Abstract
Sarcocystis spp. are cyst-forming coccidia that infect numerous animals species, including several livestock species. Despite the importance of sheep and goat production in Brazil, little it is known about the Sarcocystis species that infect small ruminants in the country and their potential impact on meat condemnation due to the presence of macroscopic cysts of the parasite. The aims of the present study were to determine the frequency of infection by Sarcocystis spp. in goats and sheep intended for human consumption in Bahia State, Brazil, as well as to identify the parasite species in selected samples. The entire tongue, esophagus, and heart were collected from 120 goats and 120 sheep. Tissues were examined for Sarcocystis spp. by macroscopic evaluation, light microscopy, electron microscopy, and molecular tests. Microscopic cysts of Sarcocystis spp. were detected in 95.8 % of sheep and 91.6 % of goats. Using either transmission electron microscopy or partial sequencing of the 18S region of the ribosomal DNA (rDNA) for species identification, Sarcocystis tenella and Sarcocystis arieticanis were observed in sheep and Sarcocystis capracanis in goats. Macroscopic cysts were not detected in the analyzed samples. We concluded that goats and sheep destined for human consumption in Bahia possess high frequencies of Sarcocystis infection. Carcass condemnation due to Sarcocystis macrocysts seems to be rare in the studied region. S. arieticanis and S. capracanis were confirmed for the first time by electron microscopy or by molecular tests in small ruminants from Brazil.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sarcocystis spp. are cyst-forming protozoan parasites with a broad host range and distributed worldwide (Levine 1986). Sarcocystis infection is commonly observed in sheep and goats, but the occurrence of disease due to sarcocystosis is not frequent in these animal species (Buxton 1998). Infected animals may present anemia, weight loss, and reduced weight gain; when the central nervous system is affected, the animals may show hind limb weakness, ataxis, paresis, myopathy, and death (Buxton 1998).
Despite the sporadic outcome of clinical sarcocystosis in small ruminants, there is a major economic impact in some regions due to condemnation of sheep and goats’ carcasses containing macroscopic cysts of Sarcocystis spp. In a study carried out in Spain, macrocysts of Sarcocystis spp. were observed in 12 % of 6065 adult sheep and goats; among the 12 % of the positive animals, 79 % of their carcasses were totally condemned, what may represent to the Spanish industry a loss of 20 million euros annually (Martínez-Navalón et al. 2012).
Sheep are infected by four Sarcocystis species: Sarcocystis tenella and Sarcocystis arieticanis, which have canids as definitive hosts, and Sarcocystis gigantea (syn. Sarcocystis ovifelis) and Sarcocystis medusiformis, with cats as definitive hosts (Collins et al. 1979; Heydorn & Mehlhorn 1987). Three Sarcocystis species are found in goats: Sarcocystis capracanis and Sarcocystis hircicanis, which have canids as definite hosts, and Sarcocystis moulei (syn. S. hircifelis) that uses cats as the definite hosts (Dubey et al. 1989; Heydorn et al. 1975; Heydorn & Matuschka 1981).
Sheep and goat production in Brazil has a significant role in the economy of the country, with populations of 17.6 million sheep and 9.3 million goats. Bahia state, which is located in the Northeast of Brazil, has a country-like territory (564,733.177 km2), which is, for example, larger than countries such as France and Germany. Bahia state possesses the second biggest population of sheep and the largest number of goats in the country (Instituto Brasileiro de Geografia e Estatística—IBGE 2011); however, the Sarcocystis species that infect small ruminants in Brazil, as well as the frequency of infection in the animals, are poorly known. Sarcocystis sp. was associated with abortion in sheep in the south of Brazil, what was confirmed by electron microscopy, but the Sarcocystis species could not be determined (Pescador et al. 2007). In another study, S. tenella DNA was detected in two animals from the south and southeast regions of the country.
The aims of our study were to determine the frequency of Sarcocystis infection in sheep and goats destined for human consumption in Brazil from an economically important region for small ruminants, as well as to accurately identify Sarcocystis species that infect these animals using a combination of ultrastructural and molecular techniques.
Materials and methods
Animals
Tissue samples from 120 sheep and 120 goats were acquired in a slaughterhouse in Bahia state, Brazil, which is under a federal inspection veterinary service and receives animals from several municipalities in Bahia state. The slaughtered animals are destined for human consumption in the internal Brazilian market. The samples were collected from January 2012 to June 2013. For the sample size calculation, a finite population for each animal species was estimated using a confidence interval of 90 %, expected prevalence of infection of 90 %, and 5 % of precision. The minimal number of animals was estimated to be 97, but 120 were obtained for each animal species (goats and sheep). The samples were collected by convenience, according to the day and time available for slaughtering. The sheep were from Santa Inês breed and Santa Inês-mixed breed. This breed is a woolless hair sheep raised for meat production. The goats consisted of mixed breed animals that are also mainly raised for meat production.
The entire tongue, heart, and esophagus from each animal were collected and individually stored in sealed plastic bags. The samples were obtained in six different days with intervals of at least 1 month between each collection. The tissues were transported to the laboratory in styrofoam boxes containing ice packs and processed during the 2 days post-collection. All tissue samples were collected from the carcasses after the animals were killed in the slaughterhouse during its regular commercial routine for meat production. Therefore, no specific ethics approval was required.
Macroscopic analysis
The macroscopic examination was carried out at the same day that the tissues were collected. At least ten transversal cuts were done using a scalpel in the tongue and heart for macroscopic cyst visualization. The whole esophagus was longitudinally sectioned to expose the esophageal lumen, and its internal and external walls were macroscopically analyzed.
Microscopic examination of fresh tissues
The microscopic survey for cysts using fresh tissues was performed according to two methods. (1) Squash preparation: separate fragments of each tissue, measuring approximately 5 mm thick, were firmly squashed between two slides and examined under an optical microscope at ×40 and ×100 magnification. This procedure was done in triplicates for each tissue. (2) Tissue grinding: approximately 50 g of each tissue was grinded in a manual meat grinder and mixed with PBS (pH 7.2). After homogenization, it was filtered in gauze and centrifuged (600×g for 10 min). The supernatant was discarded, and the sediment was added to 1.5-mL tubes. Three smears were made with a fine layer of the sediment and visualized through an optical microscope (×100 magnification). When cysts were observed, a minimum of ten cysts was measured in an Olympus BX50 microscope. With the help of a syringe needle (19G) and the use of a stereomicroscope, the cysts were individually collected and transferred to 1.5-mL DNAse/RNAse free tubes for further molecular analysis.
Histologic examination
Samples from each tissue were fixed in 10 % buffered formalin, embedded in paraffin, sectioned at 6 μm, and stained by conventional hematoxylin-eosin (HE). The tissue sections were microscopically examined for Sarcocystis spp. One section for each individual organ (tongue, esophagus, and heart) was analyzed using goat and sheep samples, totalizing 360 tissue sections.
Transmission electron microscopy
Sarcocystis spp. cysts observed by fresh tissue preparation were collected for transmission electron microscopy (TEM). Part of the cysts was collected with an adjacent muscle layer from the host, in order to better preserve the morphology of the cyst wall. The cysts were fixed in 2 % glutaraldehyde solution in sodium cacodylate buffer 0.1 M (pH 7.4) for 2 h at room temperature and stored at 4 °C until processing. After fixation, the sample was washed in sodium cacodylate buffer 0.1 M (pH 7.4), post-fixed with 1 % osmium tetroxide, dehydrated in different acetone solutions (30, 40, 50, 70, 90, and 100 %), and treated in block with 1 % phosphotungstic acid and 1 % uranyl acetate. Then, the 100 % acetone was replaced by Polybed resin, followed by paraffin embedment and polymerization in an oven at 60 °C. Semi-thin cuts were made to observe the Sarcocystis spp. cysts by light microscope. Ultrathin cuts were performed and examined using a Zeiss EM900® transmission electron microscope.
Molecular analysis
DNA was extracted from individual cysts using a commercial DNA extraction kit (Easy DNA, Invitrogen®) according to the manufacturers’ instructions. The final addition of buffer to the extracted DNA was reduced to 10 μL instead of 100 μL. The samples were maintained at −20 °C until the PCR analysis.
A conventional PCR was done to amplify part of the subunit 18S of the ribosomal DNA (rDNA) of Sarcocystis spp. using the primers 1L (forward: CCA TGC ATG TCT AAG TAT AAG C) and 3H (reverse: GGC AAA TGC TTT CGC AGT AG) (Dahlgren & Gjerde 2007; Yang et al. 2001b). For each 25-μL reaction, 1 μL of the sample DNA, 20 pmol of each primer, and 12.5 μL of PCR Master Mix (Promega®) were used. The PCR was done in a thermal cycler (Bio-Rad, USA) in the same conditions proposed by Dahlgren and Gjerde (2007). Positive control (DNA extracted from a pool of Sarcocystis spp. from a sheep) and negative control (ultrapure water) were included in all reactions. The products obtained in the PCR were observed in 1.5 % agarose gels under UV emission. The amplicons were purified from the gel and submitted for sequencing. A BLAST search (http://blast.ncbi.nlm.nih.gov) was performed using the obtained nucleotide sequences for species classification.
Statistical analysis
The McNemar test was used to compare the two techniques (tissue grinding and squash) that were employed for the detection Sarcocystis sp. animal tissues. Values of p under 0.05 were considered statistically significant. The lengths and widths of at least ten cysts from each organ (heart, tongue, and esophagus) were determined by light microscopy and expressed as mean sizes and amplitudes of variation.
Results
Macroscopic analysis
No macroscopic cysts were observed in tongue or heart tissues of the 120 sheep and 120 goats. Macroscopic cysts were also not found in the inner or outer surface of the 240 examined esophagi.
Microscopic examination of fresh tissues
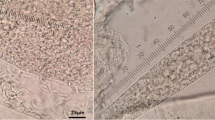
Out of 120 sheep that were examined by light microscopy using tissue squash preparation or tissue grinding, 95.8 % (115/120) were infected with Sarcocystis spp. The detected microscopic cysts were slender and highly septated. The septa of the unstained cysts presented a dark color. In contrast, the septa from cysts in histologic sections stained by HE were white (Fig. 1). The frequencies of microscopic cysts in the three employed tissues, observed through the squash method and tissue grinding, varied from 58.3 to 86.7 % (Table 1). A higher proportion of positive animals was detected using the squash technique than by means of tissue grinding (p < 0.05). A larger number of cysts in ovine tissues were found in the tongue and esophagus than the heart (p < 0.05).
Sarcocystis spp. in the tongue of a sheep. A squash preparation containing a nonstained cyst (a); the septa dividing the internal compartments are seen as dark structures. A histologic section of a cyst stained by hematoxylin and eosin (b); the septa are visualized as nonstained (light) structures. Bars = 50 μm
The histologic analysis of tongue, esophagus, and heart sections in sheep revealed sarcocysts with variable sizes and shapes; these variations were in part associated with the angle that the tissues were sectioned. The cysts presented round, elliptical, or elongated forms, with zoites separated by septa. The length of the cysts ranged from 100 to 1100 μm and the width from 30 to 130 μm (Table 2). The cyst walls were evident, but the thickness of the walls varied among cysts in the same tissue section. In higher magnification, villar protrusions could be seen in some cyst walls, although the details of these protrusions could not be accurately visualized by light microscopy (Fig. 2).
The overall frequency of microscopic Sarcocystis spp. in the tested goats was 91.6 %. The squash preparation resulted in a larger number of positive samples than the tissue grinding when hearts and esophagi were used. For the tongue, the number of positives did not statistically differ between the two techniques (Table 1). The sarcocyst appearance in goat tissues was similar to that observed for sheep, with cysts of variable sizes (Table 2) presenting thick walls and thin walls. The lengths of the cysts in goat tissues ranged from 110 to 1060 μm, and their widths varied from 30 to 400 μm (Table 2). The tongue and esophagus had higher numbers of tissue cysts than the heart (p < 0.05). The HE-stained goat cysts were similar to those observed in the ovine tissues.
Transmission electron microscopy
The ultrastructural evaluation of Sarcocystis spp. in tissues from sheep and goats was mainly targeted to the cyst walls. Ovine tissue cysts collected from two animals were examined by TEM. Two distinct types of villar protrusions were observed for the ovine cyst walls, which were characteristic of S. arieticanis and S. tenella. Cysts of S. arieticanis were observed as having snake-like protrusions, starting from the primary cyst wall, with lengths ranging between 1 and 6 μm. A granular substance was observed inside the primary cyst wall, which was approximately 0.5 μm thick (Fig. 3). The cysts were internally separated by septa in different compartments, which were filled with bradyzoites (Fig. 4). The cyst walls of S. tenella presented a ground substance and finger-like protrusions, with average lengths up to 1 μm. Numerous bradyzoites were observed underneath the walls (Fig. 5), in which amylopectin granules and micronemes were evident.
Transmission electron microscopy of Sarcocystis arieticanis in the tongue of a sheep. Hair-like villar protrusions (VP) and the primary cyst wall filled with a granular substance (GS). Different sections of bradyzoites are observed inside the cyst, in which several amylopectin granules (AG) and numerous micronemes (Mn) are visualized
Cysts analyzed by TEM from three goats were morphologically compatible with S. capracanis. Finger-shaped or palisade-like villar protrusions arising from the primary cyst walls measured from 1 to 3 μm in length. The cyst walls were filled with a granular substance (Fig. 6). There was an abundant amount of bradyzoites inside the cysts, which had a large number of micronemes, and in smaller amount, rhoptries. The appearance of the villar protrusions as finger-like or palisade-like depended on the angle in which the cyst was sectioned. In some bradyzoites, the conoid could be seen in one of the apices. Numerous amylopectin granules were noted inside the bradyzoites. Part of the cysts had muscular tissue adhered to the external layer of the cyst wall. In other cysts, the host muscle tissue was completely absent. The treatment of the nude cysts (without attached muscle) for TEM did not alter the cyst wall morphology (Fig. 7).
Transmission electron microscopy (TEM) of Sarcocystis capracanis in goat tissues. One cyst (a) was fixed for TEM with no muscle (M) from the host, while the other cyst (b) was processed for TEM with adjacent muscle to better preserve the cyst wall morphology. No differences in the morphology of the cyst walls were noted between a, b. The anterior portion of a bradyzoite (Br) is shown in a, whose micronemes (Mn), conoide (Co), and amylopectin granules (AG) are evident
Molecular analysis
PCR was carried out for 53 ovine samples of Sarcocystis collected after tissue grinding (tongue, esophagus, and heart). Out of these samples, 19 (35.84 %) were positive for the Sarcocystis spp. Amplicons of approximately 800 pb were visualized on 1.5 % agarose gels. DNA amplified from individual cysts from one animal was sequenced and matched with S. tenella, with 97 % identity with sequences deposited in GenBank using BLAST (http://blast.ncbi.nlm.nih.gov/).
A total of 270 individual cysts from 63 goats were tested by PCR. Sarcocystis DNA was amplified from 137 of the 270 examined samples. Reactions using individual cysts resulted in amplicons of approximately 800 bp. PCR products for Sarcocystis spp. were purified from the agarose gels and submitted for sequencing. Samples from two animals generated sequences with suitable sizes that were blasted (http://blast.ncbi.nlm.nih.gov/) for species classification. Two cysts corresponded to S. capracanis.
Discussion
In this study, we examined heart, tongue, and esophagus from 120 sheep and 120 goats for Sarcocystis spp. The overall frequencies for Sarcocystis spp. were 95.8 % for sheep and 91.6 % for goats. The evaluation of selected cysts by TEM and molecular analysis revealed S. arieticanis and S. tenella in ovine tissues and S. capracanis in tissues from goats. To the authors’ knowledge, this is the first confirmation of S. arieticanis and S. capracanis in Brazil and South America using ultrastructural or molecular analysis of parasite cysts. We also demonstrated S. tenella in sheep by both ultrastructural and molecular analysis. As mentioned before, the only previously confirmed report of S. tenella in Brazil was based in the detection of DNA in two animals, with no demonstration of the cysts in animal tissues (da Silva et al. 2009).
Sarcocystis spp. cysts were microscopically detected in the current work by means of tissue squash, tissue grinding, and HE staining. The tissue squash method was compared with the grinding technique; the former method, which is a fast and practical diagnostic tool, detected a higher number of positive animals than the grinding technique, although these methods did not permit the differentiation among the Sarcocystis species. There was a great variation in the lengths and widths of the observed cysts, what may be related with the age of the cysts. The sizes of the microscopic cysts do not seem to contribute for the species identification in the genus Sarcocystis that infect small ruminants.
The use of TEM was crucial for the identification of the species of Sarcocystis in some selected samples. The visualization of finger-like villar protrusions in sheep samples allowed the identification of S. tenella cysts. The villar protrusions with hair-like morphology permitted the confirmation of S. arieticanis in sheep tissues. It is interesting to note that Dubey et al. (1988) described S. arieticanis cysts from sheep in the USA as not possessing septa. In our study, S. arieticanis cysts were septated, similarly as observed by Heydorn and Mehlhorn (1987). We have noted that the septa inside some cysts are sometimes not easily visualized, especially in large cysts with large compartments. The lack of septa in S. arieticanis cysts described by Dubey et al. (1988) was probably due to the low number of analyzed cysts (n = 3).
The cysts examined by TEM in goat tissues in the present study had cyst wall morphologies characteristic of S. capracanis, which has finger-like or palisade villar protrusions (Heydorn & Haralambidis 1982), similar as the walls from S. tenella in sheep. No microcysts of S. hircicanis were observed in the current study; however, only a small number of cysts could be examined by TEM.
Macroscopic Sarcocystis in sheep, which are characteristics of S. medusiformis and S. gigantea, were not observed in any of the 120 tested animals. In goats, the species that form macroscopic cysts (S. moulei) have also not been found in the tested animals. Cats are definitive hosts for S. medusiformis, S. gigantea, and S. moulei. In Brazil, sheep and goats destined for meat production are usually raised on pasture and in closer contact with dogs than cats, what may be one of the reasons for the absence of the macroscopic cysts in the tested samples. It has been reported that macroscopic cysts of S. gigantea are found in older animals, usually over 3 or 4 years old (Munday & Obendorf 1984). The sheep used in our study were up to 2 years old. S. medusiformis has been more frequently detected in larynx, abdominal, and diaphragmatic musculature of sheep (Obendorf & Munday 1987). In our study, heart, tongue, and esophagus were employed. Macrocysts of S. moulei, which are specific of goats, are found in esophagus; however, the presence of these cysts by the naked eye is more frequent in animals from 19 months old (Heydorn & Kirmsse 1996).
The high frequencies of Sarcocystis spp. observed in goats and sheep in the current work are not surprising. Studies carried out by various authors from different countries have reported prevalences of infection in goats and sheep ranging from approximately 70 to 100 % (Dubey et al. 1988; Latif et al. 1999; Shekarforoush et al. 2005). The histologic and fresh examinations by light microscopy allowed the identification of thick wall and thin wall cysts, but they did not permit the differentiation of the species, even using high magnification (×1000).
S. arieticanis and S. capracanis have been reported in several studies conducted in different countries (Al Quraishy et al. 2014; Morsy et al. 2011; Haziroglu et al. 2003; Oryan et al. 1996; Singh et al. 1990; Heydorn & Mehlhorn 1987). In the Americas, the only reports of S. arieticanis and S. capracanis have been made in the USA (Dubey et al. 1988; Dubey & Livingston 1986). In the present study, the use of TEM allowed the identification of S. arieticanis and S. capracanis.
Besides the morphological characteristics of the cyst wall, molecular analysis provides a valuable support for the confirmation of Sarcocystis species (Yang et al. 2001a; Yang et al. 2001b). The rRNA gene subunit has been widely used to differentiate the apicomplexan from other eukaryotic species (Ellis & Morrison 1995; Ellis et al. 1995; Fischer & Odening 1998; Holmdahl et al. 1993; Jenkins et al. 1999). Other genetic markers, such as cytochrome oxidase, have been identified and shown to be useful for species confirmation in the genus Sarcocystis (Gjerde 2013). In this study, nucleotide sequencing of the PCR products using general primers for Sarcocystis spp. was performed in a small number of samples. The DNA obtained from individual cysts allowed the identification of S. tenella in sheep and S. capracanis in goats.
Although not performed in this work, bioassay methods may be applied for the differentiation among Sarcocystis that use cats or dogs as definitive hosts (Dubey et al. 1982; Heydorn & Haralambidis 1982; Heydorn & Matuschka 1981; Mehlhorn & Scholtyseck 1974; Munday & Rickard 1974).
In conclusion, we observed high frequencies of infection by Sarcocystis spp. that form microscopic cysts in sheep and goat destined for human consumption in Bahia, Brazil. We report, for the first time in Brazil and South America, the confirmation of Sarcocystis ariticanis and S. capracanis by combining ultrastructural and molecular analysis. Although Sarcocystis spp. that form macroscopic cysts in sheep (S. gigantea and S. medusiformis) and goats (S. moulei) were not found in the current work, more studies using older animals are needed to determine whether these macrocyst-forming species infect small ruminants in Brazil.
References
Al Quraishy S, Morsy K, Bashtar AR, Ghaffar FA, Mehlhorn H (2014) Sarcocystis arieticanis (Apicomplexa: Sarcocystidae) infecting the heart muscles of the domestic sheep, Ovis aries (Artiodactyla: Bovidae), from K. S. A. on the basis of light and electron microscopic data. Parasitol Res doi:10.1007/s00436-014-4050-2
Buxton D (1998) Protozoan infections (Toxoplasma gondii, Neospora caninum and Sarcocystis spp.) in sheep and goats: recent advances. Vet Res 29:289–310
Collins GH, Atkinson E, Charleston WA (1979) Studies on Sarcocystis species III: the macrocystic species of sheep. N Z Vet J 27:204–206
da Silva RC, Su C, Langoni H (2009) First identification of Sarcocystis tenella (Railliet, 1886) Moule, 1886 (Protozoa: Apicomplexa) by PCR in naturally infected sheep from Brazil. Vet Parasitol 165:332–336. doi:10.1016/j.vetpar.2009.07.016
Dahlgren SS, Gjerde B (2007) Genetic characterisation of six Sarcocystis species from reindeer (Rangifer tarandus tarandus) in Norway based on the small subunit rRNA gene. Vet Parasitol 146:204–213. doi:10.1016/j.vetpar.2007.02.023
Dubey JP, Livingston CW Jr (1986) Sarcocystis capracanis and Toxoplasma gondii infections in range goats from Texas. Am J Vet Res 47:523–524
Dubey JP, Speer CA, Epling GP (1982) Sarcocystosis in newborn calves fed Sarcocystis cruzi sporocysts from coyotes. Am J Vet Res 43:2147–2164
Dubey JP, Lindsay DS, Speer CA, Fayer R, Livingston CW Jr (1988) Sarcocystis arieticanis and other Sarcocystis species in sheep in the United States. J Parasitol 74:1033–1038
Dubey JP, Speer CA, Fayer R (1989) Sarcocystosis of animals and man. CRC Press, Boca Raton
Ellis J, Morrison D (1995) Effects of sequence alignment on the phylogeny of Sarcocystis deduced from 18S rDNA sequences. Parasitol Res 81:696–699
Ellis TJ, Luton K, Baverstock PR, Whitworth G, Tenter AM, Johnson AM (1995) Phylogenetic relationships between Toxoplasma and Sarcocystis deduced from a comparison of 18S rDNA sequences. Parasitology 110(Pt 5):521–528
Fischer S, Odening K (1998) Characterization of bovine Sarcocystis species by analysis of their 18S ribosomal DNA sequences. J Parasitol 84:50–54
Gjerde B (2013) Phylogenetic relationships among Sarcocystis species in cervids, cattle and sheep inferred from the mitochondrial cytochrome c oxidase subunit I gene. Int J Parasitol 43:579–591. doi:10.1016/j.ijpara.2013.02.004S0020-7519(13)00087-8
Haziroglu R, Guvenc T, Tunca R (2003) Electron microscopical studies on cysts of Sarcocystis arieticanis within cardiac muscle of naturally infected sheep. Parasitol Res 89:23–25. doi:10.1007/s00436-001-0549-4
Heydorn AO, Haralambidis S (1982) Development of Sarcocystis capracanis Fischer, 1979. Berl Munch Tierarztl Wochenschr 95:265–271
Heydorn AO, Kirmsse P (1996) Isolation and experimental transmission of Sarcocystis moulei Neveu-Lemaire, 1912. Berl Munch Tierarztl Wochenschr 109:440–445
Heydorn AO, Matuschka FR (1981) Final host specificity of Sarcocystis species transmitted by dogs (authors’s transl). Z Parasitenkd 66:231–234
Heydorn AO, Mehlhorn H (1987) Fine structure of Sarcocystis arieticanis Heydorn, 1985 in its intermediate and final hosts (sheep and dog). Zentralbl Bakteriol Mikrobiol Hyg A 264:353–362
Heydorn AO, Gestrich R, Mehlhorn H, Rommel M (1975) Proposal for a new nomenclature of the Sarcosporidia. Z Parasitenkd 48:73–82
Holmdahl OJ, Mattsson JG, Uggla A, Johansson KE (1993) Oligonucleotide probes complementary to variable regions of 18S rRNA from Sarcocystis species. Mol Cell Probes 7:481–486. doi:10.1006/mcpr.1993.1071
Instituto Brasileiro de Geografia e Estatística—IBGE (2011) Produção da pecuária municipal. Rio de Janeiro 39:1–63
Jenkins MC, Ellis JT, Liddell S, Ryce C, Munday BL, Morrison DA, Dubey JP (1999) The relationship of Hammondia hammondi and Sarcocystis mucosa to other heteroxenous cyst-forming coccidia as inferred by phylogenetic analysis of the 18S SSU ribosomal DNA sequence. Parasitology 119(Pt 2):135–142
Latif BM, Al-Delemi JK, Mohammed BS, Al-Bayati SM, Al-Amiry AM (1999) Prevalence of Sarcocystis spp. in meat-producing animals in Iraq. Vet Parasitol 84:85–90
Levine ND (1986) The taxonomy of Sarcocystis (Protozoa, Apicomplexa) species. J Parasitol 72:372–382
Martínez-Navalón B et al (2012) Sarcocystis infection: a major cause of carcass condemnation in adult sheep in Spain. Span J Agric Res 10:388–392. doi:10.5424/sjar/2012102-523-11
Mehlhorn H, Scholtyseck E (1974) Light and electron microscope studies on stages of Sarcocystis tenella in the intestine of cats. I. The oocysts and sporocysts (author’s transl). Z Parasitenkd 43:251–270
Morsy K et al (2011) Prevalence pattern and biology of Sarcocystis capracanis infection in the Egyptian goats: a light and ultrastructural study. Vet Parasitol 181:75–82. doi:10.1016/j.vetpar.2011.05.010
Munday BL, Obendorf DL (1984) Development and growth of Sarcocystis gigantea in experimentally-infected sheep. Vet Parasitol 15:203–211
Munday BL, Rickard MD (1974) Is Sarcocystis tenella two species. Aust Vet J 50:558–559
Obendorf DL, Munday BL (1987) Experimental infection with Sarcocystis medusiformis in sheep. Vet Parasitol 24:59–65
Oryan A, Moghaddar N, Gaur SN (1996) The distribution pattern of Sarcocystis species, their transmission and pathogenesis in sheep in Fars Province of Iran. Vet Res Commun 20:243–253
Pescador CA, Corbellini LG, Oliveira EC, Bandarra PM, Leal JS, Pedroso PMO, Dremeier D (2007) Aborto ovino associado com infecção por Sarcocystis sp. Pesqui Vet Bras 27:393–397
Shekarforoush SS, Razavi SM, Dehghan SA, Sarihi K (2005) Prevalence of Sarcocystis species in slaughtered goats in Shiraz. Iran Vet Rec 156:418–420
Singh KP, Agrawal MC, Shah HL (1990) Prevalence of sarcocysts of Sarcocystis capracanis in oesophagus and tail muscles of naturally infected goats. Vet Parasitol 36:153–155
Yang ZQ, Zuo YX, Ding B, Chen XW, Luo J, Zhang YP (2001a) Identification of Sarcocystis hominis-like (Protozoa: Sarcocystidae) cyst in water buffalo (Bubalus bubalis) based on 18S rRNA gene sequences. J Parasitol 87:934–937. doi:10.1645/0022-3395(2001)087[0934:IOSHLP]2.0.CO;2
Yang ZQ, Zuo YX, Yao YG, Chen XW, Yang GC, Zhang YP (2001b) Analysis of the 18S rRNA genes of Sarcocystis species suggests that the morphologically similar organisms from cattle and water buffalo should be considered the same species. Mol Biochem Parasitol 115:283–288
Acknowledgments
We thank the members of the Electron Microscopy section from Fundação Gonçalo Muniz (Fiocruz, Bahia) for their excellent technical assistance. The project was partially funded by Fundação de Amparo à Pesquisa do Estado da Bahia (FAPESB). L. F. Gondim was recipient of a fellowship from the National Research Council (CNPq, Brazil).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bittencourt, M.V., Meneses, I.D.S., Ribeiro-Andrade, M. et al. Sarcocystis spp. in sheep and goats: frequency of infection and species identification by morphological, ultrastructural, and molecular tests in Bahia, Brazil. Parasitol Res 115, 1683–1689 (2016). https://doi.org/10.1007/s00436-016-4909-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-016-4909-5