Abstract
Larval packet test was used for detection of resistance status against cypermethrin and deltamethrin, the most commonly used synthetic pyrethroids in Rhipicephalus (Boophilus) microplus collected from Faridkot district, Punjab (India). The slope of mortality, lethal concentration for 50 % (LC50) and resistance levels were determined from the regression graphs of probit mortality of ticks plotted against log values of increasing concentrations of cypermethrin and deltamethrin. Results indicated presence of resistance of levels I and II against cypermethrin (resistance factor (RF) = 2.82) and deltamethrin (RF = 8.44), respectively. Adult immersion test was used to assess the acaricidal activity of aqueous (MLAq), ethanol (MLE), chloroform (MLC), acetone (MLA) and hexane (MLH) extracts of leaves of Murraya koenigii against these synthetic pyrethroid (SP)-resistant engorged adult females of R. (B.) microplus by determination of per cent adult mortality, reproductive index (RI), per cent inhibition of oviposition (%IO) and hatching rate. The per cent mortality caused by various extracts at concentrations ranging from 0.625 to 10.0 % varied from 0.0 to 100.0 % with maximum per cent mortality of 10.0, 100.0, 70.0, 40.0 and 10.0 recorded against MLAq, MLE, MLC, MLA and MLH, respectively. Among all extracts, the highest acaricidal property against SP-resistant R. (B.) microplus was exhibited by the MLE as it showed the minimum LC50 [95 % confidence limit (CL)] values of 2.97 % (2.82–3.12 %), followed by MLC as 10.26 % (8.84–11.91 %) and MLA as 18.22 % (16.18–20.52 %). The average egg mass weight recorded in live ticks treated with various concentrations of different extracts was lower than the respective control group ticks and was significantly (p < 0.01) lower in ticks treated with MLH extract. However, no significant effect on hatchability of eggs of treated groups when compared to control was recorded. A significant (p < 0.05) decrease in the RI was recorded in MLH extract-treated ticks, and the %IO varied from 0.07 to 34.73 % with various extracts and was recorded maximum with highest concentration of MLH. The results of the current study indicate that the extracts of M. koenigii can be used for control of SP-resistant ticks.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The common cattle tick, Rhipicephalus (Boophilus) microplus (Canestrini 1888), is one of the major tick species responsible for negative economy impacts and losses to animal productivity in tropical and subtropical areas of the world (Jonsson 2006). In India, R. (B.) microplus is widely distributed and infests several host species including dairy animals of Punjab state (Singh and Rath 2013). Economic losses to cattle producers from ticks and tick-borne diseases (TTBDS) have been estimated at US$ 13–14 milliard globally on an annual basis (de Castro 1997), to which Brazil and Australia contribute US$ 2 milliard (Grisi et al. 2002) and Aus $ 175 million (Playford et al. 2005), respectively. In India, the control cost of TTBDS in the dairy sector has been estimated at $498.7 million per annum (Minjauw and McLeod 2003). Heavy tick infestations cause huge economic losses through anorexia, toxicosis, blood loss, general stress and irritation; decrease in productivity; depression of immune function; damage to hides; transmission of pathogens; and treatment costs (Ghosh et al. 2007).
There are inherited advantages and disadvantages of all specific technical approaches for tick control viz, chemical acaricides, biological control agents, tick-resistant animals and tick vaccines (Willadsen 2006). Chemical acaricides, if correctly applied, are efficient and cost-effective, but large-scale and repeated applications had limited their efficacy in reducing tick infestations and are often accompanied by serious drawbacks, including the development of acaricide-resistant ticks, environmental contamination and even contamination of milk and meat products with insecticide residues (Graf et al. 2004). Because of the high cost of developing new drugs, time and labour involved in many of these techniques and paucity of satisfactory immunizing agents, there is a renewed interest in the use of botanicals for control of cattle ticks (Zaman et al. 2012). The advantages of botanical acaricides include low or no toxicity to mammals, rapid degradation in the environment and less chances of development of resistance (Chungsamarnyart et al. 1991).
Presently, in order to offer a complete eco-friendly acaricide, there is a need to assess botanical extracts, especially from plants which are rustic, perennial and easily cultivable and have a better potential of extension, in addition to their inherent acaricidal properties. Among the various plant products explored for acaricidal activity, extracts and essential oils have shown significant effects against various stages of economically important tick species (Borges et al. 2003; Ribeiro et al. 2008, 2010, 2011; Magadum et al. 2009; Ravindran et al. 2011, 2012; Ghosh et al. 2011, 2013; Singh et al. 2014; Godara et al. 2014).
Murraya koenigii (L.) Spreng (Rutaceae), commonly known as ‘meethi neem’ or ‘curry leaf plant’, is an aromatic perennial shrub or a small tree commonly cultivated in India, Sri Lanka and other Asian countries. It has been reported previously to possess anti-diabetic, anti-oxidant, anti-fungal, anti-microbial, cytotoxic properties, anti-inflammatory, anti-tumour-promoting, anti-hypercholesterolemic, kidney pain-relief and hepatoprotective activities (Nayak et al. 2010). There are a few reports about the adverse effect of M. koenigii against insect pests (Devanand and Rani 2008; Malwal et al. 2009; Li et al. 2010; Senrung et al. 2014), but to the best of our knowledge till date, the acaricidal property of these plants has not been assessed particularly against synthetic pyrethroid (SP)-resistant R. (B.) microplus. Therefore, this study was executed to evaluate the acaricidal activity of M. koenigii against SP-resistant adult females of R. (B.) microplus.
Materials and methods
Ticks
Fully engorged females ticks were collected from various cattle sheds of Faridkot district, Punjab (India). After identification, these were used in the adult immersion test or were kept individually in labelled plastic tubes covered with muslin cloth and kept in desiccators placed in incubator maintained at 28 ± 1 °C and 85 ± 5 % relative humidity for oviposition for 2 weeks. These eggs when hatched under similar conditions provided the larvae used for the larval packet test.
Acaricides
Technical-grade (100 % pure) deltamethrin and cypermethrin (AccuStandard® Inc., USA) were used to prepare the stock solutions of 10,000 ppm in acetone and methanol, respectively. Different concentrations of the acaricide were prepared in distilled water from the stock solutions and tested against R. (B.) microplus.
Larval packet test
The larval packet test (LPT) was conducted according to FAO (1971) guidelines with minor modifications. Briefly, 0.5 ml of different concentrations of cypermethrin (50, 100, 200, 400 and 800 ppm) and deltamethrin (12.5, 25, 50, 100 and 200 ppm) was used to impregnate 7.0 × 7.0-cm filter paper (541 Whatman). The aqueous solution of acaricide was dried by keeping the filter paper for 30 min in incubator at 37 °C. The filter papers were then folded half diagonally and sealed on one side with adhesive tapes, forming an open-ended triangular packet to place tick larvae. After insertion of approximately 100 larvae, the open end of each packet was sealed with adhesive tape and the packets were placed in a desiccator placed in incubator maintained at 28 ± 1 °C and 85 ± 5 % RH. For each concentration of acaricide, the test was conducted in triplicate, and in control group, distilled water was used. The packets were removed after 24 h, and larval mortality was calculated.
Estimation of resistance status
Dose–response data were analyzed by probit method (Finney 1962) using GraphPad Prism 4 software. The lethal concentrations for 50 % (LC50) values of cypermethrin and deltamethrin against R. (B.) microplus were determined by applying regression equation analysis to the probit-transformed data of mortality. Resistance factors (RFs) were calculated as the quotient between LC50 of field ticks and LC50 of susceptible line of R. (B.) microplus (Castro-Janer et al. 2009). The LC50 values of cypermethrin (138.5 ppm) and deltamethrin (13.4 ppm) against reference acaricides susceptible IVRI-I line of R. (B.) microplus were used as reported earlier by Sharma et al. (2012). On the basis of RF, the resistance levels (RL) were classified as susceptible (RF <1.4), level I (RF = 1.5–5.0), level II (RF = 5.1–25.0), level III (RF = 25.1–40) and level IV (RF >40.1) (Sharma et al. 2012).
Plant material
The leaves of M. koenigii were collected from the campus of Punjab Agricultural University, Ludhiana, India, from a single tree. The plant was identified and authenticated by Dr. RK Dubey, Assistant Professor, Department of Floriculture and Landscaping, Punjab Agricultural University, Ludhiana, India.
Preparation of curry leaf extract
The leaves of M. koenigii (both tender and old) were washed and air-dried in shade at room temperature and were finely pulverized using an electric grinder. The plant extraction was carried out by maceration wherein 100 g of the powdered material was taken and added with the solvent in the ratio of 1:20 (distilled water) and 1:10 (ethanol, chloroform, acetone and hexane). The flask was agitated for frequent mixing over a period of 24 h. The mixture was filtered using muslin cloth followed by filter paper (No. 5B, Advantec®), and the filtrate was completely air-dried at 40 °C. The extract was scrapped off, transferred to an air-tight container and stored in a freezer at −20 °C till subsequent uses after determining the yields. The working concentrations (0.625, 1.25, 2.5, 5.0 and 10.0 %) of aqueous, ethanol, chloroform, acetone and hexane extracts were prepared by dissolving required quantity of extracts in distilled water, ethanol (10.0 %), dimethyl sulphoxide (DMSO) (10.0 %), DMSO (33.3 %) and DMSO (50.0 %), respectively, for testing of their acaricidal potential against R. (B.) microplus.
Bioassay
For evaluation of anti-tick activity of the various extracts, adult immersion test (AIT) (Drummond et al. 1973; Sharma et al. 2012) was adopted. Adult engorged female ticks were washed thoroughly thrice with tap water and kept on filter paper for drying. These were distributed randomly into various groups of ten each and were weighed individually. A dose-dependent response study was conducted by exposing adult ticks to various concentrations (0.625, 1.25, 2.5, 5.0 and 10.0 %) of aqueous (MLAq), ethanol (MLE), chloroform (MLC), acetone (MLA) and hexane (MLH) extracts of leaves of M. koenigii, and in each concentration, ten ticks were maintained. All the ticks were immersed for 2 min in different concentrations of the extract while control ticks of each extract were immersed in distilled water, 10.0 % ethanol, 10.0 % DMSO, 33.3 % DMSO and 50.0 % DMSO for MLAq, MLE, MLC, MLA and MLH, respectively, and then transferred to the Petri dishes padded with Whatman filter paper no. 1. After 48 h, the ticks were transferred to glass vials covered with muslin cloth and kept in desiccators placed in incubator maintained at 28 ± 1 °C and 85 ± 5 % relative humidity. The effect of the various extracts on tick mortality was recorded, and ticks which did not oviposit even after 14 days post-treatment were considered as dead (Singh and Rath 2014). The ticks which survived after exposure in various concentrations were reared subsequently for generating the data on the efficacy of plant extract on inhibition of oviposition. Eggs laid by these ticks were collected and incubated separately at same conditions for the next 30 days for visual estimation of hatching rate. The following parameters were recorded as follows:
-
1.
Mortality: recorded up to 14 days post-treatment.
-
2.
The egg mass weight laid by the live ticks.
-
3.
Reproductive index (RI) = egg mass weight / engorged female weight
-
4.
Percentage inhibition of oviposition (%IO) = [(RI control − RI treated) / RI control × 100].
Statistical analysis
Dose–response data were analyzed by probit method (Finney 1962) using GraphPad Prism 4 software. The lethal concentrations for 50 % (LC50) values of various extracts were determined applying regression equation analysis to the probit-transformed data of mortality. The differences in mean values of entomological data among the groups were analyzed by one-way ANOVA.
Results
Resistance status
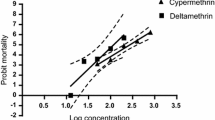
Data on slope [95 % confidence limit (CL)], goodness of fit (R 2), LC50 (95 % CL), RF values and the level of resistance against cypermethrin and deltamethrin were determined by LPT and are presented in Table 1. The regression graph of mean mortality of larvae ticks plotted against log values of progressively increasing concentrations of cypermethrin and deltamethrin is shown in Fig. 1. The dotted lines in the regression curve represented the 95 % CL. From the regression equation, the LC50 (95 % CL) values of cypermethrin and deltamethrin were calculated as 390.29 (376.0-405.1) and 113.04 ppm (108.9-117.3), respectively. Results indicated presence of resistance of levels I and II against cypermethrin (RF = 2.82) and deltamethrin (RF = 8.44), respectively.
In vitro efficacy of various M. koenigii extracts
The efficacy of various extracts of leaves of M. koenigii against SP-resistant R. (B.) microplus females was assessed by determination of per cent adult mortality, reproductive index (RI), per cent inhibition of oviposition (%IO) and hatching rate. The results of AIT using aqueous (MLAq), ethanol (MLE), chloroform (MLC), acetone (MLA) and hexane (MLH) extracts are shown in Table 2. The per cent mortality caused by various extracts at concentrations ranging from 0.625 to 10.0 % varied from 0.0 to 100.0 % with maximum per cent mortality of 10.0, 100.0, 70.0, 40.0 and 10.0 recorded against MLAq, MLE, MLC, MLA and MLH, respectively. The regression graph of mean mortality of ticks plotted against log values of increasing concentrations of different extracts is shown in Fig. 2. The LC50 (95 % CL) values, slope of mortality (95 % CL) and R 2 value of the various extracts are shown in Table 3. Among all extracts, the highest acaricidal property against SP-resistant R. (B.) microplus was exhibited by the MLE, as it showed the minimum LC50 (95 % CL) values of 2.97 % (2.82–3.12 %), followed by MLC as 10.26 % (8.84–11.91 %) and MLA as 18.22 % (16.18–20.52 %) (Table 3). Further, because of the low per cent mortality recorded, the LC50 values of MLAq and MLH extracts against R. (B.) microplus were unworkable.
In the current study, the cut-off date set for observation of adult mortality for both control and treated ticks was 14 days post-treatment (dpt). The average egg mass weight recorded 14 dpt in live ticks treated with various concentrations of different extracts was lower than the respective control group ticks, and the difference was significantly (p < 0.01) lower in ticks treated with MLH extract (Table 2). The regression graph of average egg mass weight of ticks plotted against log values of increasing concentrations of different extracts is shown in Fig. 3. The slope of average egg mass weight (95 % CL) recorded was negative, and its correlation with log concentration of various extracts was low (0.02–0.385) indicating a limited effect of various extracts on the egg laying potential of treated ticks (Table 3). Further, no significant effect on hatchability of eggs of treated groups was recorded when compared to ticks of control groups.
A significant (p < 0.05) decrease in the RI was recorded in MLH extract-treated ticks in comparison to control ticks, whereas in ticks treated with other extracts, the RI was not significantly lower than the respective control group ticks (Table 2). The regression graph of RI of ticks plotted against log values of various concentrations of different extracts is shown in Fig. 4. A low correlation of RI with log concentration of various extracts was recorded (0.012–0.621) indicating a limited effect of various extracts on the RI of treated ticks (Table 3). The per cent inhibition of oviposition observed in AIT with various extracts varied from 0.07 to 34.73 % and was recorded maximum with highest concentration of MLH (Fig. 5 and Table 2).
Discussion
The most commonly used method for the control of ticks in dairy animals is the direct application of acaricides to animals, and synthetic pyrethroids (SPs) are being used widely resulting in higher incidences of development of SP resistance in the region (Singh and Rath 2014). Among the SPs, deltamethrin and cypermethrin are, at present, the two predominant acaricides marketed aggressively by many companies for tick control in the country. Presence of widespread resistance against these SPs in R. (B.) microplus from different places of India including Punjab state has been recently reported (Singh et al. 2010, 2012; Sharma et al. 2012; Singh and Rath 2014). The commonly used bioassays for diagnosing acaricide resistance is the larval packet test (LPT) (Stone and Haydock 1962), also adopted by the FAO since 1975 as the standard test for detection and measurement of acaricide resistance (Jonsson et al. 2007). In LPT, live larvae are exposed to filter paper packets impregnated with acaricide and incubated for specific time periods, and then, larval mortality is assessed. For conducting LPT, technical-grade deltamethrin and cypermethrin were selected over commercial formulation because commercial products are prepared with many proprietary ingredients and it is difficult to assess the responses due to active ingredients (Shaw 1966).
Although among the various technical approaches for tick control, chemical acaricides, if correctly applied, are efficient and cost-effective, they are, however, often incorrectly used, and chemical resistance is a serious global problem and the presence of chemical residues in food is increasingly an issue for consumers (Willadsen 2006). Biological control agents are, in principle, highly desirable, but their efficacy, manufacture, application and stability present serious challenges and current anti-tick vaccines may lack the efficacy to be a stand-alone solution (Willadsen 2006). Experimental attempts for biological control of R. (B.) microplus as a part of integrated pest management have mainly concentrated on the use of fungal biopesticides and entomopathogenic nematodes (Polar et al. 2005). Nonetheless, other alternative methods to control this parasite have been attempted, among which, the use of phytochemical products stands out (Valente et al. 2014). These phytochemical products may also be beneficial as acaricides because of the increasing demand for pesticide-free products by consumers and the growing resistance (Fernandez-Salas et al. 2011; Ghosh et al. 2013). Recently, various plant extracts and essential oils have shown significant activity against all the stages of economically important tick species (Borges et al. 2003; Ribeiro et al. 2008, 2010, 2011; Magadum et al. 2009; Ravindran et al. 2011, 2012; Ghosh et al. 2013; Singh et al. 2014; Godara et al. 2014). Further, plants with known biological activity such as M. koenigii merit research to explore it as an alternative for the control of parasitic diseases and a potential source of new active ingredients. To the best of our knowledge, there is no previous information regarding the acaricidal activity of this plant, although preceding publications suggested its possible insecticidal effects (Devanand and Rani 2008; Malwal et al. 2009; Li et al. 2010; Senrung et al. 2014). This background prompted us to test the in vitro effects of five different extracts of leaves of M. koenigii, against SP-resistant R. (B.) microplus.
M. koenigii Spreng (curry leaf tree) is a small aromatic tree belonging to the family Rutaceae tropical to sub-tropical tree native to India. Of the 14 global species belonging to the genus Murraya, only two are available in India, namely, M. koenigii and M. paniculata, and among these, M. koenigii is more popular due to its large spectrum of medicinal properties. M. koenigii leaves have a slightly pungent, bitter and feebly acidulous taste, and these characteristics are retained even after drying and are extensively used in South Indian culinary practices for seasoning and flavouring dishes (Pruthi 1976). Different parts of the plant such as leaves, root, bark and fruit are known to possess various biological activities and are used as medicine for a variety of ailments and also used as a tonic, stomachic and carminative (Chevallier 1996). The major chemical constituents of the plant are reported as carbazole alkaloids, coumarins and flavonoids (Nayak et al. 2010). M. koenigii leaf extracts exhibit hypoglycaemic and hypolipidemic effects in experimental animals (Kesari et al. 2007), whereas carbazole alkaloids and methanolic extracts are also reported to possess anti-oxidative, anti-diarrheal and anti-trichomonal activities (Adebajo et al. 2006; Mandal et al. 2010) and anti-inflammatory and immunomodulatory activity (Shah et al. 2008). Further, hydro-methanolic extract of M. koenigii leaves is rich in phenolic content, and polyphenols have a wide spectrum of biological activities, including anti-oxidant, anti-inflammatory and metal-chelating properties (Scalbert et al. 2005). The mechanisms of action for these properties are not fully understood. The insecticidal property of curry leave extracts has been evaluated as natural insect-controlling agents (Malwal et al. 2009) against lepidopteran pests (Devanand and Rani 2008) and stored grain pest Sitophilus zeamais and Tribolium castaneum (Li et al. 2010). Recently, its anti-feedant and oviposition deterrent activity has been demonstrated against the fourth instar larvae and gravid females of Spodoptera litura, an insect pest of agricultural crops (Senrung et al. 2014)
Since, the present study is an initiative to screen the plant for acaricidal activity; it was decided to use a variety of solvents which form a range in index for polarity (Williamson et al. 1996). Cowan (1999) suggested that solvents such as chloroform and acetone would be ideally isolating the terpenoid and flavonoid fractions, whereas water and ethanol would extract a host of complex fractions composing of tannins, polyphenols, flavonol, alkaloids, terpenoids, polypeptides etc. resulting in differences between different plant extracts in their composition as well as biological activity. The extraction of hydrophilic and lipophilic compounds uses polar solvents such as water, methanol, ethanol and ethyl-acetate and non-polar solvents such as hexane or dichloromethane (Cosa et al. 2006). For this reason, five solvents were used with extreme non-polar (hexane) and polar (water) solvents. The variation in the acaricidal activity in various extracts in the current study may be attributed to the extraction process involving various polar and non-polar solvents. Results of the current study indicate that the ethanol extracts of leaves of M. koenigii can prove to be a promising herbal acaricide against SP-resistant R. (B.) microplus.
Results revealed that various extracts of M. koenigii leaves are toxic to R. (B.) microplus adults. Also, there was a reduction in the egg production in ticks treated with various extracts, and the reduction was significant with hexane extracts, as the surviving ticks were weak enough to produce optimum egg mass and thus reflect the tick control potentiality of these extracts. In ticks, the moulting hormone (ecdysteroids) plays a role in the regulation of salivary gland function, production of pheromones and oogenesis and oviposition (Rees 2004). In fully engorged adult female ticks, the level of ecdysteroids rises in the haemolymph which in turn causes salivary gland to degenerate, and as a result, the ticks lay more eggs. This hormone also triggers vitellogenesis (Sankhon et al. 1999) and inhibits reattachment to the host (Weiss and Kaufman 2001). The neuronal involvement in the control of salivary gland degeneration in the ixodid tick, Amblyomma haebraeum, has been proved (Harris and Kaufman 1984). The neurotransmitter dopamine regulates the synthesis of ecdysteroids. Dopamine was identified in the salivary gland and salivary gland nerves of R. (B.) microplus and A. haebraeum (Kaufman and Harris 1983). The probable effect of the plant extracts on these hormones and neurotransmitter may be the reason for the above observations and needs further validation by detailed studies.
Use of phytochemical products may be beneficial as acaricides to reduce the problems faced by animal owners, such as resistance and residues, also prolonging the useful life of commercial chemical products applied for parasite control through the association of bioactive plant substances with synthetic products (Chagas 2004). The medicinal effects of these phytochemicals may be due to effects of a single compound or combination of one or more active compounds present in the plant. As the acaricidal effect of the chemical constituents of plants is usually exerted in different ways including combination, therefore, the development of resistance against botanical acaricides seems to be difficult. Our results indicate that the various extracts of curry leaves and, particularly, ethanol extract could be a good source of active compound(s) that can potentially cause tick mortality specifically in SP-resistant populations, thus making them a valuable component of developing sustainable strategy for integrated tick management.
References
Adebajo AC, Ayoola OF, Iwalewa EO, Akindahunsi AA, Omisore NO, Adewunmi CO, Adenowo TK (2006) Anti-trichomonal, biochemical and toxicological activities of methanolic extract and some carbazole alkaloids isolated from the leaves of Murraya koenigii growing in Nigeria. Phytomedicine 13:246–254
Borges LM, Ferri PH, Silva WJ, Silva WC, Silva JG (2003) In vitro efficacy of extracts of Melia azedarach against the tick Boophilus microplus. Med Vet Entomol 17:228–231
Castro-Janer E, Rifran L, Piaggio J, Gil A, Miller RJ, Schumaker TTS (2009) In vitro tests to establish LC50 and discriminating concentrations for fipronil against Rhipicephalus (Boophilus) microplus (Acari: Ixodidae) and their standardization. Vet Parasitol 162:120–128
Chagas ACS (2004) Controle de parasitas utilizando extratos vegetais. Rev Bras Parasitol Vet 13:156–160
Chevallier A (1996) The encyclopedia of medicinal plants. Dorlon Kindersley Publisher, London
Chungsamarnyart N, Jiwajinda S, Ratanakreetakul C, Jasawan W (1991) Practical extraction of sugar apple seeds against tropical cattle ticks. Kasetsart J (Nat Sci) 25:101–105
Cosa P, Vlietinck AJ, Berghe DV, Maes L (2006) Anti-infective potential of natural products: how to develop a stronger in vitro ‘proof-of-concept’. J Ethnopharmacol 106:290–302
Cowan MM (1999) Plant products as antimicrobial agents. Clin Microbiol Rev 12:564–582
de Castro JJ (1997) Sustainable tick and tick-borne disease control in livestock improvement in developing countries. Vet Parasitol 71:77–97
Devanand P, Rani PU (2008) Biological potency of certain plant extracts in management of two lepidopteran pests of Ricinus communis L. J Biopest 1:170–176
Drummond RO, Ernst SE, Trevino JL, Gladney WJ, Graham OH (1973) Boophilus annulatus and Boophilus microplus: laboratory test of insecticides. J Econ Entomol 66:130–133
FAO (1971) Recommended methods for the detection and measurement of resistance of agricultural pests to pesticides-tentative method for larvae of cattle tick, Boophilus microplus spp. FAO method No. 7. FAO Plant Prot Bull 19:15–18
Fernandez-Salas A, Alonso-Diaz MA, Acosta-Rodriguez R, Torres-Acosta JF, Sandoval-Castro CA, Rodriguez-Vivas RI (2011) In vitro acaricidal effect of tannin-rich plants against the cattle tick Rhipicephalus (Boophilus) microplus (Acari: Ixodidae). Vet Parasitol 175:113–118
Finney DJ (1962) Probit analysis—a statistical treatment of the response curve. Cambridge University Press, Cambridge, pp 1–318
Ghosh S, Bansal GC, Gupta SC, Ray DD, Khan MQ, Irshad H, Shahiduzzaman M, Seitzer U, Ahmed JS (2007) Status of tick distribution in Bangladesh, India and Pakistan. Parasitol Res 101:S207–S216
Ghosh S, Sharma AK, Kumar S, Tiwari SS, Rastogi S, Srivastava S, Singh M, Kumar R, Paul S, Ray DD, Chaudhuri P, Rawat AKS (2011) In vitro and in vivo efficacy of Acorus calamus extract against Rhipicephalus (Boophilus) microplus. Parasitol Res 108:361–370
Ghosh S, Tiwari SS, Srivastava S, Sharma AK, Kumar S, Ray DD, Rawat AK (2013) Acaricidal properties of Ricinus communis leaf extracts against organophosphate and pyrethroids resistant Rhipicephalus (Boophilus) microplus. Vet Parasitol 192:259–267
Godara R, Parveen S, Katoch R, Yadav A, Verma PK, Katoch M, Kaur D, Ganai A, Raghuvanshi P, Singh NK (2014) Acaricidal activity of extract of Artemisia absinthium against Rhipicephalus sanguineus of dogs. Parasitol Res 113:747–754
Graf JF, Gogolewski R, Leach-Bing N, Sabatini GA, Molento MB, Bordin EL, Arantes GJ (2004) Tick control: an industry point of view. Parasitology 129:S427–S442
Grisi L, Massard CL, Moya Borja GE, Pereira JB (2002) Impacto economico das principais ectoparasitoses em bovinos no Brasil. A Hora Vet 21:8–10
Harris RA, Kaufman WR (1984) Neural involvement in the control of salivary gland degeneration in the ixodid tick Amblyomma hebraeum. J Exp Biol 109:281–290
Jonsson NN (2006) The productivity effects of cattle tick (Boophilus microplus) infestation on cattle, with particular reference to Bos indicus cattle and their crosses. Vet Parasitol 137:1–10
Jonsson NN, Miller RJ, Robertson JL (2007) Critical evaluation of the modified-adult immersion test with discriminating dose bioassay for Boophilus microplus using American and Australian isolates. Vet Parasitol 146:307–315
Kaufman WR, Harris RA (1983) Neural pathways mediating salivary fluid secretion in the ixodid tick Amblyomma hebraeum. Can J Zool 61:1976–1980
Kesari AN, Kesari S, Singh SK, Gupta RK, Watal G (2007) Studies on the glycemic and lipidemic effect of Murraya koenigii in experimental animals. J Ethnopharmacol 112:305–311
Li WQ, Jiang CH, Chu SS, Zuo MX, Liu ZL (2010) Chemical composition and toxicity against Sitophilus zeamais and Tribolium castaneum of the essential oil of Murraya exotica aerial parts. Molecules 15:5831–5839
Magadum S, Mondal DB, Ghosh S (2009) Comparative efficacy of Annona squamosa and Azadirachta indica extract against Boophilus microplus Izatnagar isolate. Parasitol Res 105:1085–1091
Malwal M, Sarin R, Shakeet P, Bakshi S (2009) Natural insect controlling agents from Murraya koenigii (L.) Spreng. J Herbal Med Toxicol 3:161–162
Mandal S, Nayak A, Kar M, Banerjee SK, Das A, Upadhyay SN, Singh RK, Banerji A, Banerji J (2010) Antidiarrheal activity of carbazole alkaloids from Murraya koenigii Spreng (Rutaceae) seeds. Fitoterapia 81:72–74
Minjauw B, McLeod A (2003) Tick-borne diseases and poverty. The impact of ticks and tick borne diseases on the livelihood of small scale and marginal livestock owners in India and eastern and southern Africa. Research report, DFID Animal Health Programme, Centre for Tropical Veterinary Medicine, University of Edinburgh, UK, pp 59–60
Nayak A, Banerji J, Banerji A, Mandal S (2010) Review on chemistry and pharmacology of Murraya koenigii Spreng (Rutaceae). J Chem Pharm Res 2:286–299
Playford M, Rabiee AR, Lean IJ, Ritchie M (2005) Review of research needs for cattle tick control. Phases I and II. Meat & Livestock Australia Ltd, Sydney
Polar R, Aquino de Muro M, Kairo TK, Moore D, Pegram R, John S, Roach-Benn C (2005) Thermal characteristics of Metarhizium anisopliae isolates important for the development of biological pesticides for the control of cattle ticks. Vet Parasitol 134:159–167
Pruthi JS (1976) Spices and condiments. National Book Trust India, New Delhi
Ravindran R, Juliet S, Sunil AR, Ajith Kumar KG, Nair Suresh N, Amithamol KK, Shynu M, Rawat AKS, Ghosh S (2011) Eclosion blocking effect of ethanolic extract of Leucas aspera (Lamiaceae) on Rhipicephalus (Boophilus) annulatus. Vet Parasitol 179:287–290
Ravindran R, Juliet S, Sunil AR, Ajith Kumar KG, Nair SN, Amithamol KK, Bandyopadhyay A, Rawat AK, Ghosh S (2012) Acaricidal activity of Cassia alata against Rhipicephalus (Boophilus) annulatus. Exp Appl Acarol 56:69–74
Rees HH (2004) Hormonal control of tick development and reproduction. Parasitology 129:S127–S143
Ribeiro VLS, Avancini C, Goncalves K, Toigo E, von Poser GL (2008) Acaricidal activity of Calea serrata (Asteraceae) on Boophilus microplus and Rhipicephalus sanguineus. Vet Parasitol 151:351–354
Ribeiro VLS, dos Santos JC, Bordignon SA, Apel MFA, Henriques AT, von Poser GL (2010) Acaricidal properties of the essential oil from Hesperozygis ringens (Lamiaceae) on the cattle tick Rhipicephalus (Boophilus) microplus. Bioresour Technol 101:2506–2509
Ribeiro VLS, dos Santos JC, Martins JR, Schripsema J, Siqueira IR, von Poser GL, Apel MFA (2011) Acaricidal properties of the essential oil and precocene II obtained from Calea serrata (Asteraceae) on the cattle tick Rhipicephalus (Boophilus) microplus (Acari: Ixodidae). Vet Parasitol 179:195–198
Sankhon N, Lockey T, Rosell RC, Rothschild M, Coons L (1999) Effect of methoprene and 20-hydroxyecdysone on vitellogenin production in cultured fat bodies and backless explants from unfed female Dermacentor variabilis. J Insect Physiol 45:755–761
Scalbert A, Johnson IT, Saltmarsh M (2005) Polyphenols: antioxidants and beyond. Am J Clin Nutr 81:215S–217S
Senrung A, Singh J, Sharma S, Bhutia TN, Singh AK (2014) Effect of Murraya koenigii extracts on feeding and ovipositional response of Spodoptera litura (Fab.) (Lepidoptera: Noctuidae). J Entomol Zool Stud 2:27–31
Shah AS, Wakade AS, Juvekar AR (2008) Immunomodulatory activity of methanolic extract of Murraya koenigii (L) Spreng leaves. Indian J Exp Biol 46:505–509
Sharma AK, Kumar R, Kumar S, Nagar G, Singh NK, Rawat SS, Dhakad ML, Rawat AKS, Ray DD, Ghosh S (2012) Deltamethrin and cypermethrin resistance status of Rhipicephalus (Boophilus) microplus collected from six agro-climatic regions of India. Vet Parasitol 188:337–345
Shaw RD (1966) Culture of an organophosphorus resistant strain of Boophilus microplus (Canestrini) and assessment of its resistance spectrum. Bull Entomol Res 56:398–405
Singh NK, Rath SS (2013) Epidemiology of ixodid ticks in cattle population of various agro-climatic zones of Punjab. Asian Pac J Trop Med 6:947–951
Singh NK, Rath SS (2014) Esterase mediated resistance against synthetic pyrethroids in field populations of Rhipicephalus (Boophilus) microplus (Acari: Ixodidae) in Punjab districts of India. Vet Parasitol 204:330–338
Singh NK, Jyoti, Haque M, Rath SS (2010) Studies on acaricide resistance in Rhipicephalus (Boophilus) microplus against synthetic pyrethroids by adult immersion test with a discriminating dose. J Vet Parasitol 24:207–208
Singh NK, Haque M, Jyoti, Rath SS (2012) Deltamethrin resistance in Rhipicephalus microplus in Ludhiana. Indian Vet J 89:23–25
Singh NK, Jyoti, Vemu B, Nandi A, Singh H, Kumar R, Dumka VK (2014) Acaricidal activity of Cymbopogon winterianus, Vitex negundo and Withania somnifera against synthetic pyrethroid resistant Rhipicephalus (Boophilus) microplus. Parasitol Res 113:341–350
Stone BF, Haydock P (1962) A method for measuring the acaricide susceptibility of the cattle tick Boophilus microplus (Can.). Bull Entomol Res 53:563–578
Valente PP, Amorim JM, Castilho RO, Leite RC, Ribeiro MF (2014) In vitro acaricidal efficacy of plant extracts from Brazilian flora and isolated substances against Rhipicephalus microplus (Acari: Ixodidae). Parasitol Res 113:417–423
Weiss BL, Kaufman WR (2001) The relationship between ‘critical weight’ and 20-hydroxyecdysone in the female ixodid tick, Amblyomma hebraeum. J Insect Physiol 47:1261–1267
Willadsen P (2006) Tick control: thoughts on a research agenda. Vet Parasitol 138:161–168
Williamson EM, Okpako DT, Evans FJ (1996) Volume 1: Selection, preparation and pharmacological evaluation of plant material. In: Pharmacological methods in phytotherapy research. Wiley, New York
Zaman MA, Iqbal Z, Abbas RZ, Khan MN, Muhammad G, Younus M, Ahmed S (2012) In vitro and in vivo acaricidal activity of a herbal extract. Vet Parasitol 186:431–436
Acknowledgments
Authors are thankful to the Director of Research, GADVASU, Ludhiana, for providing facilities to carry out the research work.
Conflicts of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Singh, N.K., Jyoti, Vemu, B. et al. In vitro acaricidal activity of Murraya koenigii (L.) Spreng (Rutaceae) extracts against synthetic pyrethroid-resistant Rhipicephalus (Boophilus) microplus . Parasitol Res 114, 1531–1539 (2015). https://doi.org/10.1007/s00436-015-4337-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-015-4337-y