Abstract
Malaria is still one of the most common infectious diseases and leads to various public health problems worldwide. Medicinal plants are promising sources for identifying novel agents with potential antimalarial activity. This study aimed to investigate the antimalarial and the antioxidant activities of Indigofera oblongifolia on Plasmodium chabaudi-induced spleen tissue injury in mice. Mice were divided into five groups. The first group served as a vehicle control; the second, third, fourth, and fifth groups were infected with 1 × 106 P. chabaudi-parasitized erythrocytes. Mice of the last three groups were gavaged with 100 μl of I. oblongifolia leave extract (IOLE) at a dose of 100, 200, and 300 mg IOLE/kg, respectively, once daily for 7 days. IOLE was significantly able to lower the percentage of parasitemia. The most effective dose was the 100 mg IOLE/kg, which could reduce the parasitemia from about 38 to 12 %. The infection induced spleen injury. This was evidenced by disorganization of spleen white and red pulps, appearance of hemozoin granules and parasitized erythrocytes. These changes in spleen led to the increased histological score. Also, the infection increased the spleen oxidative damage where the levels of nitrite/nitrate, malondialdehyde, and catalase were significantly altered. All these infection-induced parameters were significantly improved during IOLE treatment. In addition, the mRNA expression of inflammatory cytokines interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha were upregulated after infection with P. chabaudi, whereas IOLE significantly reduced the expression of these genes. Our results indicate that I. oblongifolia leaves extract exhibits a significant antimalarial and antioxidant effects, and protects host spleen tissue from injuries induced by P. chabaudi.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Malaria is a worldwide disease with major negative effect on economic development (Worrall et al. 2005). The disease is widespread in the tropical and subtropical regions that exist in a broad band around the equator. This includes much of Sub-Saharan Africa, Asia, and Latin America (Mehlhorn 2008). It was documented that parasite resistance to antimalarial drugs results in a delayed or incomplete clearance of parasites from the patient’s blood when the person is being treated with an antimalarial (Foster 1991; Ridley 2002). One important strategy to fight the parasite resistance is to encourage the discovery of new antimalarial compounds from various sources, especially from traditional medicinal plants where focus on medicinal plant research has been increasing all over the world. In traditional medicine, plant formulations and combined extracts of plants are used for the treatment of a wide variety of diseases (Sasidharan et al. 2011; Zhang et al. 2014).
Many studies used plant extracts against malaria infection (Al-Adhroey et al. 2010; Taherkhani et al. 2013). Recently, Toma et al. (2015) used Echinops kebericho extract as antiplasmodial agent. Also, Mubaraki et al. (2014) used pomegranate peel extract as a hepatoprotective agent from Plasmodium chabaudi infection.
Indigofera oblongifolia belongs to the family Fabaceae and is widely distributed in Asia and Africa. All parts of the plant are useful in enlargement of the spleen and liver (Kirtikar and Basu 1984). The leaves of I. oblonifolia are used in folk medicines in urinary tract infection, urolithiasis, cough, and skin infection (Ali et al. 2001). Also, Dahot (1999) reported the antimicrobial activity of the plant (Dahot 1999). The current study aimed to investigate the antimalarial activity of I. oblongifolia leaves extract and its effect on spleen immune response of P. chabaudi infection in mice.
Materials and methods
Preparation of the plant extract
I. oblongifolia leaves (Fig. S1) were obtained from Jazan Province in the southwest of the Kingdom of Saudi Arabia. The identity of this specie was confirmed by Dr. Jacob Pandalayil (Department of Botany and Microbiology, College of Science, King Saud University). Leaves were air dried at a temperature not exceeding 40 °C and ground into powder. The powdered leaves were extracted with 70 % methanol. In brief, the powder was incubated at 4 °C for 24 h with mixing from time to time. I. oblongifolia leaves extract (IOLE) was filtered and then evaporated to dryness in vacuum evaporator (Heidolph, Germany). The residue was dissolved in distilled water and used in this experiment.
Animals
This study was conducted using apparently healthy female C57BL/6 mice. The animals were 9–12 weeks old. They were obtained from The Department of Comparative Medicine, King Faisal Specialist Hospital & Research Center, Riyadh, Saudi Arabia. The mice were bred under specified pathogen-free conditions and fed a standard diet and water ad libitum. The experiments were approved by the state authorities and followed Saudi Arabian rules on animal protection.
P. chabaudi infection
Blood-stage infections of P. chabaudi were routinely kept in Swiss albino mice at weekly passages (Wunderlich et al. 1982). The experimental female C57BL/6 mice were intraperitoneally injected with 106 P. chabaudi-parasitized erythrocytes. Parasitemia was determined in Giemsa-stained smears from tail blood. Cell number was counted in a Neubauer chamber.
Experimental design
A total of 50 adult female C57BL/6 mice were divided into five groups with ten animals per each group. The first group (non-infected) served as a vehicle control. This group gavaged only with 100 μl distilled water. The second, third, fourth, and fifth groups were intraperitoneally infected with 1 × 106 P. chabaudi-infected erythrocytes. Then, after 60 min, mice of the third, fourth, and fifth groups were gavaged with 100 μl of IOLE at a dose of 100 (infected + IOLE), 200 (infected + IOLE), and 300 (infected + IOLE) mg/kg body weight of IOLE, respectively, once daily for 7 days.
Hematological studies
Mice were killed and dissected on day 7 p.i. Blood was taken from the heart into heparinized tubes for the determination of some important hematological parameters (total erythrocytes count, total leucocytes count, and hemoglobin contents) using an automatic counter (VET-530 CA Medonic; Medonic, Stockholm, Sweden).
Preparation of spleen tissue
Both non-infected and infected mice were sacrificed by cervical dislocation on day 7 p.i.. Spleens were aseptically removed from mice, cut up in small pieces, some of them were fixed in formalin for histological study and the others were washed in sterile physiological saline, and rapidly frozen and stored at −80 °C for further investigations.
Spleen histopathology
Mice spleens were fixed in 10 % neutral buffered formalin and then embedded in paraffin. Sections of 5 μm thickness were stained with hematoxylin and eosin. To evaluate the splenic histological alteration, a semi-quantitative scoring system was used (Giamarellos-Bourboulis et al. 2006). Segments of spleen were scored for the enlargement of white pulp areas (0, absent; 1, slight; 2, moderate; and 3, pronounced) and for the increased numbers of apoptotic cells, macrophages, necrotic cells, and presence of pigments (0, absent; and 1, present). Scoring of each tissue sample represented the mean score of high microscopic power fields of five different sections.
Oxidative stress in the spleen
Spleens were weighed and homogenized immediately to give 50 % (w/v) homogenate in ice-cold medium containing 50 mM Tris–HCl and 300 mM sucrose (Tsakiris et al. 2004). The homogenate was centrifuged at 500×g for 10 min at 4 °C. The supernatant (10 %) was used for the various biochemical determinations.
Nitrite/nitrate
The assay of nitrite/nitrate in spleen homogenate was done according to the method of Berkels et al. (2004). In acid medium and in the presence of nitrite, the formed nitrous acid diazotized sulfanilamide, which is coupled with N-(1-naphthyl) ethylenediamine. The resulting azo dye has a bright reddish-purple color which was measured at 540 nm.
Malondialdehyde
Lipid peroxidation in spleen homogenate was determined according to the method of Ohkawa et al. (1979) by using 1 ml of 10 % trichloroacetic acid and 1 ml of 0.67 % thiobarbituric acid, followed by heating in a boiling water bath for 30 min. Thiobarbituric acid reactive substances were determined by the absorbance at 535 nm and expressed as malondialdehyde equivalents formed.
Catalase
Catalase activity in spleen homogenate was assayed by the method of Aebi (1984). In this assay, catalase reacts with a known quantity of H2O2 and the reaction is stopped after exactly 1 min with a catalase inhibitor. In the presence of horseradish peroxidase, the remaining H2O2 reacts with 3,5-dichloro-2-hydroxybenzene sulfonic acid and 4-aminophenazone to form a chromophore with a color intensity inversely proportional to the amount of catalase in the original sample, and measured at 240 nm.
Quantitative PCR
Tissues frozen at −80 °C were thoroughly ground with a mortar under liquid nitrogen, and total RNA was isolated with TRIzol (Qiagen, Hilden, Germany). Quality and integrity of RNA were determined using the Agilent RNA 6000 Nano Kit on the Agilent 2100 Bioanalyzer (Agilent Technologies). RNA was quantified by measuring A260nm on the ND-1000 spectrophotometer (NanoDrop Technologies) (Delic et al. 2010). All RNA samples were treated with DNase (Applied Biosystems, Darmstadt, Germany) for at least 1 h and were then converted into cDNA using the reverse transcription kit following the manufacturer’s protocol (Qiagen, Hilden, Germany). Quantitative real-time PCR (RT-qPCR) was performed using the ABI Prism 7500HT sequence detection system (Applied Biosystems, Darmstadt, Germany) with SYBR green PCR master mix from Qiagen (Hilden, Germany). Genes were investigated encoding the mRNAs for interleukin 1beta (IL-1 β), interleukin-6 (IL-6), tumor necrosis factor alpha (TNF-α), and glyceraldehyd-3-phosphat-dehydrogenase (GAPDH). All primers used for RT-qPCR were obtained commercially from Qiagen. PCRs were conducted as follows: 2 min at 50 °C to activate uracil-N-glycosylase (UNG); 95 °C for 10 min to deactivate UNG; and 40 cycles at 94 °C for 15 s, 60 °C for 35 s, and 72 °C for 30 s. Reaction specificity was checked by performing dissociation curves after PCR. For quantification, mRNA levels were normalized to those of GAPDH. The threshold cycle (CT) value is the cycle number, selected from the logarithmic phase of the PCR curve, in which an increase in fluorescence above background can be detected. ΔCT is determined by subtracting the CT of GAPDH from the CT of the target. The fold change of mRNA expression was determined using the 2−ΔΔCT method.
Statistical analysis
One-way ANOVA was carried out, and the statistical comparisons among the groups were performed with Duncan’s t test using a statistical package program (SPSS version 17.0). P ≤ 0.05 was considered as significant for all statistical analysis in this study.
Results
The course of P. chabaudi infections in female C57BL/6 was previously characterized in detail (Krücken et al. 2005; Wunderlich et al. 2005). Treatment of infected mice with IOLE induced marked changes in parasitemia on day 7 p.i. with P. chabaudi-infected erythrocytes; mice treated with 100 mg of IOLE/kg appeared with a significant (P < 0.001) decrease in parasitemia compared to the infected non-treated group (infected −IOLE). The parasitemia of infected treated mice with a dose of 200 mg IOLE/kg was not significantly changed from the infected control group, while parasitemia of mice treated with 300 IOLE mg/kg of IOLE was significantly decreased by about 50 % (Fig. 1).
Anemia was diagnosed by measuring the hemoglobin content and erythrocytes count in blood of mice at day 7 p.i. with P. chaboudi. Hemoglobin of infected (−IOLE) group was 5.8 ± 1.8 g/dl while that of the non- infected mice was 16.9 ± 0.5 g/dl. Also, erythrocytes of infected (−IOLE) group was 4.5 × 1012 erythrocyte/mm3 while that of the non-infected mice reached 9.5 × 1012 ± 0.3 erythrocyte/mm3. This indicated a clear state of anemia in the infected group. I. oblongifolia significantly reduced the loss in hemoglobin as well in erythrocyte count, especially in the mice group treated with 100 mg/kg IOLE (Table 1). Also, the infection induced a marked decrease in leucocytes count but this was not significantly changed by IOLE treatment (Table 1).
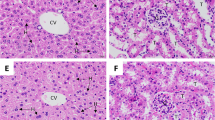
Examination of the spleen tissue sections reveal the presence of parasitized erythrocytes and hemozoin granules in the spleen of P. chabaudi-infected mice. Nevertheless, the spleen exhibits structural reorganizations (Fig. 2). The spleen revealed larger white pulp areas, and the marginal zone normally separating white and red pulp areas was highly enlarged with a more diffuse appearance, in comparison to the spleen of non-infected mice (Fig. 2). The structural reorganization of the spleen of infected mice became also evident as an increased number of apoptotic cells. Treatment of mice with 100 mg IOLE/kg induced an improvement in the spleen architecture. This was evidenced by the return of the white and the red pulps nearly to the normal appearance (Fig. 2). The number of the infected erythrocytes in the spleen section became low. Also, the hemozoin granules were much less when compared to the infected spleen. Mice treated with either 200 or 300 mg IOLE/kg did not show marked improvements in spleen histology (Fig. 2). The histological score of the splenic tissue was significantly larger in infected mice than that in non-infected mice. After treatment, only the dose of 100 mg IOLE/kg could significantly reduce the enlarged score due to P. chabaudi infection (Fig. 3).
I. oblongifolia leaves extract (IOLE) induced changes in spleen of mice infected with P. chabaudi on day 7p.i.. a, b Non-infected spleen sections with normal architecture. c, d Infected mice spleen. White pulps are fused. Infected erythrocytes (black arrow) and hemozoin granules (white arrow) are common in the red pulp. Some apoptotic bodies appeared in the spleen (white arrow head). e, f Spleen of mice treated with 100 mg/kg IOLE. Some white pulps are fused together and others are clearly separated. Infected erythrocytes and hemozoin granules are less common in the red pulp. g, h and i, j Spleen of mice treated with 200 and 300 mg/kg IOLE, respectively. White pulps are fused. Infected erythrocytes and hemozoin granules are common in the red pulp. Some apoptotic bodies appeared in the spleen. Sections are stained with hematoxylin and eosin. Scale bar = 50 μm for a, c, e, g, i and 25 μm for b, d, f, h, j
Since with the dose 100 mg of IOLE/kg mice was more efficient than the other two used doses against P. chabaudi infection, we only studied this dose for quantifying the mice spleen antioxidant activity as well as the spleen cytokine expression using QT-PCR.
P. chabaudi infection was able to significantly increase the level of nitrite/nitrate (Table 2). Treatment of mice with IOLE could induce a significant decrease in the level of nitrite/nitrate (Table 2). Furthermore, infection with P. chabaudi resulted in a significant increase in the level of malondialdehyde. On the other hand, supplementation with the plant extract significantly reduced the levels of malondialdehyde (P ≤ 0.05) in infected groups (Table 2). Moreover, the effect of the plant extract on catalase levels in the spleen of mice infected with P. chabaudi was visible on day 7 p.i. and the level of catalase in the group of mice treated with I. oblongifolia was significantly higher than (P ≤ 0.05) non-infected and infected groups (Table 2).
In this study, the mRNA expression of inflammatory cytokines, IL-1β, IL-6, and TNF-α (Fig. 4), was upregulated after infection with P. chabaudi, whereas IOLE significantly reduced the expression of these genes (Fig. 4).
Anti-inflammatory effect of I. oblongifolia leaves extract (IOLE) on P. chabaudi infected mice. Expression of IL-1β, IL-6, and TNF-α-mRNA in spleen tissues was analyzed by quantitative RT-PCR in non-infected mice, and P. chabaudi-infected mice on day 7 p.i. with and without IOLE treatment. Relative expression is given as fold increase compared to the non-infected control mice. Values are means ± SD. (a) Significance against non-infected group at P ≤ 0.05. (b) Significance against infected (−IOLE) group at P ≤ 0.05
Discussion
n an effort to find new strategies for malaria control, recent research has investigated the role medicinal plants in regulating infections with Plasmodium spp. (Mohd Abd Razak et al. 2014; Mubaraki et al. 2014; Odediran et al. 2014; Vale et al. 2015). Clearance of malaria parasites appears to be mediated by both acquired and innate immune responses (Wunderlich et al. 2014). Female C57BL/6 mice were able to heal infections with P. chabaudi and to develop long-lasting immunity against homologous rechallenge (Dkhil 2009). Medicinal plants have been used for dietary therapy for several millennia, and some of them allegedly exhibit significant antiparasitic and antioxidant activities (Mau et al. 2002; Mohd Abd Razak et al. 2014; Dkhil et al. 2015).
In this study, I. oblongifolia was used as the target natural product against malaria. Infections of P. chabaudi in mice at maximal parasitemia on day 7 p.i. induced responses in the spleen. These responses became evident as severe structural reorganizations, oxidative spleen damage, and as differential gene expression detected by microarray technology in combination with quantitative PCR. Treatment of mice with I. oblongifolia could ameliorate the altered changes due to infection.
The leaves extract was able to significantly decrease the percentage of parasitemia by approximately 68.4 % when mice were treated with 100 mg/kg of the extract. This is the first study to use such a plant against parasites. These results are comparable to that obtained by Mubaraki et al. (2014) using extract from another plant, pomegranate, which contained also rich natural ingredients (Shahjahan et al. 2005).
Dkhil et al. (2015) reported that anaemia is the major clinical sign and cause of mortality in animals with malaria infection where malaria parasites invade erythrocytes of infected animals, resulting in the destruction of the parasitized erythrocytes. In this study, I. oblongifolia was able to significantly increase the reduced number of erythrocytes as well as the hemoglobin content. Also, Kumar et al. (2007) used ethanolic extract of Indigofera trita against induced cancer in mice, and the extract was found to have a protective effect on the change in the hematological parameters including red blood cells and hemoglobin.
Histologic examination of spleen tissue of C57BL/6 mice during the course of P. chabaudi infection showed disturbed T cell areas and changes in splenic architecture (Achtman et al. 2003). Rich natural ingredients present in I. oblongifolia (Shahjahan et al. 2005) could improve the altered changes in spleen histology.
The role of the spleen as an anti-parasite effector is further substantiated by our finding that the spleen displays hemozoin granules during the acute phase of blood stage malaria. Hemozoin is generated by intraerythrocytic parasites to detoxify the lytic host ferriprotoporphyrin IX (Pagola et al. 2000) released during hemoglobin digestion by parasites (Zhang et al. 1999). The hemozoin granules are almost exclusively localized in the red pulp of the spleen, thus indicating that the red pulp predominantly harbors the antimalaria effectors of the spleen.
The role of oxidative stress in the pathophysiology of malaria is a multifactorial phenomenon and represents an important aspect of the intricate and complex host-parasite relationship. Recent studies suggest that the generation of reactive oxygen and nitrogen species (ROS and RNS) associated with oxidative stress plays a crucial role in the development of systemic complications caused by malaria. Malaria infection induces the generation of hydroxyl radicals (OH·), which most probably is the main reason for the induction of oxidative stress and apoptosis (Guha et al. 2006). Oxidative stress markers in infected animals are found in high levels compared to uninfected controls (Guha et al. 2006; Sohail et al. 2007).
In this study, oxidative stress seems to result from increased production of free radicals, a fact suggested by increased malondialdehyde, an important lipid peroxidation marker, and not from a decrease in levels of antioxidants, reinforcing the suggestion that oxidative stress is an important mechanism in parasite infection. This result is in agreement with Cabrales et al. (2011). Also, a free radical species, which appears to be involved in this disease, is nitric oxide (Pabón et al. 2002; Cabrales et al. 2011). However, its role is still controversial. Moreover, several antioxidant enzymes are important in the defense system. In this study, catalase was measured during the infection with P. chabaudi infection. This enzyme acts directly on some free radicals, making them less reactive.
Cytokines in malaria are reported to be important molecular markers of cell-mediated immune response and known to be critical players in the regulation of disease. Several studies have shown cytokines to be associated differentially with appearance of disease symptoms, levels of parasitemia, and with disease severity and complications (Malaguarnera and Musumeci 2002; Wunderlich et al. 2014). The inflammatory response that is needed to remove parasites leads to considerable tissue damage, and activation of phagocytes to kill intracellular or extracellular parasites requires the production of inflammatory cytokines. In this study, the infection of mice with P. chabaudi-parasitized erythrocytes induced a significant increase in TNF-α, IL1β, and IL-6 mRNA expression. It has been reported that increased TNF-α levels stimulated phagocytosis and thereby enhanced clearance of parasitized erythrocytes (Lyke et al. 2004; Stevenson and Riley 2004). To the contrary, proinflammatory cytokines were seen to contribute to adverse disease outcome where a strong and prolonged response of cytokines like TNF-α, IL1β, and IL-6 was associated with severe disease syndromes in both human and experimental models (Day 1999; Malaguarnera and Musumeci 2002). Moreover, Pichyangkul et al. (1994) proved that TNF-α is the first characterized parasite-induced cytokine which was induced in macrophages by erythrocytes infected by Plasmodium, malarial pigment. In this work, treatment of mice with IOLE successfully improved the change in cytokine expression in the infected mice spleen. This curative effect of the plant extract made successive usage of the whole parts of I. oblongifolia in the treatment of splenomegaly (Shahjahan et al. 2005). Other species of Indigofera named (Indigofera suffruticosa) which has nearly similar active ingredients as I. oblongifolia was used to activate the immune system through macrophage activation (Lopes et al. 2010; de ACarli et al. 2010). Activation of macrophages led to phagocytosis of the parasitized erythrocytes (Dkhil 2009). This process could lower the parasitemia and splenomegaly induced by P. chabaudi.
Collectively, our results indicate that I. oblongifolia leaves extract exhibits a significant antimalarial and antioxidant effects, and protects host spleen tissue from injuries induced by P. chabaudi. Further studies are required, however, in order to elucidate the exact mechanism of these effects and to examine its potential therapeutic effects in more detail.
References
Achtman AH, Khan M, MacLennan ICM, Langhorne J (2003) Plasmodium chabaudi chabaudi infection in mice induces strong B cell responses and striking but temporary changes in splenic cell distribution. J Immunol 171:317–324
Aebi HU (ed) (1984) Methods in enzymatic analysis. Academic, New York, pp 276–286
Al-Adhroey AH, Nor ZM, Al-Mekhlafi HM, Amran AA, Mahmud R (2010) Antimalarial activity of methanolic leaf extract of Piper betle L. Molecules 16:107–118
Ali NA, Julich WD, Kusnick C, Lindequist U (2001) Screening of Yemeni medicinal plants for antibacterial and cytotoxic activities. J Ethanopharmacol 74:173–179
Berkels R, Purol-Schnabel S, Roesen R (2004) Measurement of nitric oxide by reconversion of nitrate/nitrite to NO. J Humana Press 279:1–8
Cabrales P, Zanini GM, Meays D, Frangos JA, Carvalho LJ (2011) Nitric oxide protection against murine cerebral malaria is associated with improved cerebral microcirculatory physiology. J Infect Dis 203:1454–1463
Dahot MU (1999) Antimicrobial and antifungal activity of small protein of Indigofera oblongifolia leaves. J Ethnopharmcol 64:277–282
Day NPJ (1999) The prognostic and pathophysiologic role of pro- and antiinflammatory cytokines in severe malaria. J Infect Dis 180:1288–1297
de ACarli CB, Quilles MB, Maia DC, Lopes FC, Santos R, Pavan FR, Fujimura Leite CQ, Calvo TR, Vilegas W, Carlos IZ (2010) Antimycobacterial activity of Indigofera suffruticosa with activation potential of the innate immune system. Pharm Biol 48:878–882
Delic D, Gailus N, Vohr HW, Dkhil M, Al-Quraishy S, Wunderlich F (2010) Testosterone-induced permanent changes of hepatic gene expression in female mice sustained during Plasmodium chabaudi malaria infection. J Mol Endocrinol 45:379–390
Dkhil MA (2009) Apoptotic changes induced in mice splenic tissue due to malaria infection. J Microbiol Immunol Infect 42:13–18
Dkhil MA, Al-Quraishy S, Al-Shamrany A, Alazzouni AS, Lubbad MY, Al-Shaebi EM, Noory T Taib NT (2015) Protective effect of berberine chloridr on Plasmodium chabaudi-induced hepatic tissue injury in mice. Saudi J Biol Sci
Foster SD (1991) Pricing, distribution, and use of antimalarial drugs. Bull World Health Organ 69:349–363
Giamarellos-Bourboulis EJ, Tziortzioti V, Koutoukas P, Baziaka F, Raftogiannis M, Antonopoulou A, Adamis T, Sabracos L, Giamarellou H (2006) Clarithromycin is an effective immunomodulator in experimental pyelonephritis caused by panresistant Klebsiella pneumonia. J Antimicrob Chemother 57:937–944
Guha M, Kumar S, Choubey V, Maity P, Bandyopadhyay U (2006) Apoptosis in liver during malaria: role of oxidative stress and implication of mitochondrial pathway. FASEB J 20:439–449
Kirtikar KR, Basu BD (1984) Indian medicinal plants, 2nd edition, Vol. 1. International Book Distributors, Dehradun, pp. 712
Krücken J, Mehnert LI, Dkhil MA, El-Kadragy M, Benten WPM, Mossmann H, Wunderlich F (2005) Massive destruction of malaria parasitized red blood cells despite spleen closure. Infect Immun 73:6390–6398
Kumar RS, Jayakar B, Rajkapoor B (2007) Antitumour activity of Indigofera trita on Ehrlich ascites carcinoma induced mice. Int J Cancer Res 3:180–185
Lopes FC, Calvo TR, Colombo LL, Vilegas W, Carlos IZ (2010) Immunostimulatory and cytotoxic activities of Indigofera suffruticosa (Fabaceae). Nat Prod Res 25:1796–1806
Lyke KE, Burges R, Cissoko Y, Sangare L, Dao M, Diarra I, Kone A, Harley R, Plowe CV, Doumbo OK, Sztein MB (2004) Serum levels of the proinflammatory cytokines interleukin-1 beta (IL-1ß), IL-6, IL-8, IL-10, tumor necrosis factor alpha, and IL-12 (p70) in Malian children with severe Plasmodium falciparum malaria and matched uncomplicated malaria or healthy controls. Infect Immunol 72:5630–5637
Malaguarnera L, Musumeci S (2002) The immune response to Plasmodium falciparum malaria. Lancet Infect Dis 2:472–478
Mau JL, Lin HC, Chen CC (2002) Antioxidant properties of several medicinal mushrooms. J Agric Food Chem 50:6072–6077
Mehlhorn H (ed) (2008) Encyclopedic reference of parasitology, vol 1, 3rd edn. Springer, Berlin
Mohd Abd Razak MR, Afzan A, Ali R, Amir Jalaluddin NF, Wasiman MI, Shiekh Zahari SH, Abdullah NR, Ismail Z (2014) Effect of selected local medicinal plants on the asexual blood stage of chloroquine resistant Plasmodium falciparum. BMC Complement Altern Med 14(1):492
Mubaraki MA, Dkhil MA, Al-Shaebi EM, Lubbad MY, Ibrahim KE, Al-Quraishy S (2014) The protective effect of pomegranate, Punica granatum, on murine malaria. Pak J Zool 46:1345–1350
Odediran SA, Elujoba AA, Adebajo AC (2014) Influence of formulation ratio of the plant components on the antimalarial properties of MAMA decoction. Parasitol Res 113(5):1977–1984
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Pabón A, Carmona J, Burgos LC, Blair S (2002) Oxidative stress in patients with non-complicated malaria. Clin Biochem 36:71–78
Pagola S, Stephens PW, Bohle DS, Kosar AD, Madsen SK (2000) The structure of malaria pigment beta-haematin. Nature 404:307–310
Pichyangkul S, Saengkrai P, Webster HK (1994) Plasmodium falciparum pigment induces monocytes to release high levels of tumor necrosis factor-alpha and interleukin-1 beta. Am J Trop Med Hyg 51:430–435
Ridley RG (2002) Medical need, scientific opportunity and the drive for antimalarial drugs. Nature 415:686–693
Sasidharan S, Chen Y, Saravanan D, Sundram KM, Yoga Latha L (2011) Extraction, isolation and characterization of bioactive compounds from plants’ extracts. Afr J Tradit Complement Altern Med 8:1–10
Shahjahan M, Vani G, Shyamala Devi CS (2005) Protective effect of Indigofera oblongifolia in CCl4-induced hepatotoxicity. J Med Food 8:261–265
Sohail M, Kaul A, Raziuddin M, Adak T (2007) Decreased glutathione-S-transferase activity: diagnostic and protective role in vivax malaria. Clin Biochem 40:377–382
Stevenson MM, Riley EM (2004) Innate immunity to malaria. Nat Rev Immunol 4:169–180
Taherkhani M, Rustaiyan A, Nahrevanian H, Naeimi S, Taherkhani T (2013) Comparison of antimalarial activity of Artemisia turanica extract with current drugs in vivo. J Vector Borne Dis 50:51–g6
Toma A, Deyno S, Fikru A, Eyado A, Beale A (2015) In vivo antiplasmodial and toxicological effect of crude ethanol extract of Echinops kebericho traditionally used in treatment of malaria in Ethiopia. Malar J 14:196
Tsakiris S, Schulpis KH, Marinou K, Behrakis P (2004) Protective effect of L-cysteine and glutathione on the modulated suckling rat brain Na+, K+-ATPase and Mg2+-ATPase activities induced by the in vitro galactosaemia. Pharmacol Res 49:475–479
Vale VV, Vilhena TC, Trindade RC, Ferreira MR, Percário S, Soares LF, Pereira WL, Brandão GC, Oliveira AB, Dolabela MF, De Vasconcelos F (2015) Anti-malarial activity and toxicity assessment of Himatanthus articulatus, a plant used to treat malaria in the Brazilian Amazon. Malar J 14:132
Worrall E, Basu S, Hanson K (2005) Is malaria a disease of poverty? A review of the literature. Trop Health Med 10:1047–1059
Wunderlich F, Stübig H, Königk E (1982) Development of Plasmodium chabaudi in mouse red blood cells: structural properties of the host and parasite membranes. J Protozool 29:60–66
Wunderlich F, Dkhil MA, Mehnert LI, Braun JV, El-Khadragy M, Borsch E, Hermsen D, Benten WPM, Pfeffer K, Mossmann H, Krücken J (2005) Testosterone-responsiveness of spleen and liver in female lymphotoxin-beta receptor-deficient mice resistant to blood stage malaria. Microbes Infect 7:399–409
Wunderlich F, Al-Quraishy S, Steinbrenner H, Sies H, Dkhil MA (2014) Towards identifying novel anti-Eimeria agents: trace elements, vitamins, and plant-based natural products. Parasitol Res 113:3547–3556
Zhang J, Krugliak M, Ginsburg H (1999) The fate of ferriprotorphyrin IX in malaria infected erythrocytes in conjunction with the mode of action of antimalarial drugs. Mol Biochem Parasitol 99:129–141
Zhang XG, Li GX, Zhao SS, Xu FL, Wang YH, Wang W (2014) A review of dihydroartemisinin as another gift from traditional Chinese medicine not only for malaria control but also for schistosomiasis control. Parasitol Res 113(5):1769–1773
Acknowledgments
The authors extend appreciations to the Deanship of Scientific Research at King Saud University for funding the work through the research group project number PRG-1436-02.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. S1
Indigofera oblongifolia leaves. (JPEG 210 kb)
Rights and permissions
About this article
Cite this article
Lubbad, M.Y., Al-Quraishy, S. & Dkhil, M.A. Antimalarial and antioxidant activities of Indigofera oblongifolia on Plasmodium chabaudi-induced spleen tissue injury in mice. Parasitol Res 114, 3431–3438 (2015). https://doi.org/10.1007/s00436-015-4568-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-015-4568-y