Abstract
Pr1 is a subtilisin-like protease produced by Metarhizium spp. entomopathogenic fungi, and it is recognized as heavily involved in the initial steps of the fungal invasion of arthropod-host cuticles. In the current study, correlation was sought between mortality of tick larvae and conidial Pr1 levels of one Metarhizium anisopliae senso latu (s.l.) isolate (CG 148). Conidia with different levels of pr1 gene expression and enzymatic activity were obtained by producing them on either artificial medium (to yield low Pr1 activity) or on Rhipicephalus microplus cadavers (to yield high Pr1 activity). Conidial proteolytic activity was assessed using N-suc-ala-ala-pro-phe-ρNA as the chromogenic substrate, and pr1 expression was profiled by qPCR using three genes (gpd, try, and tef) as reference genes. Pr1 enzymatic (proteolytic) activity on conidia obtained from tick cadavers was 36 U mg−1 in comparison to 4 U mg−1 on conidia from PDA medium. Also, pr1 gene expression level was ten times higher in conidia from tick cadavers compared to PDA medium. Bioassays of M. anisopliae s.l. CG 148 spores with elevated Pr1 proteolytic activity and gene expression levels did not demonstrate increased virulence (= significant change percent mortality of tick larvae). The minimal levels of Pr1 on conidia produced on artificial medium was adequate to afford high levels of virulence, and the elevated amounts of the enzyme on tick-cadaver-produced conidia did not induce elevated larval mortality. As long as some Pr1 activity was present, fungal virulence of isolate CG 148 against tick larvae was not elevated by increased levels of conidial Pr1.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Increasing concerns about degraded environmental conditions and public health worries are stimulating research on biological control of arthropod pests. A group of insect-pathogenic fungi, the Metarhizium anisopliae species complex (Bischoff et al. 2009), has been widely studied both as biocontrol agents of arthropods and as models for elucidating host-pathogen infection processes (Chandler et al. 2000; Roberts and St Leger 2004; Fernandes et al. 2012). Arthropod-pathogenic (AP) fungi have worldwide distribution, ranging from the subarctic to the tropics (Zimmermann 2007) and hosts that include insects, ticks, and plant roots (Costa et al. 2002; Roberts and St Leger 2004; Fernandes et al. 2010; Sasan and Bidochka 2012). Laboratory tests using M. anisopliae senso latu (s.l.) species against Rhipicephalus microplus (Canestrini 1888) ticks, commonly known as the cattle tick, indicate that these fungi may be effective alternatives to chemical acaricides (Bittencourt et al. 1992, 1994; Castro et al. 1997; Camargo et al. 2012; Quinelato et al. 2012).
To infect arthropods, AP fungal conidia need to adhere, germinate, and penetrate through host cuticle, and these fungi produce enzymes (primarily proteases, lipases, and chitinases) to degrade the most abundant constituents of cuticle (Schrank and Vainstein 2010) to reach the host’s hemocoel. Fungal virulence to a targeted arthropod pest is suspected to be determined by several factors, including fungal production of enzymes and toxins, as well as the microorganism’s ability to evade or inhibit the host’s immune system. Studies have been conducted to understand, at the molecular level, infection of insects by M. anisopliae s.l. (Fang and Bidochka 2006; Wang et al. 2009); with ticks, however, there is virtually no published information on the molecular aspects of infection.
Pr1 is a protease produced by AP fungi to hydrolyze cuticle proteins of host. Pr1 is upregulated two times in the Metarhizium spp. infection cycle: firstly, during the initial stages of infection, including in the appressorium, the structure formed by the fungus in preparation for host colonization to hydrolyze host cuticular proteins (St Leger et al. 1989; St Leger et al. 1991a), and secondly, during the final stages of pathogenesis (conidiation) to facilitate hyphal emergence through insect cuticle (Small and Bidochka 2005); this emergence is necessary for completion of the fungal pathogenic cycle, viz., allowing the mass production of conidia on the surface of the arthropod cadaver, thereby facilitating wide-scale conidial dispersion—which may increase levels of biocontrol.
Multiple (serial) passages of AP fungi on artificial media is widely known to eventually lead to degradation of spore production levels and reduced virulence to its arthropod host. Interestingly, passage through arthropod hosts almost immediately restores these “lost” traits (Kawakami 1960; Fargues and Robert 1983). Accordingly, differences in conidia produced on ticks (= in vivo conidia) in contrast to those produced on artificial media (= in vitro conidia) was examined in the hope of providing critical tool(s) for improving fungal biological control of ticks. The current study evaluated pr1 expression and Pr1 proteolytic activity of M. anisopliae s.l. conidia produced on artificial medium or on R. microplus female cadavers to identify the levels of the cuticle-invasion protease Pr1 from conidia of each substrate.
Material and methods
Fungal isolate
The M. anisopliae s.l. used in the present study was CG 148 isolated from Deois flavopicta (Homoptera: Cercopidae) in Goiânia, Goiás, Brazil. It was obtained from the Brazilian Agriculture and Livestock Research Enterprise (EMBRAPA), Genetic Resources and Biotechnology (CENARGEN) station in Brasilia, DF, Brazil. Stock culture plates of potato dextrose agar medium (PDA) were incubated at 25 ± 1 °C and ≥80 % relative humidity (RH) for 14 days, and then stored at 4 °C. The isolate CG 148 was chosen based on previous information on the virulence of this fungus for tick larvae, reported by Quinelato et al. (2012). CG 148-fungus isolate yielded moderate to high virulence against tick larvae (Quinelato et al. 2012).
Fungal cultivation on artificial medium and ticks
Conidia of M. anisopliae s.l. CG 148 were produced on either PDA or R. microplus ticks. For artificial culture, conidia were inoculated on PDA medium and incubated at 25 ± 1 °C and ≥80 % RH for 15 days; for cultivation on ticks, CG 148 conidial suspension (1 × 108 conidia mL−1) was inoculated into engorged R. microplus females using a microinsulin needle (30 gauge).
Conidia were harvested from culture plates by scraping the medium surface with a scalpel blade and suspended in 100 mL of polyoxyethylene sorbitan monooleate (Tween 80®, Sigma Chemical Co., St. Louis, MO, USA) solution (0.01 % v/v). The conidial suspension was homogenized for 1 min using a vortex mixer, quantified in a hemocytometer and adjusted to 1.0 × 108 conidia mL−1. Engorged R. microplus females were from naturally infested bovines that had not been treated with chemical acaricides for more than 2 months. The engorged females were collected from stall floors and washed in a 1 % sodium hypochlorite solution for cuticular asepsis. Then, they were rinsed in sterile distilled water and dried on sterile paper towels. Inoculation was by injecting into each females tick 5 μL of fungal suspension via the foramen between the capitulum and the anterior end of scutum (Johns et al. 1998). Eight hundred females were inoculated and incubated at 25 ± 1 °C and ≥80 % RH for 15 days, which resulted to 100 % infection.
Conidial viability was determined by plating an aliquot (~50 μL) of the conidia suspension on PDA medium plus 0.05 % chloramphenicol, followed by incubation at 25 ± 1 °C and ≥80 % RH. Conidial germination was observed by microscope (×200) after 24 h (Alves 1998).
Harvest of conidia
Conidia produced on PDA medium were harvested from plates after 15 days of growth and placed in plastic microtubes. Conidia produced on ticks were removed by placing the infected ticks on sieves (final pore size = 1.18 mm; Bertel®) on a sieve shaker (Laboratory Test Sieve; Bertel®) at maximum vibration for 15 min. Conidia from PDA or from ticks were separated into seven aliquots: three aliquots of 60 mg for pr1 expression assay, two aliquots of 37 mg for proteolytic activity assay, and two aliquots of 80 mg for larval bioassay. Samples for pr1 gene expression assay were stored in 500-μL RNAlater solution (Ambion®). Conidial production experiments were performed two times.
Specific subtilisin-like protease (Pr1) activity
Conidia obtained from medium or from female ticks were suspended in an extraction buffer (Tris–HCl 50 mM, pH 8.0, containing 0.25 % Triton X-100, 1:2.5 w/v) (Santi et al. 2010). The suspensions were shaken vigorously for 5 min, and the resulting supernatant filtered through a 25-mm diameter filter, 0.2-mm pore size (Millipore® USA), to remove conidia. The obtained filtrates were used to measure conidial proteolytic activity using N-suc-ala-ala-pro-phe-ρNA as the chromogenic substrate, as follows: 5 μL of filtrate, 5 μL of 10 mM N-suc-ala-ala-pro-phe-ρNA (Sigma®) and 90 μL of 0.1 mol L−1 Tris–HCl buffer pH 8.0 (Santi et al. 2010). Kinetic assays were incubated at 37 °C for 30 min, after which absorbance was measured at 405 nm in a plate spectrophotometer (Multiskan GO® Thermo Scientific). The enzyme activity was calculated from a standard curve of ρ-nitroaniline (ρNA), with each sample expressed as micromolars per minute of reaction per milligrams of protein; the assay was repeated two times with each sample in triplicate. Total protein was quantified according to Lowry et al. (1951), using bovine serum albumin as standard. Data were analyzed via analysis of variance (ANOVA) followed by Tukey test using BioEstat® software, version 4.0. P values less than 0.05 were considered significant (Sampaio 2002).
Pr1 gene expression
The expression of the subtilisin-like protease (pr1) gene was profiled using three genes (gpd, try, and tef) as reference genes (Table 1) (Fang and Bidochka 2006). RNA was isolated using RNeasy Mini Kit (QIAGEN®) according to manufacturer’s instructions and concentrations measured using a NanoDrop 2000 spectrophotometer (Thermo Scientific® USA). Complementary DNA (cDNA) was synthesized using High Capacity RNA-to-cDNA kit (Life Technologies®) following manufacturer’s protocol. The cDNA was subsequently diluted with DEPC-treated water (Life Technologies® USA) to 25 ng μL−1. Real-time PCR (qPCR) amplification mixtures (12 μL) contained 25 ng cDNA template, 2× Power SYBR GreenPCR Master Mix (6 μL; Life Technologies®) and 150 nM each of the forward and reverse primers (Life Technologies®) (Table 1). The reaction was performed with a StepOne Plus (Life Technologies® USA) real-time PCR system. The PCR protocol was as follows: 10-min activation/denaturation step at 95 °C, followed by 40 cycles of 15 s at 95 °C and 60 s at 60 °C. Fluorescence was measured at each polymerization step. The PCR amplifications were conducted two times with each cDNA analyzed in triplicate in each of two tests.
The expression stability of the reference genes, as well as expression of the pr1 gene, was evaluated by geNorm qbasePLUS (available from http://www.biogazelle.com/). Detailed information on this application can be found in Vandesompele et al. (2002).
Larval bioassay
Engorged R. microplus females were collected and incubated for oviposition following the same methodology reported in “Fungal cultivation on artificial medium and ticks” section. Aliquots of 50 mg eggs (approximately 1000 eggs) were collected from day 1 to day 10 of oviposition, placed in test tubes sealed with cotton plugs and observed daily for 20–25 days to estimate percent hatch. Tubes with less than 98 % hatch were discarded. The larval treatment with conidia from PDA medium or from ticks was performed on the 15th day after larval hatching (approximately 40 days after oviposition). Suspensions were prepared as described in “Fungal cultivation on artificial medium and ticks” section. Conidia were quantified and adjusted to 1.0 × 108 conidia mL−1 according to Alves (1998). Conidial viability was determined as reported in “Fungal cultivation on artificial medium and ticks” section.
Each bioassay consisted of three groups (control group; group treated with conidia from PDA, and group treated with conidia from ticks). A Tween 80® solution (0.01 % v/v) was used to treat the control groups. Each group had eight test tubes, each containing approximately 1000 R. microplus larvae. Experiments were conducted by injecting 1 mL of conidial suspension into each test tube. The larvae were held immersed in the injected fluid for 3 min, and the test tube was then inverted until all of the conidial suspension had been absorbed by the cotton plug. The tubes were maintained at 27 ± 1 °C and ≥80 % RH in the dark. Larval mortality was recorded at 5-day intervals for 30 days. The percent larval mortality for each tube was visually estimated by microscopic observation (×20), with the estimates expressed as percentages varying from 0 to 100 % in 1 % intervals. Lack of larval ability to move was recorded as “dead.” Bioassays were repeated two times.
The mean mortalities of the larvae were compared using the nonparametric Kruskal-Wallis test for statistical differences, followed by Student-Newman-Keuls (SNK) test for comparison between the means. Data analyses were conducted using BioEstat® software, version 4.0. P values less than or equal to 0.05 were considered significant (Sampaio 2002).
Reisolation of Metarhizium anisopliae s.l
Dead larvae from all treatment groups were placed on PDA in Petri plates for 14 days at 25 ± 1 °C and ≥80 % RH to allow fungal growth and conidiogenesis. The macromorphology and micromorphology of the fungal colonies on fungus treated larvae were examined for the fungal characteristics (Tulloch 1976) that, if present, confirm the identity as M. anisopliae s.l.
Results
Conidial viability
Conidia of M. anisopliae isolate CG 148 used for treatment of adult females and tick larvae had approximately 100 % germination after incubation for 24 h at 25 ± 1 °C and ≥80 % RH.
Specific subtilisin-like protease (Pr1) activity
Specific Pr1 enzyme activity of surface extracts of conidia produced on artificial medium (PDA) or on infected ticks were statistically (P ≤ 0.05) different, viz., proteolytic activity of 4 U mg−1 for conidia produced on artificial medium and 36.2 U mg−1 (nine times higher) for conidia from tick cadavers.
Pr1 gene expression
The expression of three reference genes were used to clearly show the upregulation of pr1 in conidia produced on adult ticks (in contrast to artificial medium). All three reference genes demonstrated high target stability (average geNorm M ≤0. 5); i.e., geNorm M values of 0.218 for gpd, 0.237 for try, and 0.291 for tef. On the other hand, pr1 was ten times more expressed in conidia produced on tick cadavers than on artificial medium (Fig. 1). PCR efficiencies are given in Table 1.
Relative expression levels (with standard deviations) of the gene pr1 from conidia cultivated on artificial medium (PDA) or on cadavers of Rhipicephalus microplus females (ticks). The genes gpd, tef, and try were used as reference genes. Relative quantities were based on expression levels in conidia produced on PDA (geNorm qbasePLUS)
Fungal virulence against R. microplus larvae
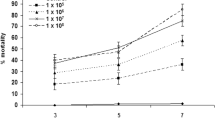
Larvae in the control group remained at least 99 % alive. Ticks exposed to conidia produced on either tick cadavers or PDA medium had significantly (P ≤ 0.05) higher mortalities than those in the control (untreated) group. Although conidia from tick cadavers induced the highest mortality levels, there was not a statistically significant difference (P ≥ 0.05) between mortalities from the two sources of conidia (Table 2).
Culture of M. anisopliae s.l. from bioassay cadavers
R. microplus larvae from the fungus-treated groups were incubated at high humidity to encourage the development of fungal colonies. The fungus morphology was characterized according to Tulloch (1976). The isolated fungus colonies universally presented the key morphological features consistent with M. anisopliae s.l. R. microplus larvae from the control group neither died nor exhibited fungal growth.
Discussion
The biological control of ticks using entomopathogenic fungi has been studied repeatedly in recent decades (e.g., Bittencourt et al. 1992, 1994, 1995, 1999; Samish and Rehacek 1999; Samish et al. 2004; Fernandes and Bittencourt 2008; Angelo et al. 2010; Fernandes et al. 2011; Perinotto et al. 2012), and this approach is frequently presented as a promising alternative to the use of chemical products. Nevertheless, further research on improving the effectiveness of these microorganisms against acari is needed, including a detailed knowledge on the key factors for the fungal virulence.
Pr1 is a subtilisin-like protease that has been proposed as an important factor in initiating fungal infection via penetration through the arthropod host’s cuticle (St Leger et al. 1986, 1988, 1989; Campos et al. 2005; Small and Bidochka 2005; Fang and Bidochka 2006; Dhar and Kaur 2010). It is well recognized that Metarhizium spp. can produce a large number of enzymes, and one of these, Pr1, probably is required to digest the arthropod-cuticle complex during the initial stages of infection, i.e., during appressorium and infection-peg formation. Thus, the question why would the presence of this enzyme be important to nongerminated conidia? According to St Leger et al. (1991b), the ease with which these enzymes can be extracted from spores using buffers, in addition to its activity on appropriate substrates, indicates that there is enzyme activity at the spore surface. This might provide ways for the germinating spore to obtain nutrients and also achieve some preliminary modifications of host cuticle surface before or at germination.
Low, but detectable, levels of subtilisin Pr1 activity were present when M. anisopliae mycelia were cultivated in liquid minimal medium (inorganic nitrogen only), the mycelium discarded, and the liquid assayed (Dhar and Kaur 2010). This suggests that Pr1 synthesis is constitutive. Growing the fungus in liquid medium containing colloidal chitin (2 %) as the sole carbon and nitrogen source afforded increased levels of Pr1 activity in the medium (Dhar and Kaur 2010). In contrast to this study on the effects of culture substrate on Pr1 presence in liquid media, the current study compared the effects of culture substrates (PDA medium or tick cadavers) on Pr1 enzyme activity detectable on the outside of conidia, which revealed considerably higher enzyme activity on conidia produced on tick-cadaver substrate. Accordingly, there was a direct relationship between culture substrate and Pr1 enzyme production levels. PDA, the artificial medium, has very low nitrogen levels in comparison to adult ticks. Confirmation of the findings on Pr1 enzyme activity in relation to culture substrates was sought by genetic analysis. Three genes were used as reference genes to analyze changes in conidial pr1 expression from PDA medium or tick cadavers. The results were as follows: pr1 gene expression was ten times higher in conidia obtained from ticks, which was in agreement with the enzymatic results presented above and, importantly, change in substrate did not alter levels of any of the reference genes (Fig. 1).
We show here, for the first time, that spores of M. anisopliae s.l. have pr1 gene expression, as well as Pr1 specific activity, increased when the fungus is cultivated in ticks. A wide arsenal of enzyme activities has been detected on M. anisopliae s.l. spore surfaces. This enzymatic diversity contributes to fungal survival and ecological fit and may be correlated to the adaptation of the fungus to a broad array of habitats (Santi et al. 2010). Nevertheless, increased Pr1activity on conidia and elevated pr1 expression levels were not associated with significant (P ≥ 0.05) changes (up or down) in larval mortality. It is well known that virulence of AP fungi may be markedly reduced after successive passages on artificial medium (e.g., Kawakami 1960; Fargues and Robert 1983). Currently, it is not known if attenuated virulence following multiple artificial medium passages involves reduction in Pr1 levels on conidia. This may be a possibility, but our findings of little correlation between conidial Pr1 levels and virulence to ticks would speak against this concept.
Experiments to identify additional differences between tick-passaged and nonpassaged fungal strains, looking for modulation of other virulence factors (such as lipases and chitinases), are needed. It is also important to note that, unlike ticks, insects are much more susceptible to entomopathogenic fungi, once conidial concentrations around 106 conidia mL−1 are already enough to successfully control insect larvae (Alves 1998). If tested against insects, tick-cadaver-produced conidia would probably yield better results in comparison with artificial-media-produced conidia. Nevertheless, conidia concentrations used for insects has no, or very little, effect against ticks (Quinelato et al. 2012).
Although Pr1 activity levels on conidia were expected to be related to fungal virulence for ticks, our findings did not concur. Similarly, recent literature shows that the presence of Pr1 genes and the activity of these proteases from some M. anisopliae isolates could not be associated with the virulence against the two insect pests (Rosas-García et al. 2014). The levels of Pr1 associated with conidia produced on artificial (PDA) medium was 9–10-fold lower than on cadaver-produced conidia; nevertheless, the minimal amount on the former was adequate to induce the same high levels of tick mortality as the latter. It is suggested that the elevated levels of Pr1 on tick-cadaver-produced conidia might have their effects merged in the virulence stability of this fungal isolate due to the enormous amount of spores (108 conidia mL−1) commonly used to control ticks. We conclude that, under the conditions of our tests, the virulence level of M. anisopliae s.l. against ticks is not directly related to conidial Pr1 levels.
References
Alves SB (1998) Fungos entomopatogênicos. In: Alves SB (ed) Controle microbiano de insetos. Fundação de Estudos Agrário Luiz de Queirós (FEALQ), Piracicaba, pp 289–382
Angelo IC, Fernandes EKK, Bahiense TC, Perinotto WMS, Moraes APR, Terra ALM (2010) Efficiency of Lecanicillium lecanii to control the tick Rhipicephalus microplus. Vet Parasitol 172:317–322
Bischoff JF, Rehner SA, Humber RA (2009) A multilocus phylogeny of the Metarhizium anisopliae lineage. Mycologia 101:512–530
Bittencourt VREP, Massard CL, Lima AF (1992) Uso do fungo Metarhizium anisopliae (Metschnikoff, 1879) Sorokin, 1883, no controle do carrapato Boophilus microplus (Canestrini, 1887). Arquivo da Universidade Rural do Rio de Janeiro 15:197–202
Bittencourt VREP, Massard CL, Lima AF (1994) Ação do fungo Metarhizium anisopliae sobre a fase não parasitária do ciclo biológico de Boophilus microplus. Revista Universidade Rural, Série Ciências da Vida 16:49–55
Bittencourt VREP, Massard CL, Lima AF (1995) Dinâmica da infecção do fungo Metarhizium anisopliae (Metschnikoff, 1879) Sorokin, 1883, sobre o carrapato Boophilus microplus (Canestrini, 1887). Revista da Universidade Rural, Série Ciências da Vida 17:83–88
Bittencourt VREP, Mascarenhas AG, Faccini JLH (1999) Mecanismo de infecção do fungo Metarhizium anisopliae no carrapato Boophilus microplus em condições experimentais. Ciência Rural, Santa Maria 29:351–354
Camargo MG, Golo PS, Angelo IC, Perinotto WMS, Sá FA, Quinelato S, Bittencourt VREP (2012) Effect of oil-based formulations of acaripathogenic fungi to control Rhipicephalus microplus ticks under laboratory conditions. Vet Parasitol 188:140–147
Campos RA, Arruda W, Boldo JT, Silva MV, Barros NM, Azevedo JL, Schrank A, Vainstain MH (2005) Boophilus microplus infection by Beauveria amorpha and Beauveria bassiana: SEM analysis and regulation of subtilizin-like proteases and chitinases. Cur Microbiol 50:257–261
Castro ABA, Bittencourt VREP, Daemon E, Viegas EC (1997) Eficácia do fungo Metarhizium anisopliae sobre o carrapato Boophilus microplus em teste de estábulo. Revista Universidade Rural, Série Ciências da Vida 19:73–82
Chandler D, Davidson G, Pell JK, Ball BV, Shaw K, Sunderland KD (2000) Fungal biocontrol of Acari. Biocontrol Sci Techn 10:357–384
Costa GL, Sarquis MIM, Moraes AML, Bittencourt VREP (2002) Isolation of Beauveria bassiana and Metarhizium anisopliae var. anisopliae from Boophilus microplus tick (Canestrini, 1887), in Rio de Janeiro State, Brazil. Mycopathol 154:207–209
Dhar P, Kaur G (2010) Cuticle-degrading proteases produced by Metarhizium anisopliae and their induction in different media. Indian J Microbiol 50:449–455
Fang W, Bidochka MJ (2006) Expression of genes involved in germination, conidiogenesis and pathogenesis in Metarhizium anisopliae using quantitative real-time RT-PCR. Mycol Res 110:1165–1171
Fargues JF, Robert PH (1983) Effects of passing through scarabeid hosts on virulence and host specificity of two strains of the entomopathogenic hyphomycete Metarhizium anisopliae. Can J Microbiol 29:576–583
Fernandes EKK, Bittencourt VREP (2008) Entomopathogenic fungi against South American tick species. Exp Appl Acarol 46:71–93
Fernandes EKK, Keyser CA, Rangel DEN, Foster RN, Roberts DW (2010) CTC medium: a novel dodine-free selective medium for isolating entomopathogenic fungi, especially Metarhizium acridum, from soil. Biol Control 54:197–205
Fernandes EKK, Angelo IC, Rangel DEN, Bahiense TC, Moraes AML, Roberts DW, Bittencourt VREP (2011) An intensive search for promising fungal biological control agents of ticks, particularly Rhipicephalus microplus. Vet Parasitol 182:307–318
Fernandes EKK, Bittencourt VREP, Roberts DW (2012) Perspectives on the potential of entomopathogenic fungi in biological control of ticks. Exp Parasitol 130:300–305
Johns R, Sonenshine DE, Hynes WL (1998) Control of bacterial infections in the hard tick Dermacentor variabilis (Acari: Ixodidae): evidence of antimicrobial proteins in tick hemolymph. J Med Entomol 35:458–464
Kawakami K (1960) On the changes of characteristics of the silkworm muscardines through successive cultures. Bull Seric Exp Stn Japan 83-99
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275
Perinotto WMS, Terra ALM, Angelo IC, Fernandes EKK, Golo PS, Camargo MG, Bittencourt VREP (2012) Nomuraea rileyi as biological control agents of Rhipicephalus microplus tick. Parasitol Res 111:1743–1748
Quinelato S, Golo PS, Perinotto WMS, Sá FA, Camargo MG, Angelo IC, Moraes AML, Bittencourt VREP (2012) Virulence potential of Metarhizium anisopliae s.l. isolates on Rhipicephalus (Boophilus) microplus larvae. Vet Parasitol 190:556–565
Roberts DW, St Leger RJ (2004) Metarhizium spp., cosmopolitan insect pathogenic fungi: mycological aspects. Adv Appl Microbiol 54:1–70
Rosas-García NM, Ávalos-de-León O, Villegas-Mendoza JM, Mireles-Marínez M, Barboza-Corona JE, Castañeda-Ramírez JC (2014) Correlation between pr1 and pr2 gene content and virulence in Metarhizium anisopliae strains. J Microbiol Biotechnol 24:1495–1502
Samish M, Rehacek J (1999) Pathogens and predators of ticks and their potential in biological control. Annu Rev Entomol 44:159–182
Samish M, Ginsberg H, Glaser I (2004) Biological control of ticks. Parasitology 129:S389–S403
Sampaio IBM (2002) Estatística aplicada à experimentação animal. Fundação de Estudo e Pesquisa em Medicina Veterinária e Zootecnia (FEPMVZ-Editora), Belo Horizonte
Santi L, Silva OB, Berger M, Guimarães JA, Schrank A, Vainstein MH (2010) Conidial surface proteins of Metarhizium anisopliae: source of activities related with toxic effects, host penetration and pathogenesis. Toxicon 55:874–880
Sasan RK, Bidochka MJ (2012) The insect-pathogenic fungus Metarhizium robertsii (clavicipitaceae) is also an endophyte that stimulates plant root development. Am J Bot 99:101–107
Schrank A, Vainstein MH (2010) Metarhizium anisopliae enzymes and toxins. Toxicon 56:1267–1274
Small CN, Bidochka MJ (2005) Up-regulation of Pr1, a subtilisin-like protease, during conidiation in the insect pathogen Metarhizium anisopliae. Mycol Res 109:307–313
St Leger RJ, Charnley AK, Cooper RM (1986) Cuticle-degrading enzymes of entomopathogenic fungi: synthesis in culture on cuticle. J Invertebr Pathol 48:85–95
St Leger RJ, Durrand PK, Cooper RM, Charnley AK (1988) Regulation of production of proteolytic enzymes by the entomopathogenic fungus Metarhizium anisopliae. Arch Microbiol 150:413–416
St Leger RJ, Butt TM, Staples RC, Roberts DW (1989) Synthesis of proteins including a cuticle-degrading protease during differentiation of the entomopathogenic fungus Metarhizium anisopliae. Exp Mycol 13:253–262
St Leger RJ, Roberts DW, Staples RC (1991a) A model to explain differentiation of appressoria by germilings of Metarhizium anisopliae. J Invertebr Pathol 57:299–310
St Leger RJ, Goettel M, Roberts DW, Staples RC (1991b) Prepenetration events during infection of host cuticle by Metarhizium anisopliae. J Invertebr Pathol 58:168–179
Tulloch M (1976) The genus Metarhizium. Trans Br Mycol Soc 66:407–411
Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F (2002) Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol Res 3:0034.1–0034.11
Wang S, Leclerque A, Pava-Ripoll M, Fang W, St Leger RJ (2009) Comparative genomics using microarrays reveals divergence and loss of virulence-associated genes in host-specific strains of the insect pathogen Metarhizium anisopliae. Eukariotic Cell 8:888–898
Zimmermann G (2007) Review on safety of the entomopathogenic fungus Metarhizium anisopliae. Biocontrol Sci Techn 17:879–920
Acknowledgments
This research was supported by grants from the Conselho Nacional de Desenvolvimento Cientifico e Tecnologico (CNPq) of Brazil and Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ) from Rio de Janeiro State, Brazil. We thank Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and CNPq for providing graduation scholarships for Patrícia S. Golo, Wendell M.S. Perinotto, Mariana G. Camargo, and Fillipe A. Sá. Vânia R.E.P. Bittencourt and Carlos L. Massard are CNPq researchers. We also very much appreciate support from USDA/APHIS (Animal Plant Health and Inspection Service), particularly Dr. Larry E. Jech and Dr. R. Nelson Foster, during the data interpretation and writing phases of this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Golo, P.S., Santos, H.A., Perinotto, W.M.S. et al. The influence of conidial Pr1 protease on pathogenicity potential of Metarhizium anisopliae senso latu to ticks. Parasitol Res 114, 2309–2315 (2015). https://doi.org/10.1007/s00436-015-4426-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-015-4426-y