Abstract
Megacolon is frequently observed in patients who develop the digestive form of Chagas disease. It is characterized by dilation of the rectum–sigmoid portion and thickening of the colon wall. Microscopically, the affected organ presents denervation, which has been considered as consequence of an inflammatory process that begins at the acute phase and persists in the chronic phase of infection. Inflammatory infiltrates are composed of lymphocytes, macrophages, natural killer cells, mast cells, and eosinophils. In this study, we hypothesized that mast cells producing tryptase could influence the migration and the activation of eosinophils at the site, thereby contributing to the immunopathology of the chronic phase. We seek evidence of interactions between mast cells and eosinophils through (1) evaluation of eosinophils, regarding the expression of PAR2, a tryptase receptor; (2) correlation analysis between densities of mast cells and eosinophils; and (3) ultrastructural studies. The electron microscopy studies revealed signs of activation of mast cells and eosinophils, as well as physical interaction between these cells. Immunohistochemistry and correlation analyses point to the participation of tryptase immunoreactive mast cells in the migration and/or survival of eosinophils at the affected organ.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The pathogenesis of Trypanosoma cruzi infection, known as Chagas disease, has been strongly debated in the literature. Given the lack of an experimental model that accurately reproduces the chronic disease, pathological analyses of tissues from infected patients have provided relevant evidence of possible immunopathological mechanisms involved in the development of the chronic disease. Symptoms usually appear 20–30 years after the initial infection, and they are related to the involvement of the cardiovascular and/or the digestive systems, and less frequently, of the central nervous system (Dias 2006). The factors leading to the establishment of different clinical forms in chronic T. cruzi infections are not completely understood, but genetic variability related to the host and/or to the parasite, as well as variable host immune responses, has been considered (Macedo et al. 2004; Manoel-Caetano and Silva 2007; Teixeira et al. 2011).
We have been especially interested in the study of the digestive clinical form, that is characterized by the development of lesions of the enteric nervous system, which sometimes culminate in peristaltic disorders, wall thickening, and dilation of the organ, mainly of the esophagus (megaesophagus) and colon (megacolon) (Köberle 1968). Inflammatory infiltrates, composed mainly of CD20+B lymphocytes, TIA-1+ cytotoxic T lymphocytes, natural killer cells, mast cells, and eosinophils are observed throughout the muscle and the myenteric plexus region (d’Avila Reis et al. 2001; da Silveira et al. 2007a, b; Côbo Ede et al. 2012). Although the precise contribution from each of those cell types remains speculative, some evidence indicates the participation of eosinophils in the pathology of Chagas disease, both at the heart (Molina and Kierszenbaum 1989) and the gastrointestinal tract (da Silveira et al. 2005). In the heart of patients with T. cruzi-induced cardiomyopathy, the presence of eosinophils correlates with disease severity, with maximal levels of infiltration occurring in necrotic lesions (Molina and Kierszenbaum 1987, 1988, 1989). Concerning the digestive clinical form, morphometric studies demonstrated increased numbers of eosinophils in the colon of T. cruzi-infected individuals, whether or not they had megacolon, suggesting the participation of this cellular population at different stages of digestive disease development (da Silveira et al. 2007b; Côbo Ede et al. 2012).
It has been well demonstrated that recruitment of eosinophils from the bloodstream into tissues could culminate in the release of a variety of products, including cytokines, chemokines, lipid mediators, and cytotoxic granule proteins, that could sustain local inflammation and also participate in the regenerative process (Kato et al. 1998; Gleich 2000; Rothenberg et al. 2001; Hogan 2007; Jacobsen et al. 2007). A range of cytokines, adhesion molecules, chemoattractants, and receptors regulate eosinophil trafficking and activation; among these, a serine protease, tryptase, that is produced specifically by mast cells. Tryptase acts on a variety of cells, such as eosinophils, monocytes, neutrophils, and endothelial cells, through linkage to its protease-activated receptor 2 (PAR), which belongs to a family of four G-protein-coupled receptors (PAR1, PAR2, PAR3, and PAR4). After interaction with tryptase, PAR2 is activated upon cleavage of its N-terminus, followed by its internalization and its targeting into lysosomes (Ramachandran et al. 2012). There is now convincing evidence for a role for tryptase in eosinophil activation, through its linkage to PAR2 (Matos et al. 2013), leading to cellular release of leukotrienes and reactive oxygen species formation (Bolton et al. 2003). The role of tryptase on eosinophil trafficking appears to be mediated by its ability to interact with endothelial cells and promote selectin-mediated eosinophil adhesion during inflammation (Steinhoff et al. 2000; Itoh et al. 2005).
In T. cruzi-infected individuals, we have previously demonstrated increased numbers of mast cells and eosinophils in colon sections, and in the present study, we aimed to search for evidence of the participation of tryptase mast cells in eosinophil recruitment and activation. We developed ultrastructural studies and quantified mast cells immunoreactive (IR) for tryptase (tryptase-IR mast cells) and eosinophils immunoreactive for PAR2 (PAR2-IR) in the colon of T. cruzi-infected individuals, with or without megacolon. We further analyzed the correlations between tryptase-IR mast cells and numbers of eosinophils, and in view of the obtained data, we discussed a putative role for tryptase and eosinophils in the immunopathology of T. cruzi-induced megacolon.
Methods
Patients and tissue collection
Colon samples from 20 infected patients (10 with and 10 without megacolon), and from 10 uninfected controls, with negative serology for T. cruzi infection and with normal colon perimeter, were analyzed in this study. The diagnosis of megacolon was performed with abdominal X-ray, digital rectal examination, and barium enema. Significant differences were not observed relative to the gender and age distributions among uninfected individuals and infected individuals (Table 1).
All biopsies were obtained from surgical procedures at Medical School of the Federal University of Goias, Brazil. Among uninfected individuals, the causes of surgery were sigmoid diverticular disease, sigmoid adenocarcinoma, and trauma. Infected individuals who did not present dilation were submitted to surgical procedures due to neoplastic diseases, diverticular disease, or complications owing to severe constipation (Table 1). Informed consent was obtained from the patients before tissue procurement. This work was approved by the Ethics and Research Committee of the Federal University of Minas Gerais, number 04939212.9.0000.5149.
The affected area of colon samples (rectum–sigmoid region) from infected individuals with megacolon was selected. In the other groups, samples of the equivalent region were collected. The perimeter of resected colon segments was measured, and the dilated portion from infected individuals with megacolon was statistically increased when compared to infected individuals without megacolon and uninfected individuals (Table 1). Morphometric analyses of inflammatory cells on hematoxylin and eosin-stained sections did not reveal any significant difference between infected and uninfected group (data not shown).
Immunohistochemistry assay
Tissue samples were fixed in 4 % buffered paraformaldehyde solution, embedded in paraffin and the sections (5 μm thick) were submitted to immunohistochemical staining. Sections were deparaffinized in xylene and then rehydrated in a graded alcohol series. For detection of tryptase, endogenous peroxidase activity was inhibited by incubation with 4 % hydrogen peroxide and 0.05 M sodium azide for 30 min. The slides were incubated with 2 % normal swine serum (Sigma, St. Louis, MO, USA) in phosphate-buffered saline for 30 min followed by incubation with the monoclonal mouse antihuman mast cell tryptase (DAKO, Denmark A/S, clone AA1, code M 7052; 1:100) overnight at 4 °C. Subsequently, the samples were incubated with peroxidase-conjugated rabbit antimouse antibodies (DAKO) for 60 min, and the immunoreaction was visualized using 0.03 % of 3-3′-diaminobenzidine (SIGMA) containing 0.5 % H2O2 in 0.01 M PBS, pH 7.4. The sections were counterstained with Gill’s Hematoxylin (SIGMA), dehydrated, and mounted using a synthetic medium. A negative control without the primary antibodies was generated for each sample.
For detection of PAR2, a different protocol was followed. After deparaffinization and rehydration, the sections were submitted to microwave antigen retrieval followed by blocking of endogenous biotin and avidin using a commercial kit (Avidin/Biotin blocking kit—Vector Laboratories, USA). The sections were incubated with 2 % bovine serum albumin for blocking nonspecific antibody binding prior to incubation with primary goat polyclonal antibody for PAR-2, a protease-activated receptor (1:200 dilution, sc-8205, SANTA CRUZ) overnight at 4 °C. After washing in PBS, the sections were exposed for 60 min to a biotinylated rabbit antigoat secondary antibody, diluted 1:100 in PBS. After this step, the sections were incubated with the avidin–biotin complex (VECTASTAIN Elite ABC kit—Vector Laboratories, USA) for 30 min and the immunoreaction was visualized using 3-3′-diaminobenzidine (SIGMA) containing 0.01 % H2O2 in 0.05 M Tris–HCl buffer, pH 7.6. Sections were counterstained with Mayer’s hematoxylin. Also, a negative control without the primary antibodies was generated for each sample.
Cell quantification
For each colon sample from infected and uninfected individuals, the total numbers of tryptase-immunoreactive (IR) mast cells and hematoxylin and eosin (HE)-stained eosinophils or PAR2-IR eosinophils were assessed in the lamina propria, inner muscle layer, and myenteric plexus’ region in the rectum–sigmoid portion of the colon. Counting of tryptase-IR mast cells was done by using a light microscope at × 400 magnification, in 20 randomly selected fields, of each region, per section (total area of 4410 × 103 μm2). HE-stained eosinophils and PAR2-IR eosinophils were counted in 50 randomly selected fields of each region per section, by using a light microscope at ×1000 magnification (total area of 2076 × 103 μm2).
In order to normalize the cell counting, a correction factor was applied. The sum of the total number of cells, calculated as described above, was multiplied by the ratio: perimeter of each individual colon/average of the circumferences of controls cases.
Electron microscopy
Colon samples from six individuals infected with T. cruzi (three with and three without megacolon) and three uninfected individuals were analyzed by electron microscopy. Samples were immersed in modified Karnovsky fixative solution (2 % paraformaldehyde, 2.5 % glutaraldehyde in 0.1 M phosphate buffer, pH 7.4), postfixed in 2 % osmium tetroxide, and routinely processed for Epon embedding. Ultrathin sections from mucosa and submucosa were analyzed in a Biotwin G2 Tecnai transmission electron microscope (FEI Company, The Netherlands). Experiments and analyses were performed in the Center of Microscopy at the Universidade Federal de Minas Gerais, Belo Horizonte, Minas Gerais, Brazil (http://www.microscopia.ufmg.br).
Statistical analysis
Analysis of variance was performed using the GraphPad PRISM (GraphPad Software Inc., San Diego, CA). The Kruskal–Wallis test and Dunn’s multiple comparison posttest were used. Data were expressed as means. The correlation analysis between number of tryptase-IR and HE-stained eosinophils in the lamina propria, inner muscle layer, and myenteric plexus’ region from infected individuals was performed using the Spearman test. Differences were considered statistically significant at P < 0.05.
Results
Morphometric analysis of tryptase-IR mast cells and eosinophils
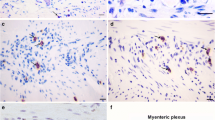
Tryptase-IR mast cells and eosinophils were observed dispersed throughout the lamina propria (Figs. 1a–c and 2a–c), myenteric plexus’ region (Figs. 1d–f and 2d–f) and inner muscle layer of the colon from T. cruzi-infected patients and uninfected individuals. In some sections, tryptase-IR mast cells were also observed in close proximity to neuronal bodies and glia cells within ganglions and in proximity to blood vessels (Fig. 1d, e). Increased numbers of eosinophils were observed in the lamina propria (Fig. 2a–c) and in the myenteric plexus’ region, both in extra- or intra-ganglion locations (Fig. 2e, f).
Tryptase-IR mast cells in colon samples from T. cruzi-infected patients and from uninfected controls. Mast cells immunostained for tryptase (arrows) are observed in the lamina propria (a–c) and in the myenteric plexus’ region (d–f) of uninfected individuals (a, d), infected individuals without megacolon (b, e), and infected individuals with megacolon (c, f). A neuronal body is indicated by an asterisk in d and a blood vessel is indicated by an arrowhead in e. Original magnification ×400, scale bar = 20 μm. Morphometric analyses were performed in 20 fields of each region (lamina propria, inner muscle layer, and myenteric plexus’ region), per section, with a ×40 objective lens. The total area analyzed per individual was 4410 × 103 μm2. The results are expressed as mean. Significant differences between groups were represented by ***P < 0.001; **P < 0.01; *P < 0.05
H&E-stained eosinophils in colon samples from T. cruzi-infected patients and from uninfected controls. Eosinophils (arrows) are observed throughout the lamina propria (a–c) and myenteric plexus’ region (d–f) of uninfected individuals (a, d), infected individuals without megacolon (b, e), and infected individuals with megacolon (c, f). A neuronal body is indicated by an asterisk in d, f. The insert (f) shows a major magnification of strongly stained eosinophils. Original magnification ×400, scale bar = 20 μm. Morphometric analyses were performed in 50 fields of each region (lamina propria, inner muscle layer, and myenteric plexus’ region), per section, with a ×100 objective lens. The total area analyzed per individual was 2076 × 103 μm2. The results are expressed as mean. Significant differences between groups were represented by ***P < 0.001; **P < 0.01; *P < 0.05
Morphometric analyses in the lamina propria, in the muscle layer or in the myenteric plexus’ region revealed that the numbers of both cellular populations, tryptase-IR mast cells and eosinophils, are increased in patients with megacolon when compared to uninfected individuals (Figs. 1g–i and 2g–i). There was a significant difference in the number of lamina propria tryptase-IR mast cells between infected individuals with and without megacolon (Fig. 1g). Patients without megacolon also presented increased numbers of tryptase-IR mast cells in the inner muscle layer when compared to control cases (Fig. 1h). Significant differences were observed between patients with and without megacolon, since in the myenteric plexus’ region, the numbers of both cell types were higher in the former (Figs. 1i and 2i).
Correlation analyses
Statistical analyses revealed a positive correlation between the numbers of eosinophils and tryptase-IR mast cells in infected individuals, with and without megacolon, whether the inner muscle layer (P < 0.0054, r = 0.5980, Fig. 3b) or the myenteric plexus’ region (P < 0.0156, r = 0.5329, Fig. 3c) was analyzed. In the lamina propria, however, no significant correlation between the numbers of those cell types was observed (P < 0.1098, r = 0.3686, Fig. 3a).
Correlation analyses. Statistical analyses revealed positive correlation between the numbers of tryptase-IR mast cells (MCs) and the numbers of eosinophils in both the inner muscle layer (P < 0.0054, r = 0.5980, b) and the myenteric plexus’ region (P < 0.0156, r = 0.5329, c). No significant correlation between these parameters was observed in the lamina propria (P < 0.1098, r = 0.3686, a). Significance was considered when P < 0.05
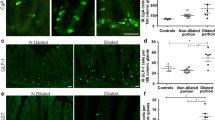
Analysis of PAR2-IR eosinophils
Most of eosinophils were strongly IR for PAR2. They were observed through the lamina propria (Fig. 4a), the inner muscle layer, and the myenteric plexus’ region, including in the interior of blood vessels (Fig. 4b, c). Morphometric analysis revealed increased numbers of PAR2-IR eosinophils in the colon of infected individuals with megacolon, when compared to the other groups, no matter the region analyzed (Fig. 4d–f).
PAR2-IR eosinophils in colon samples from T. cruzi-infected patients and from uninfected controls. PAR2-IR eosinophils (arrows) in the lamina propria (a), inner muscle layer (b), and inside a blood vessel in the myenteric plexus’ region of colon from T. cruzi-infected individuals (c). Arrowhead points to an eosinophil with no detectable reaction to PAR2 immunostaining (b). Original magnification ×1000 (a, b) and ×400 (c), scale bar = 20 μm. Morphometric analyses (d–f) were performed in 50 fields per section with a ×100 objective lens. The total area analyzed was 2076 × 103 μm2 per individual, and the results are expressed as mean. Significant differences between groups were represented by ***P < 0.0001; **P < 0.001; *P < 0.01
Ultrastructural analyses of mast cells and eosinophils
Electron microscopy revealed a close proximity of mast cells and eosinophils in the lamina propria/submucosa of the intestine (Fig. 5a), these cells sometimes presenting interdigitating plasmalemma folds at their contact surfaces (Fig. 5b). Moreover, both cell types often presented morphologic evidence of activation, as they displayed different stages of degranulation. Concerning mast cells, the most common phenotype observed was the piecemeal degranulation, characterized by the absence of granular fusion and by the presence of partially or completely empty granules. The loss of granular content could be recognized by a reduction of the granular electron density and by the appearance of clear haloes surrounding the granular content. The residual densities within granules often assumed characteristic scrolled and particulate profiles. Nevertheless, some mast cells exhibited signs of the anaphylactic degranulation phenotype, with fusion of granules being its principal aspect (Fig. 5c). Regarding eosinophils, the anaphylactic degranulation seemed to be the most common phenotype displayed, since the fusion of granules was frequently observed (Fig. 5d). Some eosinophils presented few granules into the cytoplasm, another degranulation sign (Fig. 5a). Signals of piecemeal degranulation were also observed in the cytoplasm of eosinophils (Fig. 5d).
Ultrastructures of mast cell and eosinophil in the lamina propria/submucosa of colon from a T. cruzi-infected individual. In a, the mast cell (MC) can be observed in close proximity to an eosinophil (Eos) with scarce cytoplasm granules. In b, the amplified image reveals points of contact between the plasmalemma folds (arrows) of an eosinophil and a mast cell. In c, a mast cell displays evident signs of piecemeal degranulation (asterisks) and, less frequently, of granules fusion (arrowhead). In d, an eosinophil displays evident signs of both piecemeal (asterisks) and anaphylactic (arrowheads) degranulation
Discussion
The data presented here strongly suggest a role for tryptase-IR mast cells in the immunopathology of megacolon in chronic Chagas disease. Increased numbers of tryptase-IR mast cells have been already described in several diseases of the gastrointestinal tract, such as ulcerative colitis (Stoyanova and Gulubova 2002; Albert et al. 2011; Stasikowska-Kanicka et al. 2012), Crohn’s disease (Raithel et al. 2001; Christerson et al. 2009; Smyth et al. 2013), and chagasic megaesophagus (Martins et al. 2014). Those disorders have in common an exacerbated inflammatory process, which could be maintained by high levels of tryptase. Indeed, in vitro studies have demonstrated that tryptase stimulates peripheral blood cells to synthesize and secrete pro-inflammatory cytokines, such as tumor necrosis 320 factor-α (TNF- α), interleukin (IL)-1β, and IL-6 (Malamud et al. 2003). In the current study, the local cytokine production in the organ was not addressed, but we dare speculate that a putative tryptase-mediated increasing levels of TNF-α, IL-6, and/or IL-1β could contribute to maintain the inflammatory process in the colon of patients with megacolon (Cox et al. 1991; Gröger et al. 2012; Zhang et al. 2013). Besides, tryptase activates the expression of PAR-2 by endothelial cells, which could propitiate leukocyte rolling, adhesion, and extravasation (Nystedt et al. 1996; Vergnolle 1999; Meyer et al. 2005; Cleator et al. 2006), also contributing to the inflammatory process in T. cruzi-infected individuals. We have recently demonstrated increased numbers of tryptase-IR mast cells in the esophagus of T. cruzi-infected individuals, indicating that such alteration might be a common feature of digestive pathology of Chagas disease (Martins et al. 2014).
Regarding the role of tryptase specifically in eosinophil infiltration, statistical analyses revealed a positive correlation between numbers of tryptase-IR mast cells and eosinophils, which suggests that this molecule is involved in the recruitment and/or survival of eosinophils in the colon of T. cruzi-infected individuals. In other models, such as allergic lung diseases, the participation of tryptase in eosinophil tissue infiltration has already been reported (Schmidlin et al. 2002; Bolton et al. 2003; Vliagoftis et al. 2004; Matos et al. 2013). Although the mechanisms of tryptase-induced transmigration of eosinophils are not completely understood, some experimental studies leave no doubt about the involvement of tryptase in such processes. Decreased eosinophil recruitment to tissues is observed in mast cell-deficient mice (de Boer et al. 2014), as well as in experimental models where the PAR2 receptor was blocked (Hyun et al. 2008; Matos et al. 2013). Moreover, the positive correlation observed in this study between numbers of tryptase-IR mast cells and eosinophils could be also interpreted as evidence for the role of tryptase in eosinophil survival. Corroborating with this hypothesis, studies in the literature have demonstrated that mast cells enhance eosinophil survival, in part through their activation to produce and release autocrine survival cytokines, such as granulocyte-macrophage colony-stimulating factor (GM-CSF) and IL-5 (Shakoory et al. 2004; Gröger et al. 2012).
The possibility of interactions between tryptase-IR mast cells and eosinophils was evaluated by analyzing PAR2 expression on the surface of eosinophils. Some studies have shown that eosinophils express PAR1, PAR2, and PAR3; however, only PAR2 activation seems to induce their degranulation and production of superoxide (Miike et al. 2001; Bolton et al. 2003; Miike and Kita 2003). In this study, we have observed that the numbers of PAR2-IR eosinophils, either in the lamina propria, in the inner muscle layer, or in the myenteric plexus’ region, are increased in the dilated portion of the organ, when compared to uninfected individuals or to infected cases without megacolon. Indirectly, those data point to the occurrence of interactions between tryptase and eosinophils, which could lead to their activation, degranulation, and release of inflammatory mediators and could thus contribute to the maintenance of the inflammation. One could argue that, if the interaction between tryptase and its receptor PAR2 leads to its activation and internalization, the decrease rather than increase in eosinophils expressing PAR2 should be an indication of the existence of this interaction. However, it has been demonstrated that PAR2 is constrictively expressed by eosinophils in a very low level. Once it is activated and internalized, it occurs a movement and redistribution, to the cell surface, of other stored PAR2 molecules (Miike et al. 2001; Bolton et al. 2003).
In this study, evidences of interaction between eosinophils and mast cells were also observed in ultrastructural images. In tissue samples from T. cruzi-infected individuals, the analyses revealed a close proximity between mast cells and eosinophils, pointing to the possibility of physical interactions between these cellular populations. Those cells, sometimes presented interdigitating plasmalemma folds on their contact surfaces, and different stages of degranulation suggest that they might be activated. Signs of membrane contact between eosinophils and mast cells have been recently demonstrated in human nasal polyps and asthmatic bronchi. Such studies have further demonstrated that, when in culture, mast cells and eosinophils display points of membrane contact and that mast cells regulate eosinophil survival and death, through soluble mediators and physical cell–cell contact (Elishmereni et al. 2011).
Finally, we would like to emphasize that, although this study supplies a basis for mast cell–eosinophil interaction in colon of T. cruzi-infected individuals, some other studies must be developed in order to 1) unveil the role of different mediators in such processes, and 2) evaluate whether such cellular interactions contribute to the persistence and/or to the resolution of inflammation.
References
Albert EJ, Duplisea J, Dawicki W, Haidl ID, Marshall JS (2011) Tissue eosinophilia in a mouse model of colitis is highly dependent on TLR2 and independent of mast cells. Am J Pathol 178:150–160. doi:10.1016/j.ajpath.2010.11.041
Bolton SJ, Mcnulty CA, Thomas RJ, Hewitt CRA, Wardlaw AJ (2003) Expression of and functional responses to protease-activated receptors on human eosinophils. J Leukoc Biol 74:60–68. doi:10.1189/jlb.0702351.http
Christerson U, Keita AV, Söderholm JD, Gustafson-Svärd C (2009) Increased expression of protease-activated receptor-2 in mucosal mast cells in Crohn’s ileitis. J Crohns Colitis 3:100–108. doi:10.1016/j.crohns.2008.11.003
Cleator JH, Zhu WQ, Vaughan DE, Hamm HE (2006) Differential regulation of endothelial exocytosis of P-selectin and von Willebrand factor by protease-activated receptors and cAMP. Blood 107:2736–2744. doi:10.1182/blood-2004-07-2698
Côbo Ede C, Silveira TP, Micheletti AM, Crema E, Adad SJ (2012) Research on Trypanosoma cruzi and analysis of inflammatory infiltrate in esophagus and colon from chronic chagasic patients with and without mega. J Trop Med 232646. doi:10.1155/2012/232646
Cox G, Ohtoshi T, Vancheri C, Denburg JA, Dolovich J, Gauldie J, Jordana M (1991) Promotion of eosinophil survival by human bronchial epithelial cells and its modulation by steroids. Am J Respir Cell Mol Biol 4:525–531. doi:10.1165/ajrcmb/4.6.525
d’Avila Reis D, Lemos EM, Silva GC, Adad SJ, McCurley T, Correa-Oliveira R, Machado CR (2001) Phenotypic characterization of the inflammatory cells in chagasic megaoesophagus. Trans R Soc Trop Med Hyg 95:177–178
da Silveira ABM, Arantes RME, Vago AR, Lemos EM, Adad SJ, Correa-Oliveira R, d’Avila Reis D (2005) Comparative study of the presence of Trypanosoma cruzi kDNA, inflammation and denervation in chagasic patients with and without megaesophagus. Parasitology 131:627–634
da Silveira A, Lemos E, Adad SJ, Correa-Oliveira R, Furness JB, d’Avila Reis D (2007a) Megacolon in Chagas disease: a study of inflammatory cells, enteric nerves, and glial cells. Hum Pathol 38:1256–1264. doi:10.1016/j.humpath.2007.01.020
da Silveira ABM, Adad SJ, Correa-Oliveira R, Furness J, d’Avila Reis D (2007b) Morphometric study of eosinophils, mast cells, macrophages and fibrosis in the colon of chronic chagasic patients with and without megacolon. Parasitology 134:789–796. doi:10.1017/S0031182007002296
de Boer JD, Yang J, van den Boogaard FE, Hoogendijk AJ, de Beer R, van der Zee JS, van der Poll T (2014) Mast cell-deficient kit mice develop house dust mite-induced lung inflammation despite impaired eosinophil recruitment. J Innate Immun 6:219–226. doi:10.1159/000354984
Dias JCP (2006) Chagas disease: successes and challenges. Cad Saude Publica 22:2020–2021
Elishmereni M, Alenius HT, Bradding P, Mizrahi S, Shikotra A, Minai-Fleminger Y, Levi-Schaffer F (2011) Physical interactions between mast cells and eosinophils: a novel mechanism enhancing eosinophil survival in vitro. Allergy 66:376–385. doi:10.1111/j.1398-9995.2010.02494.x
Gleich GJ (2000) Mechanisms of eosinophil-associated inflammation. J Allergy Clin Immunol 105:651–663. doi:10.1067/mai.2000.105712
Gröger M, Klemens C, Wendt S, Becker S, Canis M, Havel M, Kramer MF (2012) Mediators and cytokines in persistent allergic rhinitis and nonallergic rhinitis with eosinophilia syndrome. Int Arch Allergy Immunol 159:171–178. doi:10.1159/000336169
Hogan SP (2007) Recent advances in eosinophil biology. Int Arch Allergy Immunol 143(Suppl):3–14. doi:10.1159/000101398
Hyun E, Andrade-Gordon P, Steinhoff M, Vergnolle N (2008) Protease-activated receptor-2 activation: a major actor in intestinal inflammation. Gut 57:1222–1229. doi:10.1136/gut.2008.150722
Itoh Y, Sendo T, Oishi R (2005) Physiology and pathophysiology of proteinase-activated receptors (PARs): role of tryptase/PAR-2 in vascular endothelial barrier function. J Pharmacol Sci 1:14–19
Jacobsen EA, Taranova AG, Lee NA, Lee JJ (2007) Eosinophils: singularly destructive effector cells or purveyors of immunoregulation? J Allergy Clin Immunol 119:1313–1320. doi:10.1016/j.jaci.2007.03.043
Kato M, Kephart GM, Talley NJ, Wagner JM, Sarr MG, Bonno M, Gleich GJ (1998) Eosinophil infiltration and degranulation in normal human tissue. Anat Rec 252:418–425
Köberle F (1968) Chagas’ disease and Chagas’ syndromes: the pathology of American trypanosomiasis. Adv Parasitol 6:63–116
Macedo AM, Machado CR, Oliveira RP, Pena SD (2004) Trypanosoma cruzi: genetic structure of populations and relevance of genetic variability to the pathogenesis of Chagas disease. Mem Inst 99:1–12
Malamud V, Vaaknin A, Abramsky O, Mor M, Burgess LE, Ben-Yehudah A, Lorberboum-Galski H (2003) Tryptase activates peripheral blood mononuclear cells causing the synthesis and release of TNF-α, IL-6 and IL-1β: possible relevance to multiple sclerosis. J Neuroimmunol 138:115–122. doi:10.1016/S0165-5728(03)00090-0
Manoel-Caetano FS, Silva AE (2007) Implications of genetic variability of Trypanosoma cruzi for the pathogenesis of Chagas disease. Cad Saude Publica 23:2263–2274
Martins PR, Nascimento RD, de Souza Lisboa A, Martinelli PM, d’Ávila Reis D (2014) Neuroimmunopathology of Trypanosoma cruzi-induced megaoesophagus: is there a role for mast cell proteases? Hum Immunol 75:302–305. doi:10.1016/j.humimm.2014.02.003
Matos NA, Silva JF, Matsui TC, Damasceno KA, Duarte IDG, Lemos VS, Klein A (2013) Mast cell tryptase induces eosinophil recruitment in the pleural cavity of mice via proteinase-activated receptor 2. Inflammation 36:1260–1267. doi:10.1007/s10753-013-9664-5
Meyer MC, Creer MH, Mchowat J, Maureen C (2005) Potential role for mast cell tryptase in recruitment of inflammatory cells to endothelium. Am J Physiol 63104:1485–1491. doi:10.1152/ajpcell.00215.2005
Miike S, Kita H (2003) Human eosinophils are activated by cysteine proteases and release inflammatory mediators. J Allergy Clin Immunol 111:704–713. doi:10.1067/mai.2003.1332
Miike S, McWilliam AS, Kita H (2001) Trypsin induces activation and inflammatory mediator release from human eosinophils through protease-activated receptor-2. J Immunol 167:6615–6622
Molina HA, Kierszenbaum F (1987) A study of human myocardial tissue in Chagas’ disease: distribution and frequency of inflammatory cell types. Int J Parasitol 17:1297–1305
Molina HA, Kierszenbaum F (1988) Kinetics of development of inflammatory lesions in myocardial and skeletal muscle in experimental Trypanosoma cruzi infection. J Parasitol 74:370–374
Molina HA, Kierszenbaum F (1989) Eosinophil activation in acute and chronic chagasic myocardial lesions and deposition of toxic eosinophil granule proteins on heart myofibers. J Parasitol 75:129–133
Nystedt S, Ramakrishnan V, Sundelin J (1996) The proteinase-activated receptor 2Is induced by inflammatory mediators in human endothelial cells. Comparison with the thrombin receptor. J Biol Chem 271:14910–14915. doi:10.1074/jbc.271.25.14910
Raithel M, Winterkamp S, Pacurar A, Ulrich P, Hochberger J, Hahn EG (2001) Release of mast cell tryptase from human colorectal mucosa in inflammatory bowel disease. Scand J Gastroenterol 36:174–179
Ramachandran R, Noorbakhsh F, Defea K, Hollenberg MD (2012) Targeting proteinase-activated receptors: therapeutic potential and challenges. Nat Rev Drug Discov 11:69–86. doi:10.1038/nrd3615
Rothenberg ME, Mishra A, Brandt EB, Hogan SP (2001) Gastrointestinal eosinophils. Immunol Rev 179:139–155
Schmidlin F, Amadesi S, Dabbagh K, Lewis DE, Knott P, Bunnett NW, Stevens ME (2002) Protease-activated receptor 2 mediates eosinophil infiltration and hyperreactivity in allergic inflammation of the airway. J Immunol 169:5315–5321
Shakoory B, Fitzgerald SM, Lee SA, Chi DS, Krishnaswamy G (2004) The role of human mast cell-derived cytokines in eosinophil biology. J Interferon Cytokine Res 24:271–281. doi:10.1089/107999004323065057
Smyth CM, Akasheh N, Woods S, Kay E, Morgan RK, Thornton MA, Costello RW (2013) Activated eosinophils in association with enteric nerves in inflammatory bowel disease. PLoS One 8:e64216. doi:10.1371/journal.pone.0064216
Stasikowska-Kanicka O, Danilewicz M, Głowacka A, Wągrowska-Danilewicz M (2012) Mast cells and eosinophils are involved in activation of ulcerative colitis. Adv Med Sci 57:230–236. doi:10.2478/v10039-012-0029-3
Steinhoff M, Vergnolle N, Young SH, Tognetto M, Amadesi S, Ennes HS, Bunnett NW (2000) Agonists of proteinase-activated receptor 2 induce inflammation by a neurogenic mechanism. Nat Med 6:151–158. doi:10.1038/72247
Stoyanova II, Gulubova MV (2002) Mast cells and inflammatory mediators in chronic ulcerative colitis. Acta Histochem 104:185–192. doi:10.1078/0065-1281-00641
Teixeira ARL, Hecht MM, Guimaro MC, Guimaro MC, Sousa AO, Nitz N (2011) Pathogenesis of chagas’ disease: parasite persistence and autoimmunity. Clin Microbiol Rev 24:592–630. doi:10.1128/CMR.00063-10
Vergnolle N (1999) Proteinase-activated receptor-2-activating peptides induce leukocyte rolling, adhesion, and extravasation in vivo. J Immunol 163:5064–5069
Vliagoftis H, Lacy P, Luy B, Adamko D, Hollenberg M, Befus D, Moqbel R (2004) Mast cell tryptase activates peripheral blood eosinophils to release granule-associated enzymes. Int Arch Allergy Immunol 135:196–204. doi:10.1159/000081304
Zhang A, Chi X, Luo G, Hei Z, Xia H, ss C, Xia Z (2013) Mast cell stabilization alleviates acute lung injury after orthotopic autologous liver transplantation in rats by downregulating inflammation. PLoS One 8:e75262. doi:10.1371/journal.pone.0075262
Acknowledgments
This work was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Martins, P.R., Nascimento, R.D., Lopes, J.G. et al. Mast cells in the colon of Trypanosoma cruzi-infected patients: are they involved in the recruitment, survival and/or activation of eosinophils?. Parasitol Res 114, 1847–1856 (2015). https://doi.org/10.1007/s00436-015-4371-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-015-4371-9