by increased stocking density for ultimate production, can make fish more susceptible to stress and, consequently, to parasitic infections (Tavares-Dias et al. 2001).
For the last 10 years, research has shown the occurrence of diseases in farmed tambaquis caused by parasites of different taxonomic categories: protozoans, monogenoids, crustaceans, trematodes, cestodes, mixosporids, and acanthocephalans (Grigório 2013; Santos et al. 2013; Godoi et al. 2012; Morais et al. 2009; Araujo-Lima and Gomes 2005; Malta et al. 2001). Among these parasites, the acanthocephalan Neoechinorhynchus buttnerae (Golvan, 1956), an intestinal endoparasite, has become a constraint on Brazilian fish farming, especially in the North region, where reports informing about a reduction in health and quality of the tambaqui, due to high parasitic loads, have become more frequent (Santos et al. 2013; Martins et al. 2000). Acanthocephalans attach to the intestine of definitive hosts by means of a spiny proboscis (Nickol 2006). In the tambaqui, morphological reactions due to the presence of N. buttnerae in the intestinal lumen have been poorly studied and have only been based on gross examination (Fischer et al. 2003; Malta et al. 2001; Thatcher 1981). However, in Brazil, there are acanthocephalan reports of the Neoechinorhynchus gender, parasitizing the curimbatá Prochilodus lineatus (Martins et al. 2001) and the hake Plagioscion squamosissimus (Melo et al., 2014), where authors describe significant changes such as flaking of the intestinal epithelium, hyperplasia, hypertrophy of goblet cells, and submucosal inflammatory reaction around the proboscis attachment point. Considering the lack of morphological, clinical, and histopathological information on acanthocephalosis of economic importance among fishes in the Amazon region, the aim of the present study was to report the histological and histochemical manifestations in farmed tambaqui juveniles, C. macropomum, resulting from high infection with N. buttnerae.
Material and methods
Fish sampling
Juveniles of C. macropomum (n = 28) (weight 57.7 ± 21.1 and length 12.5 ± 1.2) were collected in Amazon State, Brazil, from an intensive fish farm nearby, Manaus city (township 2° 54″ 36.87″ S/59° 19″ 41.7″ O, September 2013). All animals were anesthetized for the analytical procedures, following the institutional guidelines for animal welfare (Animal Experimentation Ethics Committee, CEAA-UFAM protocol no. 002/2014).
Parasitic analysis
After biometry procedures, each fish was sacrificed via spinal concussion; intestines were removed after macroscopic examinations and were opened longitudinally to quantify the presence of acanthocephalan parasites under a Leica Stereomicroscope, model EZ4. The parasitic indexes (prevalence and intensity) were calculated according to the methodology established by Bush et al. (1997).
Gut histopathological analysis
Tissue samples from the second portion of the gut, immediately after pyloric caeca were collected, were fixed in 10% buffered formalin and processed according to the methodology described by Culling et al. (1985). The samples were dehydrated in a graded series of ethanol and embedded in paraffin. The sections of 5 μm were obtained, and three slides of each fish were prepared and stained with H&E (Bancroft and Gamble 2002), Periodic acid-Schiff (PAS), and Alcian Blue (pH 1.0 and 2.5) (Pinky et al. 2008). The analyses under light microscopy were performed with a Leica optical microscope, model DM 500.
Scanning electron microscopy
For scanning electron microscopy, parasites were fixed in 2.5% glutaraldehyde prepared in 0.1 M sodium cacodylate buffer (pH 7.2–7.4), dehydrated in graded ethanol and acetone series, critical point dried (CO2), metalized with gold, and observed under a scanning electron microscope (SEM) (JEOL 6490 LA, Japan) at an acceleration voltage of 8 kV, and electron micrographs were taken. All photographies and microphotographies were taken with a digital camera (model Sony DSC-W120).
Results
Parasite morphology
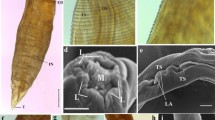
In the N. buttnerae, as described by Golvan (1956), the trunk is fusiform and curved ventrally; it has almost the entire length of the body (Fig. 1d). Scanning electron microscopic analyses showed that the parasite has a small proboscis when compared with the rest of the body. The proboscis is spherical and armed with three types of hooks arranged in six spirals, each with three hooks. The first large upper hooks are arranged in three superimposed plans. Six medium hooks are arranged in a single plan as are also the six small lower hooks (Fig. 1c). Genital pore is terminal in males and sub-terminal in females (Fig. 1a, b, respectively). In females, the vagina is peculiarly coiled (Fig. 1a).
Parasitic index and intestinal gross morphology
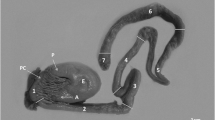
In all C. macropomum specimens analyzed, N. buttnerae were found in the intestine, with a prevalence of 100%. The average intensity of infection was 240.4, ranging from moderate to very strong, with an average of 293.8 ± 173.6 parasites per fish and minimum and maximum values of 81 and 708 parasites per fish, respectively. Infected intestines were extended, with a milky white color or with the presence of yellowish white nodules (Fig. 2b, c) in sites that were heavily infected. A cross section showed that the intestine wall was thickened with numerous parasites blocking the lumen (Fig. 2d, e).
a Lateral anatomical view of juvenile specimen of Colossoma macropomum. b Presence of nodules (arrows) throughout the bowel. c Bowel extended with milky white color due to the massive infection. d, e Transverse and longitudinal sessions, respectively, showing massive occupation of parasites in the lumen
Gut histopathology
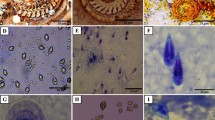
In juveniles of C. macropomum, the H&E sections of the infected intestines showed that the massive presence of N. buttnerae induced reactions in the four concentric layers that constitute the intestinal wall, the tunicas: mucosa, submucosa, muscularis, and serosa. The intensity of the damages in the intestinal wall varied according to the site where the parasites were, when free in the intestinal lumen, a situation that was more frequent; the parasite metasomas, often voluminous, caused compression of the villi (Fig. 3d), flaking of their apices, and abrasion of the epithelium (Fig. 3c). These changes in the architecture of the villi were accompanied by intense leukocyte infiltration, mainly mast cells, lymphocytes, and eosinophils in the lamina propria and in the mucosa-submucosa interface (Figs. 3e and 4a, b). In the submucosal layer, in the loose connective tissue, especially rich in fibroblasts in non-infected fish, lymphocytes and mast cells started to prevail (Fig. 3e). A clear thickening of the muscle layer was observed when compared with the thickness of this layer in fish that were uninfected by N. buttnerae (Fig. 3a, f). This finding was observed even in samples where there was no penetration of the proboscis in the host’s tissue.
Tissue changes in the intestine of C. macropomum parasitized by N. buttnerae. a Gut uninfected showing villi intact and free lumen. b Obstruction of the intestinal lumen due to occupation of numerous parasites (asterisks). c Epithelial abrasion (thin arrow), flaking of the villus (thick arrow), and mucus in the lumen (white arrowhead) adjacent of metasoma (asterisks) of parasite. d Compression (arrowheads) and hypertrophy of globet cells (thin arrows) due to the proximity of the metasoma (asterisk) of the worm. e Infiltration of leukocytes in the submucosa (arrowheads). f Thickening and edema of the muscular layer of blood vessels (arrows). g Disappearance of the villi at the site of penetration of presoma (asterisk) and metaplasia of muscle tissue by loose connective tissue with intense inflammation extending to the mucosal layer (thin arrows). h Blood vessel edema (arrows) and foci of necrosis (arrowheads). Asterisk: proboscis
Cell types found in C. macropomum gut associated with immune response to parasitism by N. buttnerae. a Mucosa. b Submucosa. c Muscle. d Serous. Hypertrophied of goblet cells (asterisks), mast cells (thick arrows), lymphocytes (black arrowheads), eosinophils (white arrowheads), fibroblasts (thin arrows), blood vessel (B), and peritoneal cavity (P)
More severe alterations, especially in the deepest tissues of the intestine wall, were observed in the sites where there were insertions of the parasite’s proboscis and hooks (Fig. 3g, h). At the site where the proboscis penetrated, the villi disappeared through the mucosa underlying the proboscis to be lined by simple squamous epithelium (Fig. 3h); in the submucosa layer, a more intense inflammatory response was observed beyond the edema of blood vessels (Fig. 3h). When the proboscis reached the muscle layer, rarely observed event, other changes besides the thickening were detected: the occurrence of metaplasia with the replacement of muscle tissue by a loose connective tissue with heavy infiltration of macrophages, lymphocytes, eosinophils, and fibroblasts (Fig. 4c, d); foci of necrosis and edema of blood vessels were also observed (Fig. 3g, h). Penetration of the proboscis in the serosa layer and perforation of the intestine wall were not observed in C. macropomum; however, severe leukocytic cell infiltration was observed in the serous regions near the muscle layer reached by the proboscis of the parasite (Figs. 3h and 4d).
Mucin histochemistry
The results on the detection of different mucins in uninfected and infected fish are summarized in Table 1. Uninfected fish showed from slight to moderate PAS-positive carbohydrates in the intestine’s goblet cells (GC) (Fig. 5a). A decrease in the intensity of PAS staining was evident in infected fish (Fig. 5b). Acidic mucins were scarce in the intestinal GC in both infected and uninfected fish in the AB 1.0 staining (Fig. 5c, d). The most evident change observed in parasitized fish as opposed to uninfected fish was the increase of the intensity in the AB 2.5 staining in parasitized fish (Fig. 5e, f). Strong AB 2.5 positive reaction was also observed in the lumen, near the parasite’s body surface. The intensity of AB (2.5) + PAS reaction in GC intestine cells changed from slight in no uninfected fish to moderate in infected fish (Fig. 5g, h).
Discussion
World aquaculture production is vulnerable, and an increase of disease outbreaks has been reported due to culture intensification, resulting in partial or total loss of production (Bondad-Reantaso et al. 2005). In this aspect, acanthocephalosis has become a great constraint in tambaqui fish farming in northern Brazil. In contrast with wild stocks, supplies of C. macropomum from aquaculture are increasing, and the species is now the most important farmed fish in northern Brazil, where it corresponds to more than 70% of the production of the region’s inland aquaculture operations (Ostrenky et al. 2008). A high infection rate by N. buttnerae in farmed C. macropomum was observed by Malta et al. (2001), and it has been observed in the present study. Much higher values for parasitic indexes by this acanthocephalan have been observed in farmed juvenile specimens; these findings have currently and commonly been reported by many fish farmers in the region. The increase in the intensity of this acanthocephalosis is worrisome and according to Chagas (2015) may possibly be due to the intensification of production systems in the region without the use of good health management practices. Studies in fish parasites are nowadays one of the most important fields of research in the Amazon region, especially when we take into account the importance that farmed fish have as a valuable source of protein, in which heavy infection may cause morphological changes that compromise production. In fishes, many infectious diseases display chronic characteristics that include clinical signs that persist for weeks, during which mortality rate increases gradually and the cumulative mortality can be high (Plumb et al. 2011). According to Thatcher (1981) intestinal occlusion, observed in the present work as well, is common in acanthocephalosis, including in C. macropomum infected with a large number of Neoechinorhynchus sp. The yellowish white nodules observed in the present work are associated with the intestine’s sites where there is a massive presence of the parasites, not corresponding, however, to the fibrotic nodules induced on the intestinal surface by deeply penetrated worms or heavy infections, which are frequent signs of infection by acanthocephalans (Sanil et al. 2010; Irshadullah and Mustafa 2012; Dezfuli 1991).
Studies on tissue changes caused by acanthocephalans in Amazonian fish are scarce (Ferraz de Lima et al. 1990; Melo et al. 2014). The occurrences of flaking, abrasion, and compression of the villi in the tambaqui, frequent when parasites are free in the lumen, were similar to changes by Neoechinorhynchus in two Amazonian fish species: Piaractus mesopotamicus (Ferraz de Lima et al., 1990) and Pl. squamosissimus; however, differently from what was observed in C. macropomum, there was the presence of hemorrhagic foci in Pi. mesopotamicus (Ferraz de Lima et al., 1990). A decrease in the number of goblet cells in Pl. squamosissimus (Melo et al., 2014) was not observed. The marked thickening of the muscle layer by hyperplasia of smooth muscle cells observed in all specimens analyzed in this study had not yet been reported in infections caused by acanthocephalans in Amazonian fish. Macroscopic evidence of this thickening was reported by Sanil et al. (2010) at the points where the acanthocephalan fixed its proboscis Tenuiproboscis sp. in the intestine of Lutjanus argentimaculatus. However, in this study, no relationship was observed between the thickening of the muscular layer and the penetration of the proboscis, since most parasites were free in the intestinal lumen. The thickening might be a response to the large amount of parasites, probably with a view to maintaining the integrity of the intestinal wall.
More drastic tissue damage occurred in the sites where the proboscis penetrated in the intestines of C. macropomum and the inflammatory responses in this case were more pronounced. Metaplasia in muscle tissue by a loose connective tissue with inflammatory infiltrate adjacent to the penetration of the proboscis of N. buttnerae was similar to changes documented by Kim et al. (2011) and Roubal (1992), but without the fibrous capsule formation described by the authors (Dezfuli et al. 2002; Sanil et al. 2010 and Dezfuli et al. 2011). The formation of the capsule is not always a result of infection by acanthocephalans, and when it occurs, it may well be associated with the presence of longer proboscises and a higher number of hooks.
Different cell types are associated with innate immunity in fish. In C. macropomum heavily parasitized by N. buttnerae, mast cells, lymphocytes, and eosinophils were present in greater quantities in places where there was a penetration of the proboscis. Mast cells, identified by their eosinophilic granules (Reite and Evensen 2006; Dezfuli et al. 2015), are commonly reported at sites of infection in fish infected by helminths (Reite and Evensen 2006; Dezfuli et al. 2007, 2008, 2009a, b, 2011) probably being involved in the induction of inflammatory responses by their effects on vasodilation, attraction of neutrophils, and macrophage activation. Lymphocytes, neutrophils, and macrophages are commonly involved in cell responses in fish infected by acanthocephalans (Roubal 1992; Dezfuli et al. 2002; Sanil et al. 2010). Works with Amazonian fish that are parasitized by acanthocephalans give little or no emphasis on the cell types involved in the inflammation process; this way, they lack more research and detailing.
The first level of defense against gastrointestinal helminths is the secretion of various substances into the lumen, which can include mucus (Wallace and Martin 2001). Regarding the composition of mucins, the most intense reaction of goblet cells to the Alcian Blue (pH 2.5), revealed by histochemical analyses in C. macropomum, indicated increased production of acidic glycoconjugates, event similar to that reported by Dezfuli et al. (2009a, b) and Bosi and Dezfuli (2015). According to Bosi and Dezfuli (2015), the function of blanket of mucus is mainly to protect the intestinal mucosa as a physical barrier against the mechanical and biochemical damages induced by parasites and Arellano et al. (2001) correlated such functions with the production of acid mucins.
The results presented here indicate that, unlike acanthocephalosis caused by specimens with a long neck and proboscis and that penetrate deep into the digestive tract of the host causing extensive damage (Taraschewski 2000), N. buttnerae was seldom observed penetrating its short proboscis into the gut wall, rarely reaching the muscle layer and never piercing the organ. Still, high infection by N. buttnerae causes morphological damages to the intestine, taking the role of the organ into account when it comes to the absorption of nutrients it can compromise the quality of C. macropomum juveniles in the stage of life in which the fish are obtained by fish farmers in the region for growth and fattening. The knowledge about the immune responses of the tambaqui to helminths is very limited. Our results point to the need for further research about the occurrence of this acanthocephalosis, with emphasis on the related pathology and its effect on production, with a view to establishing efficient control strategies.
References
Araujo-Lima CARM, Gomes LC (2005) Tambaqui (Colossoma macropomum). In: Baldisserotto B, Gomes LC (eds) Espécies nativas para piscicultura no Brasil. UFSM, Santa Maria, pp 67–104
Araujo-Lima C, Goulding M (1998) Os frutos do tambaqui: ecologia, conservação e cultivo na Amazônia. Lithera Ed. Sociedade Civil Mamirauá, CNPq, Tefé, AM, Brasil 186p
Arellano JM, Storch V, Sarasquete C (2001) Histological and histochemical observations in the stomach of the Senegal sole, Solea senegalensis. Histology and Histopathology, Murcia 16(2):511–521
Bancroft JD, Gamble M (2002) Theory and practice of histological techniques. 5th Ed. Edinburgh. Churchill Livingstone Pub. 172(5):593–620
Bondad-Reantaso MG, Subasinghe RP, Arthur JR, Ogawa K, Chinabut S, Adlard R, Tan Z, Shariff M (2005) Disease and health management in Asian aquaculture. Vet Parasitol 132:249–272
Bosi G, Dezfuli BS (2015) Responses of Squalius cephalus intestinal mucous cells to Pomphorhynchus laevis. Parasitol Int 64:167–172
Bush AO, Lafferty KD, Lotz JM, Shostak W (1997) Parasitology meets ecology on its own terms: Margolis et al. revisited. J Parasitol 83:575–583
Chagas EC (2015) Infecções por acantocéfalos: um problema para a produção de peixes. In:Tavares-Dias M, Mariano WS. (Org). Aquicultura no Brasil: novas perspectivas. São Carlos, Editora Pedro e João
CNA (2016) Ativos Aquicultura. Ano 2. Edição 7
Culling CFA, Allison RT, Barr WT (1985) Cellular pathology technique, 4th edn. Butterworths, London, p 642
Dezfuli BS (1991) Histopathology in Leuciscus cephalus (Pisces: Cyprinidae) resulting from infection with Pomphorhynchus laevis (Acanthocephala). Parassitologia 33:137–145
Dezfuli BS, Giari L, Simoni E, Bosi G, Manera M (2002) Histopathology, immunohistochemistry and ultrastructure of the intestine of Leuciscus cephalus (L.) naturally infected with Pomphorhynchus laevis (Acanthocephala). J Fish Dis 25:7–14
Dezfuli BS, Pironi F, Shinn AP, Manera M, Giari L (2007) Histopathology and ultrastructure of Platichthys flesus naturally infected with Anisakis simplex s.l. larvae (Nematoda: Anisakidae). J Parasitol 93:416–423
Dezfuli BS, Giovinazzo G, Lui A, Giari L (2008) Inflammatory response to Dentitruncus truttae (Acanthocephala) in the intestine of brown trout. Fish & Shellfish Immunology 24:726–733
Dezfuli BS, Lui A, Giovinazzo G, Boldrini P, Giari L (2009a) Intestinal inflammatory response of powan Coregonus lavaretus (Pisces) to the presence of acanthocephalan infections. Parasitology 136:929–937
Dezfuli BS, Szekely C, Giovinazzo G, Hills K, Giari L (2009b) Inflammatory response to parasitic helminthes in the digestive tract of Anguilla anguilla (L.). Aquaculture 296:1–6
Dezfuli BS, Castaldelli G, Bo T, Lorenzoni M, Giari L (2011) Intestinal immune response of Silurus glanis and Barbus barbus naturally infected with Pomphorhynchus laevis (Acanthocephala). Parasite Immunol 33:116–123
Dezfuli BS, Manera M, Giari L, DePasquale JA, Bosi G (2015) Occurrence of immune cells in the intestinal wall of Squalius cephalus infected with Pomphorhynchus laevis. Fish Shellfish Immunol. 47:556–564
Ferraz de Lima CLB, Ceccarelli PS, Reis NS (1990) Aspectos histopatológicos da acantocefalose em Piariactus mesopotamicus Holmberg, 1887. B. Téc. CEPTA, Pirassununga 3(1):55–63
Fischer C, Malta JCO, Varella AMB (2003) Fauna parasitária do tambaqui Colossoma macropomum (Cuvier, 1818) (Characiformes: Characidae) do médio rio Solimões, Estado do Amazonas (AM) e do baixo rio Amazonas Estado do Pará (PA), e seu potencial como indicadores biológicos. Acta Amaz 33(4):65–662
Godoi MMIM, Engracia V, Lizama MLAP, Takemoto RM (2012) Parasite-host relationship between the tambaqui (Colossoma macropomum Cuvier 1818) and ectoparasites, collected from fish farms in the City of Rolim de Moura, State of Rondônia, Western Amazon, Brazil. Acta Amaz 42:515–524
Golvan YJ (1956) Acanthocephales d’amazonie. Redescription d’Oligacanthorhynchus iheringi Travassos, 1916 et description de Neoechinorhynchus buttnerae n. sp. (Neoacanthocephala – Neoechinorhynchidae). Annales de Parasitologie Humaine et Compare’e 31:500–524
Grigório, MKRD (2013) Diversidade parasitária e relação parasito-hospedeiro em Colossoma macropomum e seu híbrido Tambatinga cultivados em Macapá, Dissertação (mestrado) FUFA, Programa de Pós-Graduação em Biodiversidade Tropica Macapá
Irshadullah M, Mustafa Y (2012) Pathology induced by Pomporhynchus kashmiriensis (Acanthocephala) in the alimentary canal of naturally infected Chirruh snow trout, Schizothorax esocinus (Heckel). Helminthology 49:11–15
Kim S, Lee JS, Kim J, Oh M, Kim C, Park MA, Park JJ (2011) Fine structure of Longicollum pagrosomi (Acanthocephala: Pomphorhynchidae) and intestinal histopathology of the red sea bream, Pagrus major, infected with acanthocephalans. Parasitol Res 109:175–184
Lev R, Spicer S (1964) Specific staining of sulphate groups with Alcian blue at low pH. J Histochem Cytochem 12:309
Malta JCO, Gomes ALS, Andrade SMS, Varella AMB (2001) Infestações maciças por acantocéfalos, Neoechinorhynchus buttnerae Golvan, 1956, (Eoacanthocephala: Neoechinorhynchidae) em tambaquis jovens, Colossoma macropomum (Cuvier, 1818) cultivados na Amazônia central. Acta Amaz. Manaus. 31(1):133–143
Martins ML, Moraes FR, Fujimoto RY, Onaka EM, Nomura DT, Silva CAH, Schalch SHC (2000) Parasitic infections in cultivated freshwater fishes a survey of diagnosticated cases from 1993 to 1998. Rev Bras Parasitol Vet 9(1):23–28
Martins ML, Moraes FR, Fujimoto RY, Onaka EM, Quintana CIF (2001) Prevalence and histopathology of Neoechinorhynchus curemai Noronha, 1973 (Acanthocephala: Neoechinorhynchidae) in Prochilodus lineatus Valenciennes, 1836 from Volta Grande Reservoir, MG, Brazil. Brazil J Biol 61(3):517–522
Melo FTV, Rodrigues RAR, Giese EG, Gardner SL, Santos JN (2014) Histopathologic aspects in Plagioscion squamosissimus (HECKEL, 1940) induced by Neoechinorhynchus veropesoi, metacestodes and anisakidae juveniles. Rev Bras Parasitol Vet 23(2):224–230. doi:10.1590/S1984-29612014048
McManus JFA (1948) Histological and histochemical uses of periodic acid. Stain Technol 23:99–108
Morais AM, Varella AMB, Correa MAV, Malta JCO (2009) A fauna de parasitos em juvenis de tambaqui Colossoma macropomum (Cuvier, 1818) (Characidae: Serrasalminae) criados em tanques-rede em lago de várzea da Amazônia central. Biologia Geral Experimental:914–923
Mowry RW (1956) Alcian blue techniques for the histochemical study of acidic carbohydrates. J Histochem Cytochem 4:407–408
Mowry RW (1963) The special value of methods that colour both acidic and vicinal hydroxyl groups in the histochemical study of mucins with revised directions for the colloidal iron stain, the use of alcian blue 8GX, and their combination with the periodic acid-Schiff reaction. Ann N Y Acad Sci 106:402–423
Nickol BB (2006) Phylum Acanthocephala. In: Woo PTK (ed) Fish diseases and disorders, second edition: protozoan and metazoan infections, vol I. CAB International, Wallingford, pp 444–465
Ostrenky A, Boeger WA, Chammas MA (2008) Potencial para o desenvolvimento da aquicultura no Brasil. In: Ostrensky A, Borghetti JR, Soto D. Aquicultura no Brasil: O desafio é crescer. Brasília. 276p
Pinky, Mittal S, Mittal AK (2008) Glycoproteins in the epithelium of lips and associated structures of a hill stream fish Garra lamta (Cyprinidae, Cyprinoformes): a histochemical investigation. Anat Histol Embryol 37:101–113
Plumb JA, Larry A, Hanson LA (2011) Health maintenance and principal microbial diseases of cultured fishes, 3th edn. Blackwell Publishing Ltd., Iowa, p 492
Reite OB, Evensen O (2006) Inflammatory cells of teleostean fish: a review focusing on mast cells/eosinophilic granule cells and rodlet cells. Fish Shellfish Immunol 20(2):192–208. doi:10.1016/j.fsi.2005.01.012
Ribeiro SC, Castelo AS, Silva BMP, Cunha AS, Proietti Júnior AA, Oba-Yoshioka ET (2016) Hematological responses of tambaqui Colossoma macropomum (Serrassalmidae) fed with diets supplemented with essential oil from Mentha piperita (Lamiaceae) and challenged with Aeromonas hydrophila. Acta Amaz 46(1):99–106 ISSN 0044-5967
Roubal FR (1992) Comparative histopathology of Longicollum (Acanthocephala: Pomphorhynchidae) infection in the alimentary tract and spleen of Acanthopagrus australis (Pisces: Sparidae). Int J Parasitol 23:391–397
Sanil NK, Asokan PK, John L, Vijayan KK (2010) Pathological manifestations of the acanthocephalan parasite, Tenuiproboscis sp. in the mangrove red snapper (Lutjanus argentimaculatus) (Forsskål, 1775), a candidate species for aquaculture from Southern India. Aquaculture. doi:10.1016/j.aquaculture.2010.10.027
Santos EF, Tavares-Dias M, Pinheiro DA, Neves LR, Marinho RGB, Dias MKR (2013) Fauna parasitária de tambaqui Colossoma macropomum (Characidae) cultivado em tanque-rede no Estado do Amapá, Amazônia Oriental. Acta Amaz Manaus 43(1):106–112
Taraschewski H (2000) Host-parasite interactions in Acanthocephala: a morphological approach. Adv Parasitol 46:1–179
Tavares-Dias M, Moraes FR, Martins ML, Kronka SN (2001) Fauna parasitária de peixes oriundos de pesque-pagues do município de Franca, São Paulo Brasil. II. Metazoários. Revista Brasileira de Zoologia 18:81–95
Thatcher VE (1981) Neoechinorhynchus pterodoridis n. sp. (Acanthocephala: Neoechinorhynchidae) do pacu liso (Pterodoras granulosus) da Amazônia brasileira. Acta Amaz 11:443–446
Wallace JL, Martin GR (2001) Inflammatory mediators in gastrointestinal defence and injury. Exp Biol Med 22:1003–1015
Acknowledgments
The authors would like to thank the Desenvolvimento da Aquicultura e de Recursos Pesqueiros da Amazônia (DARPA) Project and Development project of a herbal dewormer with anthelmintic action for the treatment of diseases in farmed fish for the financial support and Microscopy Laboratory in the National Institute of Amazonian Research for the assistance with scan microscopy analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Statement on the welfare of animals
All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Rights and permissions
About this article
Cite this article
de Matos, L.V., de Oliveira, M.I.B., Gomes, A.L.S. et al. Morphological and histochemical changes associated with massive infection by Neoechinorhynchus buttnerae (Acanthocephala: Neoechinorhynchidae) in the farmed freshwater fish Colossoma macropomum Cuvier, 1818 from the Amazon State, Brazil. Parasitol Res 116, 1029–1037 (2017). https://doi.org/10.1007/s00436-017-5384-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-017-5384-3