Abstract
Bovine theileriosis, a tick-borne protozoan disease caused by Theileria annulata, Theileria orientalis and Theileria sinensis, is widespread in China and is a serious economic problem for the Chinese livestock industry. In this study, recombinant major piroplasma surface proteins (MPSP) of T. annulata, T. orientalis and T. sinensis based on MPSP genes were expressed in Escherichia coli BL21(DE3). The immunogenicity and specificity of the three purified recombinant MPSP proteins were evaluated with the reference positive sera of T. annulata, T. orientalis, T. sinensis, Babesia bovis, B abesia bigemina, Babesia major, Babesia motasi, Theileria luwenshuni, Theileria uilenbergi and Anaplasma ovis using an enzyme-linked immunosorbent assay (ELISA) or western blotting. The results showed that all three of the rMPSP proteins had a strong reaction with the sera from cattle infected with T. annulata, T. orientalis and T. sinensis via western blotting but not with other piroplasma and Anaplasma species. Then, the rMPSP protein of T. sinensis was used to develop an iELISA for detecting the three Theileria species infections. The specificity and sensitivity were 95.7 and 95.5 %, respectively, with a threshold of 28.8 % of the specific mean antibody rate (AbR). Finally, 2473 field-collected bovine sera, from 42 prefectures of 17 provinces in China, were tested using the ELISA to evaluate the prevalence of bovine theileriosis, and the average positive rate was 43.6 %. The developed iELISA could be a suitable tool to detect the three bovine Theileria species, and the data also provided important information regarding the current prevalence of bovine theileriosis in China.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bovine theileriosis is a tick-borne haemoprotozoan disease caused by the parasites of the genus Theileria, which infect bovine leukocytes and erythrocytes (Liu et al. 2009). The main symptoms of the disease are fever, anaemia, jaundice and superficial lymph node enlargement (Xu et al. 1997). The disease has a worldwide distribution. It is known that Theileria parva, Theileria annulata, Theileria mutans, Theileria taurotragi, Theileria velifera and Theileria orientalis can cause bovine infection (Onuma et al. 1998; Sugimoto et al. 1991). T. annulata, a malignant Theileria species causing tropical theileriosis, can be transmitted by ticks of the genus Hyalomma, such as Hyalomma detritum and Hyalomma anatolicum anatolicum (Dolan 1989), which are mainly distributed in North Africa, Southern Europe, India, the Middle East and Asia (Bilgic et al. 2010). This species mainly affects crossbred cattle, and infection is characterized by lymphadenopathy, splenomegaly, fever, anaemia, weakness, loss of body weight and sometimes death, which causes large economic losses (Omer et al. 2002; El-Deeb and Younis 2009). T. orientalis belongs to the group formerly known as the Theileria sergenti/Theileria buffeli/T. orientalis complex and is widely distributed throughout Japan, the Korean Peninsula, New Zealand and Australia (Wang et al. 2010a; Liu et al. 2010; Kamau et al. 2011a; Eamens et al. 2013; Pulford et al. 2016b). Animals infected by T. orientalis mainly exhibit apathy, fever, anaemia and lymphadenectasis (Jin et al. 2007). In China, T. annulata is distributed in the North Yangtze River region, where ticks of the Hyalomma genus are abundant. T. orientalis is the most prevalent bovine Theileria species, as its vector tick Haemaphysalis longicornis is the most widely distributed tick species in China. In addition, Bai et al. (2002) reported a new Theileria species infective to cattle and yaks, T. sinensis, that is transmitted by Haemaphysalis qinghaiensis and is thought to originate from Gansu Province. Usually, T. sinensis infection does not result in death, and due to limited geographical distribution, infections do not contribute to large economic losses. Thus, it is generally thought that the above-mentioned three Theileria species are responsible for bovine theileriosis in China.
Compared to traditional pathogenic identification through observations of blood smears, serological and molecular diagnostic methods are more sensitive and convenient for the detection of bovine Theileria infection. 18S rRNA, ITS, major piroplasma surface protein (MPSP) and T. annulata merozoite surface protein 1 (Tams1) genes were selected as target genes to develop a species-specific PCR assay for different Theileria species (Tanaka et al. 1993; d’Oliveira et al. 1995; Han et al. 2009; Kamau et al. 2011b). Recently, real-time PCR was utilized in the quantitative detection of Theileria infection (Ros-Garcia et al. 2012; Chaisi et al. 2013; Bogema et al. 2015; Pulford et al. 2016a). Additionally, reverse line blotting (RLB) was also used for the simultaneous detection of different Theileria infections (Salih et al. 2007; Iqbal et al. 2013). However, these detection techniques are time-consuming and require complex procedures and equipment, such as thermal cyclers, which resulted in the development of loop-mediated isothermal amplification (LAMP) for detecting Theileria infection (Wang et al. 2010a; Liu et al. 2013). These molecular diagnostic methods can detect and differentiate nearly all known Theileria species with a high sensitivity. These types of serodiagnostic techniques are essential tools for performing epidemiological studies of theileriosis and evaluating preventive measures. Compared to other serodiagnostic methods, such as complement fixation tests (CFT) and indirect fluorescent antibody tests (IFAT), enzyme-linked immunosorbent assays (ELISA) are more sensitive, non-subjective, easier to standardize, high throughput and suitable for large-scale epidemiological surveys. Thus, a number of ELISAs have been designed for the diagnosis of Theileria spp. infection (Schnittger et al. 2002; Salih et al. 2005; Wang et al. 2010b; Mohamed et al. 2012; Rajendran and Ray, 2014). Few studies, however, have focused on the development of simple, rapid, stable and routine serological techniques to simultaneously detect T. annulata, T. orientalis and T. sinensis to evaluate the prevalence of bovine theileriosis.
The MPSP homologues are a group of conserved glycoproteins on the surface of merozoites and piroplasms of Theileria species ranging from 30 to 34 kDa. They have excellent immunogenicity and can stimulate a strong immune response in infected animals, which suggests that these proteins are biologically significant in parasite development (Shiels et al. 1995; Onuma et al. 1997). These proteins are, thus, candidate antigens for vaccine and serodiagnostic development. Thus, in this study, rMPSPs of T. annulata, T. orientalis and T. sinensis were evaluated as candidate antigens for ELISA. A universal ELISA method that detects the three bovine Theileria infections in one reaction was developed based on the rMPSP protein of T. sinensis, and this assay was used in an epidemiological survey of bovine theileriosis in China.
Material and methods
Parasites
Bovine blood samples infected by T. annulata (Liaoyang strain from Liaoning Province), T. orientalis (Lintao strain from Gansu Province) and T. sinensis (Dingxi strain from Gansu Province) were cryopreserved in liquid nitrogen at the Lanzhou Veterinary Research Institute (LVRI), China.
Sera and genomic DNAs
Reference positive sera and genomic DNA of bovine piroplasms (T. annulata, T. orientalis, T. sinensis, Babesia bovis, Babesia bigemina and Babesia major), ovine piroplasms (Babesia motasi, Theileria luwenshuni and Theileria uilenbergi) and A. ovis were provided by the Vectors and Vector-borne Diseases (VVBD) Laboratory of LVRI.
Animal experimentation was approved (permit SYXK2010-0001) by the Science and Technology Department of Gansu Province, China. In this experiment, three calves were purchased from Gansu Province in China (Nos. 08840, 322 and 353) and were negative for Theileria species by microscopic examination of Giemsa-stained smears and PCR using primer 989/990 (Allsopp et al. 1993). The three animals were then infected with either 10 ml bovine blood containing T. annulata (Liaoyang strain), T. orientalis (Lintao strain) or T. sinensis (Dingxi strain). Sera, DNA extracts from blood and blood smears were made from the three infected calves during 19 weeks post-infection (wpi) for evaluation of the infections and antibody kinetics. The presence of the parasite was evaluated using microscopic examination and PCR, in which species-specific primer sets AnCb, SerITS and Tsin F/R were used to detect T. annulata, T. orientalis and T. sinensis, respectively (Liu et al. 2010, 2015).
Sera (n = 47) were from 47 cattle purchased from the Gansu Province of China and tested negative for T. annulata, T. orientalis and T. sinensis by microscopic examination of Giemsa-stained smears and PCR using primer 989/990 prior to the experiment. These samples were collected to evaluate the specificity of ELISA and mixed for use as a standard negative serum. Reference positive sera (n = 22) against bovine Theileria spp. provided by VVBD and sera (n = 45) collected from T. annulata and T. sinensis-infected calves during 4–19 wpi and that of T. orientalis during 7–19 wpi were used to evaluate the sensitivity of ELISA, and a mixture of these sera was used as standard positive serum.
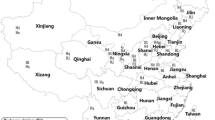
Field-collected bovine sera were collected from 2473 cattle and yaks in 42 prefectures of 17 provinces from 2008 to 2013. The provinces sampled included Guangdong (Qingyuan, Maoming), Jinlin (Jilin, Changchun), Sichuan (Panzhihua, Luzhou), Liaoning (Anshan, Dalian and Benxi), Hunan (Huaihua, Yongzhou and Changde), Yunnan (Jinghong, Menghai, Ruili, Qujing, Wenshan and Honghe), Qinghai (Haibei, Xining), Shanxi (Yan’an), Gansu (Linxia, Zhangye and Maqu), Inner Mongolia (Hulunbeier, Baotou, Tongliao, Xing’anmeng and Xilinguolemeng), Tibet (Ihasa), Hebei (Tangshan), Guizhou (Kaili, Tongren, Anshun and Xingyi), Henan (Sanmenxia), Chongqing (Jiangjin, Wanzhou), Xinjiang (Zhaosu) and Guangxi (Chongzuo, Baise and Guilin).
Cloning and expressing MPSP gene
The specific primers were designed to amplify MPSP CDS based on the sequences of T. annulata, T. orientalis and T. sinensis (accession nos. JX683683.1, GQ180194.1 and GQ180193.1) in GenBank (Table 1). Subsequently, the PCR products were purified, digested with restriction enzymes EcoR I and Xho I and finally inserted into a pET-30a vector. The recombinant pET-MPSP vectors were confirmed by double restriction enzyme digestion and sequencing. The recombinant vectors were then transformed into chemically competent E. coli BL21(DE3) to express the rMPSPs as His-fusion proteins. Three hundred microliters of recombinant E. coli was inoculated into 30 ml fresh LB medium with 50 μg/ml of kanamycin and cultivated at 37 °C, 200 rpm. When the OD600 of the bacterium culture reached 0.6, IPTG was added at a final concentration of 1.0 mmol/L to induce protein expression at 37 °C for 8 h. Finally, the rMPSPs were identified and analysed by SDS-PAGE.
The rMPSPs were purified according to the instructions of the Ni-NTA Purification System (Catalogue No. K950-01, Invitrogen, Carlsbad City, CA, USA). Briefly, the E. coli were pelleted and resuspended in native binding buffer (denaturing buffer for protein supernatant) and then lysed by freeze thawing and sonication. Following this, the supernatant was collected. After denaturation by guanidinium lysis buffer, the supernatant is soluble. The soluble proteins from supernatant were combined with the Ni-NTA resin for 1–2 h under gentle rotation. Following five washes with native or denaturing wash buffer, the eluate was collected into separate tubes for identification by SDS-PAGE.
Western blotting
Western blotting was performed using the method described by Guan et al. (2012). The three rMPSPs were separated by 12 % SDS-PAGE and electro-blotted onto nitrocellulose (NC) membranes (RPN303E, Amersham). The NC sheet was cut into strips and blocked with 10 % skimmed milk powder in 0.1 M Tris-buffered saline (pH 7.6) with 0.1 % Tween 20 (TBST), overnight at 4 °C. The NC strips were probed with each reference serum and mouse monoclonal anti-poly histidine antibody (H1029, Sigma). Following three washes with TBST, secondary antibodies, monoclonal alkaline phosphatase-conjugated goat anti-mouse IgG (A3562, Sigma), alkaline phosphatase-conjugated mouse anti-goat/sheep (A8062, Sigma) and alkaline phosphatase-conjugated rabbit anti-bovine IgG (A0705, Sigma) were applied. Following the four washes with TBST, positive signals were revealed using the 5-bromo-4-chloro-3-indolyl phosphate/nitro blue tetrazolium (BCIP/NBT) liquid substrate system (B1911, Sigma).
ELISA procedure
An ELISA procedure described previously by Chauvin et al. (1995) and Guan et al. (2010) was used in the present study. Briefly, 96-well microtiter plates (Nunc, Roskilde, Denmark) were coated with 50 μl recombinant antigen at a concentration of 2.8 μg/ml in a coating buffer (0.1 M carbonate–bicarbonate buffer, pH 9.6). The plates were washed three times with PBS containing 0.1 % Tween 20 (PBST) and incubated with 100 μl/well of a blocking solution (1 % BSA in PBST) for 1 h at 37 °C. After drying the plate, the samples, blanks (PBST) and standard positive and negative controls (dilution of 1:200) were distributed in duplicate and the plates were incubated at 37 °C for 1 h. After washing as described above, a peroxidase conjugate of rabbit anti-bovine IgG (A-5295, Sigma) diluted at 1:40,000 with PBST was added to each well and the plates were again incubated at 37 °C for 1 h. The plates were washed three times as described above, and subsequently, 50 μl of TMB (T0440-1L, Sigma, USA) was added to each well and incubated at room temperature for 20 min. The reaction was stopped by the addition of 50 μl of 0.2 M H2SO4 and the optical density (OD) was measured with an ELISA reader (microplate reader Model 680, Bio-Rad, USA) at a wavelength of 450 nm. The results are expressed as the percentage of the specific mean antibody rate (AbR%), determined using the formula, AbR% = (Sample mean OD − Negative control mean OD) / (Positive control mean OD − Negative control mean OD) × 100 %. Cutoff points, specificity and sensitivity of the ELISA were evaluated by receiver operating characteristic (ROC) analysis using MedCalc statistical software with 47 negative sera and 67 positive sera against T. annulata, T. orientalis and T. sinensis from cattle.
Results
Cloning MPSP gene, expression and purification of recombinant proteins
The complete MPSP CDSs of T. annulata, T. orientalis and T. sinensis were amplified successfully by PCR from genomic DNA, at the sizes of 846, 852 and 873 bp, encoding 281 (32 kDa), 283 (32 kDa) and 290 (33 kDa) amino acid residues predicted by an online software analysis, respectively. The SDS-PAGE results showed that the rMPSPs were 37 kDa (T. annulata), 37 kDa (T. orientalis) and 38 kDa (T. sinensis) in size including His-tag protein (approximately 5 kDa in size) (Fig. 1). T. orientalis and T. sinensis rMPSPs existed both in the supernatants (soluble protein) and precipitates (inclusion bodies) but T. annulata rMPSP only as inclusion body. The rMPSPs were purified according to the instruction of Ni-NTA Purification System. The concentrations of purified rMPSPs of T. orientalis and T. sinensis from supernatants were 0.2 and 0.28 mg/ml and that of T. annulata purified from inclusion bodies was 0.1 mg/ml.
Reactivity of recombinant proteins
The cross-reactivity between the three rMPSPs and positive reference sera against T. annulata, T. orientalis, T. sinensis, T. luwenshuni, T. uilenbergi, B. bovis, B. bigemina, B. major, B. motasi and A. ovis, were evaluated by western blotting. Each rMPSP had strong reactions with the positive sera against T. annulata, T. orientalis and T. sinensis but no cross-reactions with other piroplasm and A. ovis positive sera (Fig. 2). Meanwhile, all the three rMPSPs had a strong reaction with anti-polyhistidine antibody demonstrated by western blot, which indicates the exact expression of the three rMPSPs in the prokaryotic expression system. These results show that all the three rMPSPs can be used to develop a universal serodiagnostic method for evaluating infection with the three bovine Theileria species. However, this method does not differentiate between the three species.
Western blotting analysis of the three rMPSPs with sera against piroplasms and Anaplasma. Lane M the molecular weight markers; lane 1 anti-His-tag protein; 2 Negative sera; 3–5 Positive sera against T. annulata, T. orientalis, T. sinensis; 6-12 Positive sera against B. bovis, B. bigemina, B. major, ovine babesia, T. luwenshuni, T. uilenbergi, A. ovis
Evaluating the cross-reactivity, specificity, sensitivity and threshold value of ELISA
Given the concentration of the purified rMPSPs, the rMPSP of T. sinensis was used as an antigen to develop a universal ELISA for detecting the infection of the three bovine Theileria species. The mean AbRs and standard diversity, calculated with Excel 2007 for ELISA results, showed the rMPSP of T. sinensis had strong reactions with the positive sera against T. annulata, T. orientalis and T. sinensis but no cross-reactions with other piroplasm and A. ovis positive sera (Fig. 3), which was consistent with the results from the western blot. Following the above procedure, 47 negative sera and 67 positive sera against T. annulata, T. orientalis and T. sinensis were used to evaluate the specificity, sensitivity and threshold value of the ELISA. The AbRs were catalogued in MedCalc statistical software. Results demonstrate the specificity of the assay is 95.7 % (0.855∼0.995 under 95 % CI) and the sensitivity is 95.5 % (0.875∼0.991 under 95 % CI) when the threshold value is 28.8 % (Fig. 4).
Kinetics of anti-Theileria spp. antibodies in experimentally infected cattle
Three calves (nos. 08840, 322 and 353) were infected with bovine blood of either T. annulata (Liaoyang strain), T. orientalis (Lintao strain) or T. sinensis (Dingxi strain), and the sera during 1–19 wpi were collected and used to assess antibody kinetics. The antibodies of T. annulata and T. sinensis were detected at 3 wpi by ELISA, and the high level of antibodies lasted until 19th wpi. The antibodies against T. orientalis were positively detected between 6 and 19 wpi except in weeks 7, 9, 10 and 11 and the antibody levels were low and irregular (Fig. 5). Based on PCR tests, the three Theileria species were detected at 3 wpi, but the PCR test of T. annulata was negative from 14 to 19 wpi. In comparing blood smears to serological and PCR tests, blood smears showed T. annulata and T. sinensis were first observed at 4 wpi, T. annulata was not observed from 10 to 19 wpi and. T. orientalis was first observed at 7 wpi (Table 2).
Serological epidemiology
In this study, a large-scale investigation of bovine Theileria infection in China was conducted using ELISA. Field-collected bovine sera (2473) from 42 prefectures of 17 provinces were evaluated. The results show 12.9 to 67.5 % of sera tested positive for Theileria infection with an average positive rate of 43.6 % in these 17 provinces (Table 3).
Discussion
Bovine theileriosis is a widespread haemoprotozoan disease that causes great economic losses and can seriously hinder the development of cattle. Yearly, approximately 250 million animals are exposed to the risk of T. annulata infection (Radositis et al. 1997; Sudan et al. 2014, 2015), which results in roughly $800 million in losses worldwide. Recently, many diagnostic methods have been developed for detecting the infection of bovine Theileria. A multiplex PCR assay was used to detect and discriminate between T. annulata and T. orientalis (Liu et al. 2015). Since LAMP is a technique that has the advantage of high sensitivity and easy manipulation, Liu et al. (2013) developed a LAMP assay to identify the infection of T. orientalis and T. sinensis. Salih et al. (2007) developed an RLB assay to detect T. parva, T. mutans, T. velifera, T. taurotragi, T. buffeli and T. annulata infections in cattle, and Iqbal et al. (2013) applied an RLB assay to detect Theileria and Babesia sp. infections in goat and sheep. All of these methods can be used to detect two or more Theileria species simultaneously, but they are time consuming and inconvenient for large-scale epidemiological surveys due to the need for nucleic acid extraction compared to serodiagnostic methods. Examples of serodiagnostic methods include the assay developed by Gubbels et al. (2000), which used an indirect Tams1 ELISA for diagnosis of T. annulata infection in cattle, demonstrated that the antigen had no cross-reactivity with antisera positive for T. velifera, T. taurotragi, T. orientalis, B. bovis, and B. bigemina. Based on another membrane protein TaSP, Renneker et al. (2008) developed a competitive ELISA for specific detection of T. annulata infection. In 2010, Wang et al. (2010b) established an indirect ELISA to detect T. orientalis in water buffalo using recombinant P33, and cross-reactivity of the iELISA indicated there were no cross-reactions with the positive sera against B. orientalis, Schistosomiasis japonica, A naplasma marginale and Toxoplasma gondii in water buffalo. However, there is no serodiagnostic method to detect T. sinensis infection in cattle at present. Thus, to evaluate the infection status of bovine theileriosis in China, it is necessary to develop a serodiagnostic method for detecting T. annulata, T. orientalis and T. sinensis infection simultaneously.
In this study, we expressed the rMPSPs of T. annulata, T. orientalis and T. sinensis in a prokaryotic expression system. The three rMPSPs had strong reactivity with the positive sera against the three Theileria species but no cross-reactivity with other piroplasms and A. ovis as evaluated by western blotting. In addition, the rMPSP of T. sinensis had a high concentration of soluble protein in the supernatant. Thus, a universal ELISA method was developed using the rMPSP of T. sinensis for detecting the infections of the three bovine Theileria species simultaneously. The calculated specificity and sensitivity of the ELISA were 95.7 and 95.5 %, respectively, with a threshold value of 28.8 %.
Piroplasm-free calves, nos. 08840, 322 and 353, were infected experimentally with T. annulata, T. orientalis and T. sinensis to evaluate seroconversion using the ELISA. The results showed positive ELISA and PCR tests for T. annulata from the third wpi. Both the ELISA and PCR assays were more sensitive than microscopic examination of blood smears that first observed the parasite in the fourth wpi. However, the PCR tests were negative from the 14th wpi, but ELISA tests remained positive until 19 wpi.
It is well known that the piroplasm stage of T. annulata is the only stage of infection in recovered carrier animals that can be detected, and antibody titres of infected animals are typically higher in the later stages of infection (Renneker et al. 2009; Mohamed et al. 2012). Additionally, Seitzer et al. (2007) tested sera collected from experimentally infected cattle using a TaSP ELISA, which detected the parasite from 3 to 20 wpi. The PCR and ELISA results in this study are consistent with the results reported by Seitzer et al. (2007) which demonstrates that the developed ELISA method is reliable and useful for detecting animals with prior exposure to the parasite. It is also suitable for diagnosis in the later phase of T. annulata infection in contrast with PCR testing. Jenkins and Bogema (2016) utilized qPCR to test for T. orientalis, demonstrating detection from 3 wpi to the end of the experiment, which is consistent with our results. Meanwhile, Hammer et al. (2016) also obtained similar qPCR results in a high-dose group. In contrast, the antibody titre reached maximum in 6 wpi but then dropped sharply from 6 to 11 wpi. The serological response was stead from 12 wpi to the end of the experiment. However, the titres of four sera samples from 7, 9 10 and 11 wpi were under the cutoff value for positivity. In general, the kinetics of anti-T. orientalis antibodies in this study was similar to the results reported by Jenkins and Bogema (2016). However, the developed ELISA could result in underestimation of the prevalence of T. orientalis, because the antibody of some cases was negative but the animal still carried T. orientalis. According to the seasonal distribution of ticks in China, the ticks become active from March, so testing the field-collected bovine sera after June could reflect the prevalence of T. orientalis better. In a word, PCR and ELISA are more sensitive than microscopic examination, which first observed the parasite at 7 wpi. For T. sinensis, the data showed that ELISA results correlated well with PCR and microscopic examination, suggesting that ELISA may be useful for detecting active infection by T. sinensis. The results showed ELISA and PCR methods are more sensitive in detecting the three Theileria species infection in cattle or yak, compared to microscopic examination. However, during the later phase of the infection when parasitemia is low, it is difficult to detect the infection with microscopic examination and PCR. At this stage, the animals still have high antibody titres; therefore, the ELISA is a more suitable detection method for this condition.
In conclusion, the developed ELISA is a good choice to detect Theileria spp. infections, and it will be useful for large-scale sero-epidemiological investigations of bovine theileriosis.
To date, there have been no reports regarding large-scale epidemiological investigations of bovine theileriosis in China. Most of the surveys focus on one or several provinces. Jian et al. (2011) studied the prevalence of T. annulata in the Xinjiang regions using a recombinant Tams1 ELISA, demonstrating a positive rate of approximately 11 %. Hu et al. (2012) investigated T. orientalis infection using a PCR method in 206 cattle from Yanji, Jilin Province, and showed a positive rate of 62.6 %. Yang et al. (2014) detected the Theileria infection in 1235 cattle from 10 provinces using a genus-specific pan-Theileria FRET-qPCR. The results demonstrated a positive rate of 30.8 %. In the present study, we performed a large-scale sero-epidemiological survey of bovine theileriosis using a universal ELISA for three Theileria species. In total, 2473 field-collected bovine sera from 42 prefectures of 17 provinces were examined using the ELISA. Infection was found in all provinces investigated. The positive rates ranged from 12.9 to 67.5 % and the average positive rate was 43.6 % in these provinces, which indicates that bovine Theileria spp. are common in China. These data provide important information about bovine Theileria infection that is beneficial for management programmes of bovine theileriosis.
References
Allsopp BA, Baylis HA, Allsopp MT, Cavalier-Smith T, Bishop RP, Carrington DM, Sohanpal B, Spooner P (1993) Discrimination between six species of Theileria using oligonucleotide probes which detect small subunit ribosomal RNA sequences. Parasitol 107(Pt2):157–165
Bai Q, Liu GY, Yin H, Zhao QZ, Liu DK, Ren JX, Li X (2002) Theileria sinensis sp nov: a new species of bovine Theileria-classical taxonomic studies. Acta Vet Zootech Sin 33(1):73–77 (in Chinese)
Bilgic HB, Karagenc T, Shiels B, Tait A, Eren H, Weir W (2010) Evaluation of cytochrome b as a sensitive target for PCR based detection of T. annulata carrier animals. Vet Parasitol 174:341–347
Bogema RD, Deutscher AT, Fell S, Collins D, Eamens GJ, Jenkins C (2015) Development and validation of a multiplexed hydrolysis probe qPCR assay for the detection and quantification of Theileria orientalis and differentiation of clinically-relevant subtypes. J Clin Microbiol 53:941–950
Chaisi ME, Janssens ME, Vermeiren L, Oosthuizen MC, Collins NE, Geysen D (2013) Evaluation of a real-time PCR test for the detection and discrimination of Theileria species in the African buffalo (Syncerus caffer). PLoS One 8:e75827
Chauvin A, L’Hostis M, Valentin A, Precigout E, Cesbron-Zeggane N, Gorenflot A (1995) Babesia divergens: an ELISA with soluble parasite antigen for monitoring the epidemiology of bovine babesiosis. Parasite 2:257–262
Dolan TT (1989) Theileriasis: a comprehensive review. Revue Scientifique et Technique, Office International Des Epizooties 8:11–78
d’Oliveira C, van der Weide M, Habela MA, Jacquiet P, Jongejan F (1995) Detection of Theileria annulata in blood samples of carrier cattle by PCR. J Clin Microbiol 33(10):2665–2669
Eamens GJ, Bailey G, Jenkins C, Gonsalves JR (2013) Significance of Theileria orientalis types in individual affected beef herds in New South Wales based on clinical, smear and PCR findings. Vet Parasitol 196:96–105
El-Deeb WM, Younis EE (2009) Clinical and biochemical studies on Theileria annulata in Egyptian buffaloes (Bubalus bubalis) with particular orientation to oxidative stress and ketosis relationship. Vet Parasitol 164(2):301–305
Guan GQ, Ma ML, Liu AH, Ren QY, Wang JM, Yang JF, Li AY, Liu ZJ, Du PF, Li YQ, Liu Q, Zhu H, Yin H, Luo JX (2012) A recently identified ovine Babesia in China: serology and sero-epidemiology. Parasitol Int 61:532–537
Guan GQ, Chauvin A, Rogniaux H, Luo JX, Yin H, Moreau E (2010) Merozoite proteins from Babesia sp. BQ1 (Lintan) as potential antigens for serodiagnosis by ELISA. Parasitol 137:927–938
Gubbels MJ, d’Oliveira C, Jongejan F (2000) Development of an indirect Tams1 enzyme-linked immunosorbent assay for diagnosis of Theileria annulata infection in cattle. Clin Diagn Lab Immunol 7:404–411
Hammer JF, Jenkins C, Bogema D, Emery D (2016) Mechanical transfer of Theileria orientalis: possible roles of biting arthropods, colostrum and husbandry practices in disease transmission. Parasit Vectors 9:34
Han JI, Jang HJ, Na KJ (2009) Molecular detection of Theileria sp. in wild Chinese water deer (Hydropotes inermis argyropus). J Wildl Dis 45:1213–1216
Hu SY, Qian NC, Wang YN, Jia LJ, Zhang SF (2012) Molecular epidemiological survey of Theileria sergenti infections in cattle in Yanbian prefecture. Chinese Veterinary Science 42:647–650 (in chinese)
Iqbal F, Khattak R, Ozubek S, Khattak M, Rasul A, Aktas M (2013) Application of the reverse line blot assay for the molecular detection of Theileria and Babesia sp. in sheep and goat blood samples from Pakistan. Iran J Parasitol 8:289–295
Jenkins C, Bogema DR (2016) Factors associated with seroconversion to the major piroplasm surface protein of the bovine haemoparasite Theileria orientalis. Parasit Vectors 9:106
Jian ZJ, Ma SZ, Huang JY, Sun QZ, Shen JY, Miao ZQ, Lv W (2011) Development of an improved indirect Tams1 ELISA for detection of Theileria annulata in cattle. Xinjiang Agricultural Sciences 3:578–583 (in chinese)
Jin CM, Xu YT, Zhang SF, Lu C, Yu LZ (2007) Cloning and sequence analysis of the cattle Theileria sergenti P33 surface protein gene. Chinese Journal of Preventive Veterinary Medicene 29:112–119 (in chinese)
Kamau J, de Vos AJ, Playford M, Salim B, Kinyanjui P, Sugimoto C (2011a) Emergence of new types of Theileria orientalis in Australian cattle and possible cause of theileriosis outbreaks. Parasit Vectors 4:22
Kamau J, Salim B, Yokoyama N, Kinyanjui P, Sugimoto C (2011b) Rapid discrimination and quantification of Theileria orientalis types using ribosomal DNA internal transcribed spacers. Infection Genetics and Evolution 11:407–414
Liu AH, Guan GQ, Du PF, Gou HT, Zhang J, Liu ZJ, Ma ML, Ren QY, Liu JL, Yang JF, Li YQ, Niu QL, Bai Q, Yin H, Luo JX (2013) Rapid identification and differentiation of Theileria sergenti and Theileria sinensis using a loop-mediated isothermal amplification (LAMP) assay. Vet Parasitol 191:15–22
Liu AH, Guan GQ, Liu ZJ, Liu JL, Leblanc N, Li YQ, Gao JL, Ma ML, Niu QL, Ren QY, Bai Q, Yin H, Luo JX (2010) Detecting and differentiating Theileria sergenti and Theileria sinensis in cattle and yaks by PCR based on major piroplasm surface protein (MPSP). Exp Parasitol 126:476–481
Liu AH, Yin H, Guan GQ, Liu ZJ, Liu JL, Ma ML, Dang ZS, Li YQ, Luo JX (2009) Studies on comparison of 18S rRNA gene sequences of Theileria species infective to cattle. Acta Vet Zootech Sin 40:1063–1068 (in Chinese)
Liu JL, Li YQ, Liu AH, Guan GQ, Xie JR, Yin H, Luo JX (2015) Development of a multiplex PCR assay for detection and discrimination of Theileria annulata and Theileria sergenti in cattle. Parasitol Res 114:2715–2721
Mohamed AM, Abdel-Rady A, Ahmed LS, El-Hosary A (2012) Evaluation of indirect TaSP enzyme-linked immunosorbent assay for diagnosis of tropical theileriosis in cattle (Bos indicus) and water buffaloes (Bubalus bubalis) in Egypt. Vet Parasitol 186:486–489
Omer OH, El-Malik KH, Mahmoud OM, Haroun EM, Hawas A, Sweeney D, Magzoub M (2002) Haematological profiles in pure bred cattle naturally infected with Theileria annulata in Saudi Arabia. Vet Parasitol 107(1):161–168
Onuma M, Kakuda T, Sugimoto C (1998) Theileria parasite infection in East Asia and control of the disease. Comp Immunol Microbiol Infect Dis 21:165–177
Onuma M, Kubota S, Kakuda T, Sako Y, Asada M, Kabeya H, Sugimoto C (1997) Trop Anim Health Prod 29:119S–123S
Pulford DJ, Gias E, Bueno IM, McFadden A (2016a) Developing high throughput quantitative PCR assays for diagnosing Ikeda and other Theileria orientalis types common to New Zealand in bovine blood samples. N Z Vet J 64:29–37
Pulford DJ, McFadden A, Hamilton JS, Donald J (2016b) Investigation of the index case herd and identification of the genotypes of Theileria orientalis associated with outbreaks of bovine anaemia in New Zealand in 2012. N Z Vet J 64:21–28
Radositis OM, Blood DC, Gay CL (1997) Veterinary medicine a text book of the diseases of cattle, sheep, pigs, goats and horses. Saunders, London
Rajendran C, Ray DD (2014) Diagnosis of tropical bovine theileriosis by ELISA with recombinant merozoite surface protein of Theileria annulata (Tams1). Parasit Dis 38:41–45
Renneker S, Abdo J, Ahmed JS, Seitzer U (2009) Field validation of a competitive ELISA for detection of Theileria annulata infection. Parasitol Res 106:47–53
Renneker S, Kullmann B, Gerber S, Dobschanski J, Bakheit MA, Geysen D, Shiels B, Tait A, Ahmed JS, Seitzer U (2008) Development of a competitive ELISA for detection of Theileria annulata infection. Transbound Emerg Dis 55:249–256
Ros-Garcia A, Nicolas A, Garcia-Perez AL, Juste RA, Hurtado A (2012) Development and evaluation of a real-time PCR assay for the quantitative detection of Theileria annulata in cattle. Parasit Vectors 5:171
Salih DE, Ahmed JS, Bakheit MA, Ali EB, El Hussein AM, Hassan SM, Shariff OE, Fadl M, Jongejan F (2005) Validation of the indirect TaSP enzyme-linked immunosorbent assay for diagnosis of Theileria annulata infection in cattle. Parasitol Res 97:302–308
Salih DE, El Hussein AM, Seitzer U, Ahmed JS (2007) Epidemiological studies on tick-borne diseases of cattle in Central Equatoria State, Southern Sudan. Parasitol Res 101:1035–1044
Schnittger L, Katzer F, Biermann R, Shayan P, Boguslawski K, McKellar S, Beyer D, Shiels BR, Ahmed JS (2002) Characterization of a polymorphic Theileria annulata surface protein (TaSP) closely related to PIM of Theileria parva: implications for use in diagnostic tests and subunit vaccines. Mol Biochem Parasitol 120:247–256
Seitzer U, Bakheit MA, Salih DE, Ali A, Haller D, Yin H, Schnittger L, Ahmed J (2007) From molecule to diagnostic tool: Theileria annulata surface protein TaSP. Parasitol Res 101(Suppl 2):S217–S223
Shiels BR, d’Oliveira C, McKellar S, Ben-Miled L, Kawazu SI, Hide G (1995) Selection of diversity at putative glycosylation sites in the immunodominant merozoite/piroplasm surface antigen of Theileria parasites. Mol Biochem Parasitol 72:149–162
Sudan V, Jaiswal AK, Parashar R, Shanker D (2015) A duplex PCR-based assay for simultaneous detection of Trypanosoma evansi and Theileria annulata infections in water buffaloes. Trop Anim Health Prod 47:915–919
Sudan V, Sharma RL, Yadav R, Borah MK (2014) Turning sickness in a riverine buffalo naturally infected with Theileria annulata and its successful therapeutic management. Comp Clin Pathol 23:39–42
Sugimoto C, Kawazu S, Kamio T, Fujisaki K (1991) Protein analysis of Theileria sergenti/buffeli/orientalis piroplasms by two-dimensional polyacrylamide gel electrophoresis. Parasitol 102(Pt 3):341–346
Tanaka M, Onoe S, Matsuba T, Katayama S, Yamanaka M, Yonemichi H, Hiramatsu K, Baek BK, Sugimoto C, Onuma M (1993) Detection of Theileria sergenti infection in cattle by polymerase chain reaction amplification of parasite-specific DNA. J Clin Microbiol 31:2565–2569
Wang LX, He L, Fang R, Song QQ, Tu P, Jenkins A, Zhou YQ, Zhao JL (2010a) Loop-mediated isothermal amplification (LAMP) assay for detection of Theileria sergenti infection targeting the p33 gene. Vet Parasitol 171:159–162
Wang LX, Zhao JH, He L, Liu Q, Zhou DN, Zhou YQ, Zhao JL (2010b) An indirect ELISA for detection of Theileria sergenti antibodies in water buffalo using a recombinant major piroplasm surface protein. Vet Parasitol 170:323–326
Xu YT, Zhang SF, Li SY, Jin MH (1997) Progress in diagnosis and prevention research of bovine theileriosis. Journal of Agricultural Science Yanbian University 19:271–275 (in Chinese)
Yang Y, Mao Y, Kelly P, Yang Z, Luan L, Zhang J, Li J, El-Mahallawy HS, Wang C (2014) A pan-Theileria FRET-qPCR survey for Theileria spp. in ruminants from nine provinces of China. Parasit Vectors 7(1):413–420
Acknowledgements
This work was financially supported by the 973 Program (2015CB150300); Supporting Program (2013BAD12B03, 2013BAD12B05), MOST, China; NSFC (No. 31402189, No. 31372432, No. 31201899, No. 31272556, No. 31471967), ASTIP, FRIP (2014ZL010) and CAAS; Creative Research Groups of Gansu Province (No. 1210RJIA006); “948” (2014-S05), NBCIS CARS-38, Special Fund for Agro-scientific Research in the Public Research (No. 201303035, No. 201303037), MOA; and Jiangsu Co-innovation Center program for Prevention and Control of Important Animal Infectious Diseases and Zoonoses, State Key Laboratory of Veterinary Etiological Biology Project. The work was also facilitated by CRP No. 16198/R0 IAEA.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Zhao, S., Liu, J., Zhao, H. et al. Evaluating an indirect rMPSP enzyme-linked immunosorbent assay for the detection of bovine Theileria infection in China. Parasitol Res 116, 667–676 (2017). https://doi.org/10.1007/s00436-016-5332-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-016-5332-7