Abstract
Rhinoestrus purpureus (Brauer, 1858) (Diptera: Oestridae) is an economically important parasite that can cause severe nasal myiasis in equids or even attacking humans. The antennae of R. purpureus were examined using stereoscopic microscopy and scanning electron microscopy. The general morphology was provided detailedly, together with distribution, type, size, and ultrastructure of antennal sensilla. All the three antennal segments, antennal scape, pedicel, and funiculus, are interspersed by microtrichiae. Only mechanoreceptors are detected on antennal scape and pedicel. On antennal funiculus, three types of sensilla were observed, including basiconic sensilla, coeloconic sensilla and clavate sensilla. Two features are characterized of this host-specific bot fly: (1) numerous sensory pits with branched basiconic sensilla on antennal funiculus and (2) the absence of trichoid sensilla. The function of these distinctive traits are discussed in association with the life history. We suggest that more sensory pits with branched sensilla could increase the sensitivity of olfactory system for host orientation, while the capability of pheromone identification might be reduced due to the absence of trichoid sensilla. Besides, we support both thermo- and chemo-functions of coeloconic sensilla.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rhinoestrus purpureus is an obligatory parasite of nasal-pharyngeal cavities in horses, donkeys, and zebras (Zumpt, 1965), which irritates the mucous membrane of nasopharyngeal cavity and injures both olfactory nerves and cerebral membrane, inducing encephalomyelitis and high mortality of the hosts (Zumpt 1965; Zayed 1992; Kaboret et al. 1997; Otranto et al. 2004, 2005; Colwell et al. 2006, 2007). Attacks on humans causing ophthalmomyasis and conjunctivitis by this species are also reported (Peyresblanques 1964). Myiasis caused by R. purpureus is considered to be confined to Asiatic and African countries (Zumpt 1965; Zayed et al. 1993; Deconinck et al. 1996; Kaboret et al. 1997; Tibayrenc et al. 1999); however, infestations has extended to Europe, specifically in southern Italy (Otranto et al. 2004, 2005).
As the crucial sensory organs involved in feeding, mating, and spawning (Fernandes et al. 2005; Amer and Mehlhorn 2006; Smallegange et al. 2008; Guha et al. 2012; Wang et al. 2012), antennal sensilla of flies have managed to evolve various features to enhance their sensitivity and adaptability to the ever-changing environment (Ross 1992; Sukontason et al. 2004; Zhang et al. 2012a, b, 2013a, b, c; Wang et al. 2014). Little is known about the antennal sensilla of adult R. purpureus. Thus, the antennal investigation of this mouthparts atrophied and host-specific species (Catts and Garcia 1963; Papavero 1977; Colwell et al. 2006) can provide more valid information that might further our understanding of parasitic adaptation, especially host-parasite interaction and co-evolution (Hall 1995; Colwell et al. 2007; Zhang et al. 2012a, b). In this study, we characterize the antennal structures and distribution, type, size, and ultrastructure of antennal sensory organs of R. purpureus, evaluate distinctive antennal characters by comparing with the previous findings, and discuss their potential function in the life history of this parasite.
Materials and methods
Both male and female R. purpureus were obtained from Siziwang Banner, Ulanchap, Inner Mongolia, North China, 1971, identified and pinned as museum samples, and air-dried on site.
The morphology of antenna was studied using an Olympus SZX16 stereoscopic microscope (Olympus Corp., Tokyo, Japan). A series of photographs of continuous sequences taken by a Cannon 500D digital camera (Canon, Inc., Tokyo, Japan) were superimposed on a standard Windows 7 platform by Adobe Photoshop CS4 (Adobe Systems, Inc., San Jose, CA, USA).
To prepare for observation through the scanning electron microscope (SEM), the heads of flies were first excised and rinsed in phosphate-buffered saline (PBS) buffer (pH 7.4) to remove surface debris. For further processing, the antennae were dissected from the head and dehydrated in a graded ethanol series (two times 15 min each with 60, 70, 80, 90, 95, and 100 % ethanol), mounted on stubs with double-sided adhesive tape, left in a desiccator overnight to dry completely, and then covered with gold and observed by HITACHI S3400 SEM (Hitachi Corp., Tokyo, Japan) at the Microscopy Core Facility, Biological Technology Center, Beijing Forestry University (Beijing, China). The length, basal diameter, and distal dilatation diameter of antennal sensilla were measured, combined with comparison of their dimension and distribution between males and females.
The terminology and nomenclatures used to identify antennal sensilla types and describe antennal morphology in this study follow the ones used by Zhang et al. (2012a, b, 2013a, b, c, 2015).
Results
General description of antenna
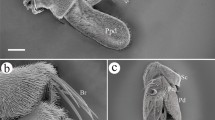
Adult R. purpureus bears a pair of aristate antennae that locate on the frontal region of the head between the compound eyes (Fig. 1a). The antenna is composed of three segments, a proximal scape (Sc), a pedicel (Pd), and a distal flagellum, which consists of funiculus (Fn) and arista (Ar) (Fig. 1a–c, e, f).
Features on the head of adult Rhinoestrus purpureus. a Antennae are located centrally between compound eyes. b Magnification of whole antenna. c. Magnification of antennal scape and pedicel. d. Magnification of the mechanoreceptor on antennal pedicel. e. Anterodorsal surface of antennal funiculus. f. Antennal arista. Scale bars: a = 1.00 mm, b = 150 μm, c = 60 μm, d = 5 μm, e = 100 μm, f = 150 μm. Ad, anterodorsal surface, Ar arista, Mr mechanoreceptor, Dl dorsolateral margin, Fn funiculus, Pd pedicel, Pv posteroventral surface, Sc scape
Scape and pedicel
Scape is the most proximal and shortest segment (Fig. 1a–c), whose surface is interspersed by hair-like and acuminate microtrichiae (Fig. 1b, c).
Pedicel is the second segment of antenna, on which the only sensilla are morphologically similar mechanoreceptors (Mrs). Mr is a long and relatively straight bristle with longitudinal grooved wall, inserting into a socket (Fig. 1c, d). Microtrichiae are also spread around the cuticular surface of antennal pedicel.
Funiculus
The antennal funiculus is the most important segment of the antenna (Fig. 1a, b, e) to which has numerous sensilla attached. For orientation, antennal funiculus has been divided into three regions: the anterodorsal surface (Ad), the dorsolateral margin (Dl), and the posteroventral surface (Pv) (Fig. 1b, c, e, f). A total of three types of antennal sensilla are distributed on it, including basiconic sensilla (Figs. 2a–c and 3a, b), coeloconic sensilla (Fig. 3a, c), and clavate sensilla (Fig. 3a, d). The length, basal diameter, and distal dilatation diameter of these antennal sensilla for both male and female species are summarized in Table 1, and their distribution is also examined, displayed in Fig 4. The arista consists of one short basal segments and one long distal segment, with sparse microtrichiae on basal part (Fig. 1a, b, e, f).
Scanning electron micrographs of basiconic sensilla on the antennal funiculus in Rhinoestrus purpureus.a Overview of basiconic sensilla I and clavate sensilla in sensory pits. b Basiconic sensilla I and basiconic sensilla II in sensory pits. c Magnification of basiconic sensilla I. Scale bars: a = 30 μm, b = 10 μm, c = 2.5 μm. Ba I basiconic sensilla I, Ba II basiconic sensilla II, Cl clavate sensilla
Scanning electron micrographs of basiconic sensilla, coeloconic sensilla, and clavate sensilla on the antennal funiculus in Rhinoestrus purpureus. a Overview of basiconic sensilla II, coeloconic sensilla, and clavate sensilla in sensory pits. b Magnification of basiconic sensilla II in sensory pits. c Magnification of coeloconic sensilla. d Magnification of clavate sensilla. Scale bars: a = 25 μm; b, c, d = 2.5 μm. Ba II basiconic sensilla II, Cl clavate sensilla, Co coeloconic sensilla
Distribution of antennal sensilla and sensory pits on each side of the antennal funiculus in Rhinoestrus purpureus. a Anterodorsal surface and dorsolateral margin of male R. purpureus. b. Posteroventral surface of male R. purpureus. c Anterodorsal surface and dorsolateral margin of female R. purpureus. d Posteroventral surface of female R. purpureus
Sensilla on antennal funiculus
A majority of the antennal sensilla are seated in sensory pits, which resemble the antennal sensilla on surface of the funiculus morphologically.
Basiconic sensilla
In adult R. purpureus examined, two subtypes of basiconic sensilla (Ba) can be distinguished by their shape and size. Basiconic sensilla I (Ba I) are sharp tipped (Fig. 2a–c) and distributed widely, especially middle and distal part on antennal funiculus (Fig. 4a–d), while basiconic sensilla II (Ba II) are only observed in sensory pits, with short branches at their apical part (Figs. 2b and 3a, b), and longer and wider than Ba I in general (Table 1).
Coeloconic sensilla
Coeloconic sensilla (Co), which are located in slightly sunken depressions of antennal funiculus, are relatively short sensilla characterized by distinct longitudinal ridges on their cuticular walls (Fig. 3a, c), with lower density than Ba (Fig. 4a–d).
Clavate sensilla
Clavate sensilla (Cl) are club like and featured by distal dilatations, which are seated in a superficial cavity with wall pierced by numerous pores (Fig. 3a, d) and observed only on the most proximal regions of antennal funiculus (Fig. 4a–d).
Discussion
As an obligatory parasite with rather short life span, typically about 1 week (Zumpt 1965; Papavero 1977; Zayed 1992; Zayed et al. 1993; Deconinck et al. 1996; Kaboret et al. 1997; Tibayrenc et al. 1999; Otranto et al. 2004, 2005; Colwell et al. 2006), the antennal sensilla in R. purpureus are distinctive in many respects compared with other species in Calyptratae.
An unusual aspect of R. purpureus antenna is the decreasing number of surface sensilla but numerous sensory pits and pit sensilla on antennal funiculus. The semblable examples were found in Hypoderma bovis (Hypodermatinae) (Hunter and Adserballe 1996), Oestrus ovis (Oestrinae) (Poddighe et al. 2010), and Gasterophilus nigricornis (Gasterophilinae) (Zhang et al. 2012a). Meanwhile, the branched antennal sensilla, Ba II, are detected in these sensory pits, which show a resemblance among some other dipterans, such as branched trichoid sensilla in Portschinskia magnifica (Zhang et al. 2012b) and Delia radicum (Ross and Anderson 1987), and mosquito larvae from later developmental stages (McIver and Beech 1986; Green and Hartenstein 1997). Based on previous studies, sensory pits could support a more efficient way to capture odor molecules in vicinity, and branched sensilla enlarge the surface area of antennal sensilla (Ross 1992; Hunter and Adserballe 1996; Bruyne et al. 2001; Sukontason et al. 2004; Poddighe et al. 2010; Zhang et al. 2012a, b, 2013a, b, c); thus, the capacity of odor detection is highly facilitated in combination of these two features. Considering R. purpureus are known to aggregate and mate at specified landmarks on mountain tops (Ullrich 1939; Grunin 1959; Catts 1964; Papavero 1977; Hall 1995; Colwell et al. 2006), large number of sensory pits and branched antennal sensilla might contribute significantly to finding the vital station (Ross 1992; Hunter and Adserballe 1996; Bruyne et al. 2001; Sukontason et al. 2004; Poddighe et al. 2010; Zhang et al. 2012a, b, 2013a, b). In R. purpureus, more sensory pits in females were observed than in males. Since the high specialization of R. purpureus larvae to equid hosts (Colwell et al. 2006), more sensory pits on female antennae could provide a more sensitive olfactory system for host orientation (Hall 1995; Ross 1992; Hunter and Adserballe 1996; Bruyne et al. 2001; Sukontason et al. 2004; Poddighe et al. 2010; Zhang et al. 2012a, b, 2013a, b).
Trichoid sensilla, as one of the basic type of antennal sensilla in many other flies (Ross 1992; Shanbhag et al. 1995; Hunter and Adserballe 1996; Shanbhag et al. 2000; Sukontason et al. 2004, 2007; Smallegange et al. 2008; Liu et al. 2013; Zhang et al. 2013a, b), are undiscovered in R. purpureus. In some hippoboscids (Hippobosca equina, Hippobosca longipennis, Melophagus ovinus) (Zhang et al. 2015) and phorids (Megaselia scalaris) (Loew) (Sukontason et al. 2005), trichoid sensilla are also absent. Clyne et al. (1999) confirm the pheromone sensitivity of trichoid sensilla by electrophysiological studies. In consideration of the fact that male bot flies try to chase any flies about their own size passing in front of them when waited at mating sites (Grunin 1959; Catts 1964; Papavero 1977; Colwell et al. 2006), this relatively violent mating strategy might be the consequence of reduced pheromone identification capability due to the absence of trichoid sensilla.
The function of grooved coeloconic sensilla is usually a controversy, which is involved in thermo- or hygroreception (McIver 1969; Altner et al. 1983; Zacharuk 1985; Ochieng et al. 2000) or possibly chemo- (CO2) reception (Olson and Andow 1993; Renthal et al. 2003). As temperature can greatly impact the behavior of this nasal botfly and the carbon dioxide direct the females to hunt healthy hosts (Papavero 1977; Hall 1995; Colwell et al. 2006), the coeloconic sensilla on antennal funiculus of R. purpureus appear to have both thermo- and chemo-functions.
Myiasis caused by oestrid flies is a global health hazard for ungulates, especially for those endangered (Zumpt 1965; Papavero 1977; Hunter and Adserballe 1996; Otranto et al. 2004, 2005; Colwell et al. 2006; Poddighe et al. 2010). Clearly, great potentials for further investigations of antennal sensilla based on more oestrid samples are demonstrated, which would contribute to the development of synthetic attractants for the monitoring studies and ultimately in fly populations control (Hall 1995; Colwell et al., 2006; Poddighe et al. 2010; Zhang et al. 2012a, b, 2015).
References
Altner H, Schalle L, Stetter H, Wohlrab I (1983) Poreless sensilla with inflexible sockets: a comparative study of a fundamental type of insect sensilla probably comprising thermo- and hygroreceptors. Cell Tissue Res 234:279–307
Amer A, Mehlhorn H (2006) The sensilla of Aedes and Anopheles mosquitoes and their importance in repellency. Parasitol Res 99:491–499
Bruyne M, Foster K, Carlson JR (2001) Odor coding in the Drosophila antenna. Neuron 30:537–552
Catts EP, Garcia R (1963) Drinking by adult Cephenemyia (Diptera: Oestridae). Ann Entomol Soc Am 56:660–663
Catts EP (1964) Field behavior of adult Cephenemyia (Diptera: Oestridae). Can Entomol 96:579–585
Colwell DD, Hall MJR, Scholl PJ (2006) The Oestrid flies: biology, host-parasite relationships, impact and management. CABI Publishing, Cambridge
Colwell DD, Otranto D, Horak IG (2007) Comparative scanning electron microscopy of Gasterophilus third instars. Med Vet Entomol 21:255–264
Clyne PJ, Certel SJ, de Bruyne M, Zaslavsky L, Johnson WA, Carlson JR (1999) The odor specificities of a subset of olfactory receptor neurons are governed by Acj6, a POU-domain transcription factor. Neuron 22:339–347
Deconinck P, Pangui LJ, Githego A, Dorchies P (1996) Prévalence de Rhinoestrus usbekistanicus (Gan 1947) chezl’âne (Equus asinus) au Sénégal. Rev Elev Med Vet Pays Trop 49:38–40
Fernandes FF, Freitas EPS, Linardi PM, Pimenta PFP (2005) Ultrastructure of contact-chemoreceptor sensilla found among the genae of female Gasterophilus nasalis. J Parasitol 91:1218–1220
Green P, Hartenstein V (1997) Structure and spatial pattern of the sensilla of the body segments of insect larvae. Microsc Res Tech 39:470–478
Grunin KYA (1959) Aggregation of bot fly males on the highest points in the locality and its cause. Zool Zhurn 38:1683–1688
Guha L, Seenivasagan T, Bandyopadhyay P, Thanvir IS, Sathe M, Sharma P, Parashar BD, Kaushik MP (2012) Oviposition and flightorientation response of Aedes aegypti to certain aromatic aryl hydrazono esters. Parasitol Res 111:975–982
Hall MJ (1995) Trapping the flies that cause myiasis: their responses to host-stimuli. Ann Trop Med Parasit 89:333–357
Hunter FF, Adserballe CF (1996) Cuticular structures on the antennae of Hypoderma bovis De Geer (Diptera: Oestridae) females. Int J Insect Morphol Embryol 25:173–181
Kaboret Y, Deconinck P, Pangui J, Akakpo J, Dorchies P (1997) Lésion de larhinoestrose spontanéeà Rhinoestrus usbekistanicus (Gan 1947) chezlesâne (Equus asinus) au Sénégal. Rev Med Vet 148:123–126
Liu XH, Zhang M, Shi JN, Li K, Zhang D (2013) Ultrastructure of antennal sensilla of a parasitoid fly, Pales pavida Meigen (Diptera: Tachinidae). Micron 2013:36–42
McIver SB (1969) Antennal sense organs of female Culex tarsalis (Diptera: Culicidae). Ann Entomol Soc Am 62:1455–1461
McIver S, Beech M (1986) Prey finding behavior and mechanosensilla of larval Toxorhynchites brevipalpis Theobald (Diptera: Culicidae). Int J Insect Morphol Embryol 15:213–225
Ochieng SA, Park KC, Zhu JW, Baker TC (2000) Functional morphology of antennal chemoreceptors of the parasitoid Microplitis croceipes (Hymenoptera: Braconidae). Arthropod Struct Dev 29:231–240
Olson DM, Andow DA (1993) Antennal sensilla of female Trichogramma nubilale (Ertle and Davis) (Hymenoptera: Trichogrammatidae) and comparisons with other parasitic Hymenoptera. Int J Insect Morphol Embryol 22:507–520
Otranto D, Colwell DD, Milillo P, Marco VD, Paradies P, Napoli C, Giannetto S (2004) Report in Europe of nasal myiasis by rhinoestrus spp. in horses and donkeys: seasonal patterns and taxonomical considerations. Vet Parasitol 122:79–88
Otranto D, Milillo P, Traversa D, Colwell DD (2005) Morphological variability and genetic identity in Rhinoestrus spp. causing horse nasal myiasis. Med Vet Entomol 19:96–100
Papavero N (1977) The world Oestridae (Diptera) mammals and continental drift. Springer, Hague
Peyresblanques J (1964) Myases oculares. Annuales d’Oculistic 197:271–295
Poddighe S, Dekker T, Scala A, Angioy AM (2010) Olfaction in the female sheep botfly. Naturwissenschaften 97:827–835
Renthal R, Velasquez D, Olmos D, Hampton J, Wergin WP (2003) Structure and distribution of antennal sensilla of the red imported fire ant. Micron 34:405–413
Ross KTA, Anderson M (1987) Morphology of the antennal sensilla of the cabbage root fly, Delia radicum L. (Diptera: Anthomyiidae). Int J Insect Morphol Embryol 16:331–342
Ross KTA (1992) Comparative study of the antennal sensilla of five species of root maggots: Delia radicum L., D. floralis F., D. antique Mg., D. platura Mg. (Diptera: Antomyiidae), and Psila rosae F. (Diptera: Psilidae). Int J Insect Morphol Embryol 21:175–197
Shanbhag SR, Singh K, Singh RN (1995) Fine structure and primary sensory projections of sensilla located in the sacculus of the antenna of Drosophila melanogaster. Cell Tissue Res 282:237–249
Shanbhag SR, Muller B, Steinbrecht RA (2000) Atlas of olfactory organs of Drosophila melanogaster. 2. Internal organization and cellular architecture of olfactory sensilla. Arthropod Struct Dev 29:211–229
Smallegange RC, Kelling RJ, Den Otter CJ (2008) Types and numbers of sensilla on antennae and maxillary palps of small and large houseflies, Musca domestica (Diptera, Muscidae). Microsc Res Tech 71:880–886
Sukontason K, Sukontason KL, Piangjai S, Boonchu N, Chaiwong T, Ngern-klun R, Sripakdee D, Vogtsberger RC, Olson JK (2004) Antennal sensilla of some forensically important flies in families Calliphoridae, Sarcophagidae and Muscidae. Micron 35:671–679
Sukontason K, Sukontason KL, Vogtsberger RC, Boonchu N, Chaiwong T, Piangjai S, Disney H (2005) Ultrastructure of Coeloconic Sensilla on Postpedicel and Maxillary Palp of Megaselia scalaris (Diptera: Phoridae). Ann Entomol Soc Am 98:113–118
Sukontason K, Methanitikorn R, Chaiwong T, Kurahashi H, Vogtsberger RC, Sukontason KL (2007) Sensilla of the antenna and palp of Hydrotaea chalcogaster (Diptera: Muscidae). Micron 38:218–223
Tibayrenc R, Garba D, Dorchies P (1999) Prévalence de Rhinoestrus usbekistanicus (Gan 1947) chezl’âne (Equus asinus) dans larégion de Niamey, Niger. Rev Elev Med Vet Pays Trop 52:113–115
Ullrich H (1939) Zur Biologie der Rachenbremsen unseres einheimischen Wildes, Genus Cephenomyia Latreille und Genus Pharyngomyia Schiner. Verh VII int Kongr Ent 3:2149–2171
Wang X, Zhong M, Wen J, Cai J, Jiang H, Li Y, Al SM, Xiong F (2012) Molecular characterization and expression pattern of an odorant receptor from the myiasis-causing blowfly, Lucilia sericata (Diptera: Calliphoridae). Parasitol Res 110:843–851
Wang QK, Yang YZ, Liu MQ, Zhang D (2014) Fine structure of Delia platura (Meigen) (Diptera: Anthomyiidae) revealed by scanning electron microscopy. Microsc Res Tech 77:619–630
Zacharuk RY (1985) Antennal sensilla. In: Kerkut GA, Gilbert LI (eds) Comparative insect physiology, biochemistry and pharmacology, vol 6. Pergamon, Oxford, pp 1–69
Zayed AA (1992) Studies on Rhinoestrus purpureus (Diptera: Oestridae) larvae infesting donkeys (Equus asinus) in Egypt. III. Pupal duration under controlled conditions. Vet Parasitol 44:285–290
Zayed AA, Hilali ME, Metenawy TM (1993) Studies on Rhinoestrus purpureus (Diptera: Oestridae) larvae infesting donkeys (Equus asinus) in Egypt. J Equine Vet Sci 13:46–49
Zhang D, Wang QK, Hu DF, Li K (2012a) Sensilla on the antennal funiculus of the stomach bot fly, Gasterophilus nigricornis (Diptera: Oestridae). Med Vet Entomol 26:314–322
Zhang D, Wang QK, Hu DF, Li K (2012b) Cuticular structures on antennae of the bot fly, Portschinskia magnifica (Diptera: Oestridae). Parasitol Res 111:1651–1659
Zhang D, Wang QK, Yang YZ, Chen YO, Li K (2013a) Sensory organs of the antenna of two medically and hygienically important Fannia species (Diptera: Fanniidae). Parasitol Res 112:2177–2185
Zhang D, Liu XH, Li XY, Zhang M, Li K (2013b) Antennal sensilla of the green bottle fly, Lucilia sericata (Meigen) (Diptera: Calliphoridae). Parasitol Res 112:3843–3850
Zhang D, Wang QK, Liu XH, Li K (2013c) Sensilla on antenna and maxillary palp of predaceous fly, Lispe neimongola Tian et Ma (Diptera: Muscidae). Micron 49:33–39
Zhang D, Liu XH, Li XY, Cao J, Chu HJ, Li K (2015) Ultrastructural investigation of antennae in three cutaneous myiasis flies: Melophagus ovinus, Hippobosca equina, and Hippobosca longipennis (Diptera: Hippoboscidae). Parasitol Res doi:10.1007/s00436-015-4376-4
Zumpt F (1965) Myiasis in man and animals in the Old World. Butterworths, London
Acknowledgments
This study was supported by the Beijing Higher Education Young Elite Teacher Project (no. YETP0771), Program for New Century Excellent Talents in University (no. NCET-12-0783).
Author information
Authors and Affiliations
Corresponding author
Additional information
X. H. Liu and X. Y. Li contributed equally to this work.
Rights and permissions
About this article
Cite this article
Liu, X.H., Li, X.Y., Li, K. et al. Ultrastructure of antennal sensory organs of horse nasal-myiasis fly, Rhinoestrus purpureus (Diptera: Oestridae). Parasitol Res 114, 2527–2533 (2015). https://doi.org/10.1007/s00436-015-4453-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-015-4453-8