Abstract
By definition, parasites cause harm to their hosts. But, considerable evidence from ancient traditional medicine has supported the theory of using parasites and their products in treating many diseases. Maggots have been used successfully to treat chronic, long-standing, infected wounds which failed to respond to conventional treatment by many beneficial effects on the wound including debridement, disinfection, and healing enhancement. Maggots are also applied in forensic medicine to estimate time between the death and discovery of a corpse and in entomotoxicology involving the potential use of insects as alternative samples for detecting drugs and toxins in death investigations. Leeches are segmented invertebrates, famous by their blood-feeding habits and used in phlebotomy to treat various ailments since ancient times. Leech therapy is experiencing resurgence nowadays in health care principally in plastic and reconstructive surgery. Earthworms provide a source of medicinally useful products with potential antimicrobial, antiviral, and anticancer properties. Lumbrokinases are a group of fibrinolytic enzymes isolated and purified from earthworms capable of degrading plasminogen-rich and plasminogen-free fibrin and so can be used to treat various conditions associated with thrombotic diseases. Helminth infection has been proved to have therapeutic effects in both animal and human clinical trials with promising evidence in treating many allergic diseases and can block the induction of or reduce the severity of some autoimmune disorders as Crohn’s disease or ulcerative colitis. What is more, venomous arthropods such as scorpions, bees, wasps, spiders, ants, centipedes, snail, beetles, and caterpillars. The venoms and toxins from these arthropods provide a promising source of natural bioactive compounds which can be employed in the development of new drugs to treat diseases as cancer. The possibility of using these active molecules in biotechnological processes can make these venoms and toxins a valuable and promising source of natural bioactive compounds. The therapeutic use of helminthes and insects will be of great value in biomedicine and further studies on insect toxins will contribute extensively to the development of Biomedical Sciences.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Insects and helminthes have been used globally in traditional medicine for centuries in East Asia, Africa, and South America and still used in parts of the world where conventional medical care is scare. This folk medicine provides a cheap method in economically challenged world (Pechter and Sherman 1983; Cooper et al. 2012). The research on maggots, helminthes, and insect therapy is highly successful promising aspects in biomedicine (Bonn 2000; Amendt et al. 2011). Furthermore, insect’s ingredients have been evaluated experimentally and preliminary reports proved their beneficial properties (Marsh and Williams 2005; Kini 2006). Insect-based products that purify arthropod-derived constituents have been developed recently and will yield further benefits (Chaim et al. 2011; Oršolić 2012).
Maggot
Maggot therapy is an inexpensive natural way combating wound infection, and it is under research to obtain more benefits from its implementation in the new alternative medicine. William Baer pioneered the use of sterile maggots as a biosurgical method of wound therapy in the late 1930s, after observing its value in traumatic wounds on the battlefield in France during World War I (Baer 1931). By the mid-1940s maggot therapy was neglected after the era of antibiotics therapy and improved surgical techniques. Later on, maggot therapy made its second resurgence with the emergence of antibiotic-resistant bacteria strains in the 1980s (Sherman et al. 2000). Nowadays, larvae of the green bottle blowfly Lucilia sericata are used worldwide as a debridement agent for chronic and infected wounds such as for the treatment of venous stasis leg ulcers (Wayman et al. 2000), diabetic foot ulcers, especially neuropathic ulcers (Marineau et al. 2011; Wilasrusmee et al. 2014), wound bed preparation prior to surgical closure (Sherman and Shimoda 2004), traumatic and post-surgical wounds (Namias et al. 2000), osteomyelitis (van der Plas et al. 2008), and burns (Robinson and Norwood 1933). Moreover, the larval excreta/secreta are used as potent agents against methicillin-resistant Staphylococcus aureus infection (Bexfield et al. 2004). In 2004, the US Food and Drug Administration (FDA) allowed production and marketing of maggots as a medical tool. Many types of larvae were used as a debridement therapy like Lucilia ceaesar (Baer 1931), the large blue bottle flies (Calliphora vomitans and Calliphora erythrocephala) (Weil et al. 1933), and Lucilia cuprina (Fine and Alexander 1934). But maggots of L. sericata are the most commonly used probably because of its starvation on clean granulation tissue (Weil et al. 1933). The most common adverse effects associated with maggot therapy are discomfort which is reported in 5–30 % of treated wounds (Sherman 2003), delivery problems, and escaping maggots (Sherman 2009).
The outstanding effects of medicinal maggot therapy attributed to the synergistic actions of these main activities: (1) Debridement (Fleischmann et al. 1999; Wayman et al. 2000; Sherman et al. 2007), which means the removal of foreign material and dead contaminated tissue from wound bed to expose healthy tissue, and this is the most known and accepted mechanism of action by maggots. It occurs by secreting a rich cocktail of digestive enzymes during its feeding like leucine aminopeptidase, carboxypeptidases A and B (Vistnes et al. 1981), serine proteases (trypsin-like and chymotrypsin-like enzymes) (Chambers et al. 2003), and collagenase (Ziffren et al. 1953). Moreover, four proteolytic enzymes, containing two serine proteases, a metalloproteinase and an aspartyl proteinase, were detected in its secretions with molecular weights ranging from 20 to 40 kDa, with activity across a wide pH range (Chambers et al. 2003). The significant role of a chymotrypsin-like serine proteinase in the larva of Lucilia in degradation of wound matrix components laminin, fibronectin, and collagen types I and III has been defined. Moreover, the wriggling of maggots have mechanical effect which promotes debridement as these possess mouth hooks which probes and macerate the necrotic tissue (Barnard 1977). (2) Disinfection (Sherman and Shimoda 2004; Armstrong et al. 2005; Bowling et al. 2007), the application of maggots to an infected wound results in rapid elimination of many anaerobic and aerobic bacteria (Bowler and Davies 1999) including Gram-negative bacteria like Escherichia coli, Pseudomonas aeruginosa, and Salmonella spp, as well Gram-positive bacteria such as S. aureus, Staphylococcus epidermis, Listeria monocytogenes, and clinical isolates of methicillin-resistant S. aureus (Bexfield et al. 2004; Daeschlein et al. 2007; Kerridge et al. 2005). Moreover, maggots produce a large quantity of ammonia which alters wound pH from acidic to neutral or slightly alkaline at pH 7 or 8 which discourage bacterial growth (Mumcuoglu 2001); (3) Stimulation of healing (Sherman et al. 1995; Horobin et al. 2005), maggots have ability to physically stimulate granulation tissue by its crawling motions (Buchman and Blair 1932). Also, it may be due to the nitrogenous waste of Lucilia containing 10 % allantoin (2,5-Dioxo-4-imadazolidinyl urea) (Robinson 1935) and 90 % ammonia biocarbonate (Robinson 1940) which are responsible for the abundant growth of granulation tissue; and (4) Eradication and inhibition of Staphylococcus epidermidis (Harris et al. 2009) S. aureus and, to a lesser extent, P. aeruginosa and biofilms (van der Plas et al. 2008).
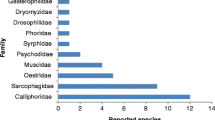
Another interesting use of maggots was in forensic entomology, which is the science of collecting and analyzing insect evidence for forensic and legal purposes (Amendt et al. 2007). Most important task in forensic medicine is estimating the minimum post-mortem interval (PMI) that refers to the time between the death and discovery of a corpse (Catts 1992). Insects are the most powerful method to estimate this time quickly and perfectly (Amendt et al. 2011). Depending on environmental conditions, necrophagous insects will colonize a fresh corpse quickly. Usually, the body will be attacked firstly by flies (Diptera), mainly the blow fly of family Calliphoridae (Sukontason et al. 2007), which can locate an odor source with great spatial precision and deposit their eggs on a corpse within minutes of death. Larvae hatch from the eggs and feed on the underlying tissues. They grow and moult twice, and when third instar larvae finish feeding, they enter the post-feeding stage, puparium, and migrate away from the body to find shelter. Decomposition occurs in corpse as a result of continuous process of insect activity in and on the corpse. The degree of this decomposition can be measured, allowing accurate minimum PMI estimates to be made up to several months after death depending on the circumstances and temperature profile of the region (Campobasso et al. 2001; Reibe et al. 2010). The hypothesis behind these estimates is that by calculating the age of developing insects on a body, it is possible to calculate the time of colonization, which infers a minimum PMI (PMImin), and because blowflies are the usual first group to colonize a body, the focus of PMImin estimates is often on them when using entomological evidence (Catts 1992). There is a different rate of development regarding each Calliphoridae species according to its specific energy requirements and the nutritional composition of each type of food (Thyssen et al. 2014).
Entomotoxicology is another relatively new branch of forensic entomology. It involves the potential use of insects as alternative samples for detecting drugs and toxins (Kintz et al. 1990). In death investigations, Diptera and other arthropods can be reliable alternate specimens for toxicological analyses in the absence of tissues and fluids normally taken for such purposes. Entomotoxicology also investigates the effects caused by drugs and toxins on arthropod development in order to assist the forensic postmortem interval estimates (Nolte et al. 1992; Introna et al. 2001). Most of the substances implicated in drug-related deaths are detectable through analyses of maggots: opiates such as morphine and codeine, cocaine and benzoylecognine, amphetamines, tricyclic antidepressants, phenothiazines and benzodiazepines, steroids, barbiturates, and several salicylates (Introna et al. 2001; Carvalho 2010). Drugs and toxins have also been detected by analyzing of puparial cases (Pien et al. 2004), and moreover, it is founded in beetle exuviae and fecal material (Miller et al. 1994). Bourel et al. (2001) have demonstrated the accumulation of morphine inside the cuticle of Calliphora vicina maggots.
Helminthes
Although parasites are harmful to their hosts, there is a growing evidence from experimental studies using animal models of human disease supporting that helminthes infection play a role in suppressing the progression and incidence of other diseases (Garg et al. 2014) (Table 1). Moreover, it has demonstrated that helminth infections have reduced the incidences of autoimmune and allergic diseases (McKay 2008). It is known that, in helminths infection, Th2 is the predominant immunological response. This Th2 stimulation can ameliorate disorders in which Th1 is the dominant event (McKay 2009). The dependable theory for most researchers to use helminth infection in blocking the induction of or reducing the severity of some diseases is predicting the immunological Th2 events evoked by helminth infection which can ameliorate disorders in which Th1 events predominated (McKay 2009). This is supported by observing the strong correlation between reduction in parasite exposure and the dramatic increase in atopic, autoimmune, and autoinflammatory disorders in developed countries (Rook 2009). Moreover, clinical evidence support the theory of helminth therapy for unrelated diseases; such as using Trichuris suis therapy in Crohn’s disease (Summers et al. 2005; Reddy and Fried 2007, 2009) and ulcerative colitis since its colonization promotes goblet cell hyperplasia and mucus production through Th2 cytokines and IL-22 (Broadhurst et al. 2010), Necator americanus in the treatment of Crohn’s disease (Croese et al. 2006) and protection from asthma (Mortimer et al. 2006), and Schistosoma haematobium in decreasing atopy (van den Biggelaar et al. 2000). However, studies oppose this trend not only for psychological reasons, but also for safety measures against risk of zoonosis or tissue damage by accidental worm migration to unwanted sites in the human body (Van Kruiningen and West 2005). On the other hand, studies favor the use of helminth infection as a therapy but considering other factors that determine the relationship between helminth and allergic disease as (1) the duration of infection as early and/or chronic infections are more efficient in down-modulation of allergic disease, but sporadic or transient infections may enhance allergic clinical symptoms (Cooper et al. 2006); (2) infection intensity since light infections may promote allergic disease whereas a heavy one may induce immune suppression (Smits et al. 2010); (3) host Genetics, which is responsible for induction of the specific host immune regulatory mechanisms; therefore, individuals genetically susceptible to atopic disease may be more liable to develop allergic responses to helminth and allergens and will be genetically more resistant to infection (Moller et al. 2007); and (4) concurrent infections and environmental differences affect the outcome of analyses of natural infections in humans (McKay 2009).
Leech
Medicinal leech therapy is an earliest skill that dates back to ancient Egypt. Leeches was one of the most common therapeutic for human illness in Greek, Greco-Roman, and Byzantine medicine (Whitaker et al. 2004). The popularity of the use of leeches reached the peak in the early nineteenth century in Europe (Eldor et al. 1996).
Leeches are sanguivorous or blood-sucking annelid worms, usually black or brown in color and about 10 cm in length. Of the more than 650 known species in the Hirudo genus, Hirudo medicinalis is the commonly used in medical therapy. The mouth of the leech has three jaws; each has about 100 tiny teeth, in addition to a posterior suction device, which is used for stability. Leech therapy involves an initial painless bite, then sucking about 5 to 15 mL of blood by dissecting the skin with its sharp teeth (Godekmerdan et al. 2011) and simultaneously injecting of a cocktail of several medicinally useful bioactive molecules in their saliva (Zaidi et al. 2011). Once they are detached, the site of bite continues to bleed for several hours (Ikizceli et al. 2005). The most important substance in their saliva is a pure anti-coagulating called ‘Hirudine’ which is discovered by Haycraft 1884. In 1955, Markwardt have isolated and characterized Hirudin from leech pharyngeal glands. Subsequently, this effective anticoagulant was produced by genetic engineering (Fields 1991). Moreover, the leech also secretes hyaluronidase, which allows the anticoagulant to spread throughout the wound, and antihistamines, which vasodilate and contribute to prolongation of bleeding following a bite (Whitaker et al. 2004).
The most serious complication of leech therapy is infection, which occur in 2.4–20 % of plastic surgical repairs (de Chalain 1996). The leech’s digestive system contains a symbiote, Aeromonas hydrophila, which is the most common organism found in leech infections (Whitaker et al. 2003; Ardehali et al. 2006). Infections can arise from 2 to 11 days after therapy begins and can result in abscesses and cellulitis, which can progress in some cases to sepsis (Abdelgabar and Bhowmick 2003). von Rheinbaben et al. (2014) recommended making a quarantine period of 4–6 months after the lasting feeding of leech which supports microbial degradation inside leech gut and so decrease risk of viral infection for treated patients. Also, excessive bleeding can occur with leech therapy which may necessitate blood transfusion (Whitaker et al. 2003).
Leeching is applied to head for treatment of headache or mania and in cases of inflammation of the throat, with their application to the neck (Adams 1988). Currently, leech is extensively used in surgery, such as plastic surgery to aid wound healing (Whitaker et al. 2004), in the treatment of ring avulsion injuries in plastic surgery (Tuncali et al. 2004), after breast surgery to relieve the possible complication of venous congestion (Gross and Apesos 1992), treat venous congestion and hematoma after plastic reconstructive surgery (Riede et al. 2010), alleviate post-phlebitis syndrome, in which venous valves are obliterated by a deep vein thrombosis (Eldor et al. 1996), reduce symptoms caused by osteoarthritis (Michalsen et al. 2003; Andereya et al. 2008), symptomatic relief of refractory cancer pain (Kalender et al. 2010), with replanted digits (Foucher et al. 1981), ears (Cho and Ahn 1999), lips (Walton et al. 1998), and nasal tips (Mortenson et al. 1998), and for treatment coronary ischemia (van de Loo and Bode 2002).
Recently, specific substances from leeches have been refined and utilized to prevent thrombosis (Markwardt 2002). Also, a topical cream has been manufactured from hirudin and used to shrink hematomas caused by musculoskeletal injuries (Stamenova et al. 2001). Recombinant hirudins are undergoing clinical trials to treat deep venous thrombosis and acute coronary syndromes and also as an adjuvant to thrombolysis in myocardial infarction (Markwardt 1956).
The most serious complication of leech therapy is infection that occurs in 2.4–20 % of plastic surgical repairs (de Chalain 1996) due to the presence of symbiote, A. hydrophila in the leech’s digestive system (Ardehali et al. 2006). Infections can arise within 11 days after therapy begins and can result in abscesses and cellulitis, which may progress to a life-threatening sepsis (Abdelgabar and Bhowmick 2003). Other reported complications include allergic reactions, anemia, scarring, and bleeding that may necessitate blood transfusion (Whitaker et al. 2003).
Earthworms
Earthworms have been widely used in traditional medicine for thousands of years (Sun et al. 1997) since they contain substances with potential antimicrobial, antiviral, and anticancer properties (Cooper et al. 2004). Earthworms have been used to treat inflammatory, hematological, oxidative, and nerve diseases (Chen et al. 2010; Liu et al. 2013). Baking earthworms with bread have been used to treat kidney stones; in addition, ingestion of dried or burnt ashes of earthworms has been used to improve alopecia and relieve jaundice. Moreover, topical application of dead earthworms alleviates arthralgia and treat injuries (Cooper et al. 2004). Furthermore, treatment of some blood diseases and supporting circulatory health was recorded with oral ingestion of earthworm as powder (Pan et al. 2011).
A strong fibrinolytic enzyme from the earthworm, Lumbricus rubellus has been purified and is capable of degrading plasminogen-rich and plasminogen-free fibrin. This result suggests that earthworm powder represents a possible oral thrombolytic agent and may thus be applicable for treating patients with thalassemia (Mihara et al. 1991). It was also reported that oral administration of a saline suspension of L. rubellus earthworm powder (EWP) to rats could reduce the formation of venous thrombus, suggesting that EWP may be valuable for the prevention and/or treatment of thrombotic diseases (Hahn et al. 1997). Glycolipoprotein mixture (G-90), prepared from the tissue of the earthworm Eisenia foetida, was examined in vivo, in an experimental study on Wistar rats, to determine its fibrinolytic and anticoagulation activity. The results have shown that G-90 have a similar effect like heparin, a known anticoagulant, on blood coagulation. Thus, G-90 considered as a new thrombolytic agent of use in veterinary and human medicine (Matausic-Pisl et al. 2011). Additionally, the weight of thrombus has been reduced upon feeding a rat with an arterio-venous shunt on dried powder from L. rubellus earth worm (Kim et al. 1998).
Prakash and Gunasekaran (2010) have studied the effect of various doses of earthworm paste (Lampito mauritii, Kinberg) on aspirin-induced gastric ulcer and compared with ranitidine. The earthworm paste showed significant ulcer protective effects due to its anti-secretory action and its effects on mucosal glycoprotein. It is proven to be more effective in gastric cytoprotection than ranitidine in preventing lesion formation. Balamurugan et al. (2009) have reported the anti-inflammatory and anti-pyretic properties of earthworm extract which is similar to glycoprotein complex (G-90). An earthworm-derived Factor Xa (FXa) inhibitor, eisenstasin-derived small peptide (ESP), has been isolated from the midgut of the Eisenia andrei worm and found to reduce the expressions of pro-inflammatory cytokines (IL-1 α, IL-1β, IL-8, IL-16, MCP-1, MIP-1α, and MIP-1β) by cultured cells treated with both ESP and FXa. So, ESP might interrupt coagulation cascades by inhibiting FXa and thus may be effective control the bidirectional alternation between coagulation and inflammation (Joo et al. 2009).
Lumbricin-PG, a novel lumbricin-like antimicrobial peptide, has been isolated from skin secretions of the earthworm, Pheretima guillelmi (Michaelsen) and has exerted potential antimicrobial activities against bacteria and fungi (Li et al. 2011). The Earthworm decoction can lighten the airway inflammation in asthmatic guinea pigs. It acts by inhibition of eosinophil infiltration, acceleration of eosinophil apoptosis, and improvement of the bronchial tube and the lung tectology changes (Li et al. 2007). Earthworm paste (EP) derived from an indigenous species Lampito mauritii (Kinberg) reduce inflammation, restore the levels of antioxidants-reduced glutathione, glutathione peroxidase, superoxide dismutase, catalase, and thiobarbituric acid reactive substances, normalize the values of erythrocyte, leukocyte, differential levels of neutrophils, lymphocytes, eosinophils, hemoglobin, and serum biochemical contents when applied to inflamed Wistar albino rat. It seems to be powerful than standard anti-inflammatory drug, aspirin (Balamurugan et al. 2007).
Regarding its anti-cancer activity, the polysaccharide–protein complex isolated from metabolites of bacteria inhibiting gut of Raoultella ornithinolytica earthworms have anti-fungal and anticancer activity against breast ductal carcinoma and endometrioid ovarian cancer in vitro (Fiołka et al. 2013). Furthermore, earthworm aqueous extracts have been utilized in the green synthesis of gold nanoparticles (AuNPs). Earthworm extracts act as reducing agents to convert Au3+ to AuNPs. The earthworm–AuNPs is capable of reinforcing the anticoagulant activities of heparin. So, the newly prepared AuNPs are promising candidates for novel gold nanomedicines (Kim et al. 2013).
Venom
Insect venoms constituents have anti-inflammatory properties that may make them valuable in the treatment of several medical disorders. Research in venom has increased over recent years, and the challenges and opportunities are still enormous. Studies on insect toxins have contributed extensively to the development of Biomedical Sciences. Many bioactive molecules found in insect’s venoms can exert important pharmacological activities in human physiology. So, it is of great value to characterize these active components for development of new drugs capable of directly and specifically acting upon cell physiology. Some of major molecules isolated from in these venoms are introduced in Table 2.
Scorpion
Scorpions are an ancient terrestrial venomous lineage. Scorpion’s venom exerts toxic activities on a wide range of biological targets (Gantenbein and Largiadèr 2003; Bryson et al. 2013).Venoms from scorpion is a complex mixture of a large variety of molecules rich in proteins and peptides (Kuhn-Nentwig 2003). Over 1,500 scorpion species have been identified, each producing a different type of venom; each venom is estimated to be composed of 50–100 different toxic polypeptides (Possani et al. 2000). In contrast to other venoms, scorpion venom displays low levels of enzymatic activity (Gwee et al. 2002). Among several toxin types present in scorpion venoms are the CSα/β scaffold cysteine-stabilized α/β which include sodium ion channel (NaV) modulators (Quintero-Hernández et al. 2013).
Scorpion venom has been used in traditional Chinese medicine to relieve pain and treat meningitis, epilepsy, stroke, and rheumatic diseases (Wang et al. 2009). Scorpion toxins are a promising approach to fight cancer. The most studied peptides from scorpion venom are the long-chain toxins composed of 60–70 amino acid residues cross-linked by four disulfide bridges and act mostly by activating Na channels (Goudet et al. 2002). A protein called BmBKTx1 created from Chinese scorpion venom prevents the breakdown of the tumor suppressor protein, and another called “AGAP” retards the growth of intra-peritoneal tumors in mice (Liu et al. 2003). Chinese scorpion venom contains a hyaluronidase, which was used to suppress hyaluronan, a cell adhesion factor that promoted metastasis in an in vitro human breast cancer cell line (Feng et al. 2008).
Chlorotoxin (Cltx), a peptide isolated from the species Leiurus quinquestriatus, is one of most active components present in scorpion venom (Lippens 1995). Chlorotoxin inhibit the activity of human glioma cells in vitro by inhibiting chloride influx in glioma cells membrane (Soroceanu et al. 1999) and binding matrix metalloproteinase II (MMP-2) which is involved in tumor invasion (Veiseh et al. 2007). Gao et al. (2008) isolated a serine proteinase-like protein (BMK-CBP) from BmK venom, which could bind to the breast cancer cell line MCF-7 in a dose-dependent manner, showing the potential of BMKCBP as a delivering drug for cancer treatment. A protein from the Indian black scorpion Heterometrus bengalensis, bengalin, caused human leukemic cells to undergo apoptosis in vitro through mitochondrial pathway against the human histiocytic lymphoma cell line U937 and the human chronic myelogenous leukemia cell line K562 (chronic myelogenous leukemia), not affecting normal human lymphocytes (Gupta et al. 2010). Moreover, venom from the Venezuelan scorpion, Tityus discrepans, has antimicrobial and antiparasitic activity and is found to suppress the growth of Leishmania species in vitro (Borges et al. 2006). In addition to another promising toxin in the treatment of sleep apnea, α-toxin retards inactivation of sodium channels at the neuromuscular junction that might increase neuromuscular reflexes and airway contraction (Conduit et al. 2007).
Spider
Spiders are the most diverse group of arthropods, but relatively few toxins have been studied so far (Escoubas 2006). Spider venom contains a collection of peptides and other compounds that include substances altering neuronal sodium, calcium and potassium channels, and glutamate and acetylcholine receptors (Ori and Ikeda 1998). Regarding the biochemistry of spider venoms, they present a variety of ion channel toxins, novel non-neurotoxins, enzymes, and low-molecular-weight compounds (Rash and Hodgson 2002).
The venom of the brown Loxosceles spiders contains a complex mixture of protein and peptide toxins with a molecular mass profile ranging from 1 to 40 kDa (Futrell 1992). Several molecules from the Loxosceles spider crude venoms have been identified, including alkaline phosphatase (Sales and Santoro 2008), 5′-ribonucleotide phosphohydrolase (Futrell 1992), sulfated nucleosides (Schroeder et al. 2008), hyaluronidase (da Silveira et al. 2007), fosfolipases-D (Kalapothakis et al. 2007), metalloproteases, serine proteases (Barbaro et al. 2005), and insecticide toxins (de Castro et al. 2004). Brown spider hyaluronidase could be used therapeutically in many fields (Senff-Ribeiro et al. 2008). It can be used to promote absorption of excess fluids, to increase the efficiency of local anesthesia, and to decrease tissue destruction by subcutaneous and intramuscular injection of fluids (Girish and Kemparaju 2007). Hyalurondase has also been used to reduce the extent of tissue damage following extravasation of parental nutrition solution, electrolyte infusions, antibiotics, aminophyline, mannitol, and chemotherapeutic agents, including Vinca alkaloids (Goolsby and Lombardo 2006). Additionally, technological advancement has led to synthesis of recombinant human hyaluronidase (rHuPH20) which has been used in the management of chronic pain, improving systemic absorption and bioavailability of drugs (Etesse et al. 2009; Muchmore and Vaughn 2010). Moreover, testicular hyaluronidase (HAase) has been added to drug regimens of cancer therapy to improve drug penetration and to enhance the efficacy of vinblastin in the treatment of malignant melanoma and Kaposi’s sarcoma, among other cancers (Lokeshwar and Selzer 2008). Furthermore, it has been found that, when the level of HA decreases under conditions in which hyaluronidase activity increases, the moisture and tension of the skin are reduced, and histamine is released from mast cells (Barla et al. 2009), so, the future recognition and characterization of hyaluronidase inhibitors will be relevant to the development of contraceptives, anti-tumor, anti-microbial, anti-venom, anti-wrinkle, anti-aging agents, and allergy and inflammation suppressors (da Silveira et al. 2007; Barla et al. 2009). From this context, it is obvious that Loxosceles recombinant hyaluronidases are associated with numerous potential applications (Gremski et al. 2010).
TCTP protein has been identified in Loxosceles intermedia venom gland, and it has been proposed as a potential cancer biomarker (Slaby et al. 2009) as TCTP protein levels are upregulated in cancer cells and in human tumors (Ma et al. 2009) and downregulated in biological models of tumor reversion (Tuynder et al. 2004). Also, this protein is the target of various anticancer drugs (Efferth 2005), and so it could be used as a potential target for therapy (Zhu et al. 2008). Astacins from L. intermedia could also be used as starting materials to design new drugs, as agonists and/or inhibitors. The possible therapeutic use of astacins from L. intermedia is the vascular diseases (acute myocardial infarction, acute ischemic stroke, thrombosed aortic aneurysms, pulmonary embolism, etc.) and as thrombolytic agents (Chaim et al. 2011). Meprins, which are members of the astacin family, hydrolyze and inactivate several endogenous vasoactive peptides. Recently, it has shown that a meprin inhibitor suppresses the formation of atherosclerotic plaques (Gao et al. 2009). The recombinant astacins could also be used as reagents for laboratorial tests to diagnose Loxoscelism, anti-loxosceles serum production, and in the treatment of envenomation (Chaim et al. 2011).
The toxin Tx2–6 from Phoneutria nigriventer (Brazilian Wandering spider) venom causes an improvement in the level of nitric oxide in penile tissue in rats, and so it can be investigated as a tool in the treatment of erectile dysfunction (Villanova et al. 2009). Additionally, other peptides were identified in Cupiennius salei spider venom with antibacterial properties. These peptides act as channel-forming toxins within the bacteria wall. Therefore, it is expected that analogous synthetic molecules would have great potential, especially in the emergence of multiple-antibiotic-resistant bacteria (Haeberli et al. 2000). The oxyopinins from the wolf spider Oxyopes kitabensis, forming pores in lipid membranes (Belokoneva et al. 2003), considered the anti-tumor action of other pore-forming peptides, oxyopinins could also be considered as good candidates for anti-cancer therapy (Shaposhnikova et al. 1997). Gao et al. (2007) have verified the effects of the venom of Macrothele raven (Araneae, Hexathelidae) upon the proliferation and cytotoxicity of human cervical carcinoma cells (2005) and on the human breast carcinoma cell line MCF-7. Psalmotoxin 1, a component of the venom of a West Indies tarantula, is a 40-amino acid peptide that inhibits cation currents mediated by acid-sensing ion channels (ASIC) (Escoubas et al. 2000).
Ants and centipedes
Ant venom has also been used to treat diseases especially arthritis. A partially purified extract of the South American tree ant Pseudomyrmex sp. venom was tested by Altman et al. (1984) in a case–control study of patients with rheumatoid arthritis. It has been demonstrated that venom-treated patients have an improvement in overall efficacy and a significant reduction in the number of tender/painful joints and swollen joints. Another study showed that solenopsin A, a primary alkaloid from the fire ant Solenopsis invicta, exhibits anti-angiogenic activity. Authors investigated the ability solenopsin A for inhibiting a series of kinases involved in the process of angiogenesis. Mouse embryonic fibroblast cell lines (3 T3-L1 and NIH3T3) were used, and solenopsin prevented the activation of PI3K, the phosphorylation of Akt-1 at both Thr308 and Ser473, and the phosphorylation and subsequent subcellular localization of forkhead box O1a (FOXO1a), a physiologic substrate of Akt. The selective inhibition of Akt by solenopsin in vitro is of great interest since few Akt inhibitors have been developed so far, and Akt is a key molecular target in the pharmacological treatment of cancer (Arbiser et al. 2007). An inhibitor of PI3K/Akt, Perifosine is being used in clinical assays to treat patients with advanced stages of cancer (Chee et al. 2007).
Regarding centipede, a study has reported the anti-tumoral action of centipede venom (Sonoda et al. 2008). It was shown that a synthetic compound, Manbβ (Fuca(1–3))Glcb 1-Cer,(glycosphingolipid 7), which was identified in the centipede Parafontaria laminate armigera, had an antiproliferative effect on melanoma cells. The effect of glycosphingolipid 7 on the proliferation of melanoma cells was through suppression of the activation of FAK-Akt and Erk1/2, the phosphorylation of pRb, and the expression of cyclinD1 and CDK4. The authors concluded that Glycosphingolipid 7 might be a candidate as an inhibitor of cell proliferation in melanomas. Moreover, μ-SLPTX-Ssm6a, a unique 46-residue peptide from centipede venom, has a promising effect as a novel analgesic treating a broad range of pain conditions exceeding morphine in rodent pain models (Yang et al. 2013).
Snail
Snail has been used in medicine since ancient times and prepared according to several formulations. The venoms of these snails contain a cocktail of up to 200 pharmacologically active components that mainly target different voltage- and ligand-gated ion channels (Becker and Terlau 2008). The first description of snails as medication was during the Roman Empire when it was used in the mental illness, syncope, vertigo, and infectious diseases (Bonnemain 2005). In the eighteenth century, American investigators have assessed hundreds of neurotoxins derived from sea snails which are utilized by snail to immobilize their prey and induce a neuro-muscular blockage. That is why Ziconotide (SNXIII), a synthetic equivalent to a 25-amino-acid peptide derived from snail venom, has been under FDA review since 1999 to be administered intrathecally as an alternative to opioids in cases of severe pain due to its powerful anesthetic effect (Vitale et al. 2008). In twentieth century, a neurotoxin called conotoxin TVIIA, was extracted from Conus magus, a fish-eating sea snail and approved for the treatment of intractable pain (Stix 2005; Bingham et al. 2010), and it has been demonstrated to reduce the size of the myocardial infarct in an ischemia/reperfusion model in rabbits, rats, and dogs in vivo (Lubbers et al. 2005).
Beetles (Cantharidin)
Mylabris is the dried body of the Chinese blister Mylabris phalerata and Mylabris cichorii beetles. Its use as a traditional medicine in China traced back more than 2,000 years (Wang 1989). Dried bodies of beetles are ingredients of traditional Chinese medicine used to treat esophageal cancer, hepatoma, and skin diseases (Efferth et al. 2005). The active constituent of mylabris is cantharidin. Cantharidin is a vesicant found in all body fluids of blister beetles, including Mylabris phalerata and M. cichorii (Chinese blister beetles) and Lytta vesicatoria (Spanish fly) (Ellenhorn 1997). In Asia, cantharidin was used in the treatment of furuncles and piles, ulcers, and tuberculous scrofuloderma (Wang 1989). In Europe, Hippocrates prescribed cantharidin as a treatment for dropsy (Cheng et al. 1990). In South Africa, the Tswanas grind cantharidin-producing beetles into a powder and use it in a medicine called seletsa as an aphrodisiac, as an abortifacient, and for purifying the blood (Ellenhorn 1997).
Cantharidin may be a useful candidate to develop novel strategies for cancer therapy. Cantharidin has anti-cancer activity inhibiting human leukemia cell lines growth in vitro (Rauh et al. 2007). Unlike other chemotherapeutic agents, cantharidin acts on leukemia progenitor and stem cells (Dorn et al. 2009). Derivatives of cantharidin also inhibit the growth of prostate, colon, oral, cervical, and gall bladder cancer cell lines (Hill et al. 2007; Liu and Chen 2009). Moreover, Cantharidin stopped the production of P-gp, a membrane transport protein that produces chemotherapeutic drug resistance in a hepatoma cell line (Zheng et al. 2008). More than that, cantharidin has anti-metastatic effect in A549 human lung cancer cells (Kim et al. 2013). There is growing evidence that Norcantharidin (NCTD), a water-soluble synthetic small-molecule derivative of naturally occurring cantharidin from the medicinal insect blister beetle (Mylabris phalerata Pallas) is a demethylated analog of cantharidin (Wang 1989). Norcantharidin is capable of chemoprevention and tumor inhibition (Hsieh et al. 2013) and also promotes tumor cell adhesion and metastasis (Chen et al. 2005). Lee et al. (2013) suggest that norcantharidin exhibits anticancer effects against non-small cell lung cancer cells in vitro, and his report supports the potential use of Norcantharidin as a chemotherapeutic agent for treating non-small cell lung cancer. Topical cantharidin in a collodion vehicle has been used by dermatologists in the treatment for warts and molluscum contagiosum since the 1950s (Funt 1961). Moye et al. (2014) has reported that Cantharidin is a safe treatment modality for molluscum contagiosum and should be considered when symptomatic infection necessitates treatment (Kadioglu et al. 2014).
Bees
The therapeutic application of bee venom has been used in traditional Oriental medicine to treat many diseases as arthritis, rheumatism, and pain (Jang et al. 2009). Bee venom possesses radioprotective (Varanda and Tavares 1998), antimutagenic (Varanda et al. 1999), anti-inflammatory (Nam et al. 2003), antinociceptive (Son et al. 2007), and anticancer activities (Moon et al. 2006). The anti-inflammatory effect of bee venom is attributable to suppression of phospholipase A2, free radical production, alpha-1 acid glycoprotein gene expression, and activation of nitrous oxide (Son et al. 2007; Yoon et al. 2008). Other anti-inflammatory mechanisms include suppression leukocyte migration, inhibition of inflammatory gene activation, reduction of cyclooxygenase-2 (COX-2) activation, mRNA expression, decrease of inflammatory cytokines tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β), and superoxide production and direct inhibition of NF–kB (Becker and Terlau 2008; Hama and Sagen 2009).
Regarding its role in treatment in rheumatoid arthritis, a study enrolling rheumatoid arthritis patients showed an additive effect when bee sting was used simultaneously with classical oral drugs such as methotrexate, sulfasalazine, and meloxicam (Liu et al. 2008). An in vitro human study showed that bee venom induced apoptosis of synovial fibroblasts in subjects with rheumatoid arthritis (Zhang et al. 2003). Darwish et al. (2013) have concluded that bee venom potentiates the anti-arthritic effects of methotrexate, possibly by increasing its bioavailability. Also, it provides a potent anti-nociceptive effect. Furthermore, bee venom protects against methotrexate-induced hepatotoxicity mostly due to its inhibitory effect on TNF-a and NF–kβ.
Depending on the fact that people perceive bee stings as being painful or hyperesthetic, bee venom can be used to influence pain regulation as it induces pain through the activation of spinal neurons (Son et al. 2007). In an experimental study in rats, bee venom application by plantar injection increases a marker of neuronal activity, c-fos gene expression, in nociceptive neurons of the dorsal horn of the spinal cord (Luo et al. 1998). On the other hand, other study showed that bee venom promotes the expression of genes in spinal neurons that downregulate pain. The acupuncture needle coated with bee venom has been utilized to treat pain (Son et al. 2007). A clinical trial in Korea has been performed using apipuncture to treat arthritis. Subjects had significantly fewer tender and swollen joints and less pain and morning stiffness after therapy (Lee et al. 2005). Another study reported the efficacy and safety of bee venom acupuncture in patients with chronic non-specific low back pain (Seo et al. 2013). In a pilot study, both acupuncture and bee venom acupuncture showed promising results as adjuvant therapies for idiopathic Parkinson’s disease (Cho et al. 2012).
Moreover, purified bee venom (PBV) has been used in treating acne vulgaris. The anti-microbial activity of PBV were translated in vivo study which showed that cosmetics containing PBV provided a certain degree of efficacy in terms of lesion counts and skin microorganism concentration compared with cosmetics without PBV in subjects with acne vulgaris. This study proved that PBV could be a good candidate compound for developing therapeutic drug for the treatment of acne vulgaris (Han et al. 2013). Also, bee venom has been used in treatment of polycystic ovary by its anti-inflammatory effect. It decreases levels of IL-6, COX-2, VEGF, and serum androgens and increased number of corpora lutea in Wistar rats (Karimzadeh et al. 2013). Furthermore, Honeybee venom (apitoxin) can be used as therapeutic option to lower blood glucose and lipids in diabetic rats (Mousavi et al. 2012). Another use of bee venom was in the treatment of multiple sclerosis. A preliminary human study showed that subjects treated with venom injection have improvement in neurological examinations with no side effects (Castro et al. 2005).
Considering the anti-cancer activity of bee venom, it is the most studied venom due mainly to two substances that have been isolated and characterized: melittin and phospholipase A2(PLA2) which are derived from the venom of species Apis mellifera (Ownby et al. 1997). Melittin is a small amphiphilic peptide containing 26 amino acid residues having the greatest antitumor activity (Gevod and Birdi 1984). Many cancer cells lines, including leukemia, renal, lung, liver, prostate, bladder, and mammary cancer cells, can be targets of melittin (Son et al. 2007). Moreover, Chueng (1982) has shown that melittin have the capablity of binding calmodulin, which has a role in cellular proliferation. Melittin have a powerful inhibitory effect on calmodulin activity, and so it is considered as an inhibitor of cell growth and clonogenicity of human and murine leukemic cells (Lee and Hait 1985). Another study by Gest and Salomon (1987) showed that melittin inhibits the melanotropin receptor in M2R melanoma cell membranes. Other mechanism of action of melittin is acting as pore-forming agent-killing malignant cells (Shaposhnikova et al. 1997). Arora et al. (1996) also reported that melittin, as a PLA2 activator, increased the calpain activity and cell necrosis in the hepatocellular carcinoma cell lines N1S1 and McA-RH7777. More recent studies have shown that melittin kills tumor cells by apoptosis through many cancer cell death mechanisms, together with the activation of caspase and matrix metalloproteinases (MMP) (Moon et al. 2006). Kollipara et al. (2014) suggested a new approach that can enhance the anti-cancer effect of bee venom on NSCLC cells at a much lower concentration by co-culture of bee venom with NK-92MI cells through inactivation of NF–κβ.
It is reported that bee venom significantly inhibited mammary carcinoma metastasis in mice (Orsolić et al. 2003), exhibited morphological changes typical of apoptotic cells on NCI-H1299 lung cancer cells Jang et al. (2003), inhibits proliferation and induces apoptosis of SMMC-7721 human hepatoma cells Hu et al. (2006), decreased the viability of human lymphoma cell line HL-60 and human lymphocytes after 24 h (Lee et al. 2007), induces apoptosis in human breast cancer MCF-7 cells (Ip et al. 2008a) and human cervical cancer Ca Ski cells (Ip et al. 2008b), potentiates the apoptotic effects of TRAIL (TNF-related apoptosis-inducing ligand) on human hepatocellular carcinoma (HCC) cells and may be a promising therapeutic approach in the treatment of TRAIL-resistant human cancer (Wang et al. 2009).
Caterpillar
Caterpillars are the larval form of members of the order Lepidoptera (the insect order comprising butterflies and moths) (Hossler 2009). The first isolation of caterpillar peptide was from the hemolymph of the giant silk moth, Hyalophora cecropia. This peptide named Cecropins and proved to have antimicrobial activity (Andreu et al. 1985). Cecropin exert strong antibiotic activity against both Gram-positive and Gram-negative bacteria in micromolar concentrations (Boman et al. 1991). Besides their antimicrobial activity, recent studies have demonstrated that Cecropin have anti-cancer activity against multiple tumor cell lines including mammalian leukemia, lymphoma, and colon carcinoma cell lines (Chen et al. 1997), more to the point, small cell lung cancer (Shin et al. 1997) and gastric cancer cells (Chan et al. 1998).
Also, Cecropins A and B (two well characterized members of the Cecropin-family) exert selective cytotoxic and antiproliferative efficacy in bladder cancer cells while sparing targets of benign murine or human fibroblast origin. Both peptides may offer novel therapeutic strategies for the treatment of bladder cancer with limited cytotoxic effects on benign cells, and thus, this is considered as promising novel therapeutic strategies for the treatment of bladder cancer with limited cytotoxic effects on benign cells (Suttmann et al. 2008). In vivo, Cecropin B improves survival of mice with ascitic colon adenocarcinomas (Moore et al. 1994). Transfection of human bladder cancer cells with Cecropin genes reduces their tumorigenicity in nude mouse models (Winder et al. 1998). Both cecropins A and B inhibit the viability and proliferation of bladder cancer cells, but with no effect on fibroblasts (Suttmann et al. 2008). Thus, these novel peptides offer new therapeutic strategies for the treatment of bladder cancer with limited cytotoxic effects on benign cells. Moreover, many active components from the venom of L. oblique have been isolated and characterized, including fibrinogenases (Pinto et al. 2006), hyaluronidases (Gouveia et al. 2005), a phospholipase A2 (Seibert et al. 2006), a factor X activator (Alvarez Flores et al. 2006), a prothrombin activator (Reis et al. 2001), and an antiapoptotic protein (Souza et al. 2005). Hyaluronidases, named lonogliases, have been identified from L. oblique venom (Gouveia et al. 2005). These hyaluronidases have been reported to affect cancer cell growth as well as tumor invasion; thus, they bear a potential as tools in studies of cancer cell biology (Matsushita and Okabi 2001) and in the pharmaceutical industry (Smith et al. 1997).
Lonomia obliqua caterpillar bristle extract (LOCBE) induces in vitro calcium-dependent pro-coagulant activity both in human and rat plasma (Kelen et al. 1995). It may be due to the activation of prothrombin and factor X, with no effects on platelets (Donato et al. 1998). In vivo, it causes blood incoagulability in laboratory animals (Reis et al. 1999). LOCBE proved also to be effective in preventing experimental venous thrombosis in rats, justifying further studies using purified fractions of the extract to clarify the mechanisms of this effect (Prezoto et al. 2002).
Wasp
Wasp stings cause severe pain and tissue damage and may be fatal (Argiolas and Pisano 1984). Although wasp stings may cause serious health problems, studies have paid attention to its bioactive compounds such as biogenic amines, peptides, and proteins (Nakajima et al. 1986). Mastoparan is a 14-amino-acid amphipathic peptide obtained from wasp venom (Zimmerberg and Parsegian 1986). It have a wide spectrum of pharmacological activities including mast cell degranulation (Hirai et al. 1979), activation of G-protein-mediated mechanisms (Ozaki et al. 1990), inhibition of calmodulin-mediated mechanisms (Wheeler-Jones et al. 1992), stimulation of phospholipases A2 and C (Wallace and Carter 1989), and stimulation or inhibition of cation-specific channels (Glavinovic et al. 1992). Mastoparan have been reported to have anti-cancer effect by inducing potent mitochondrial permeability transition in the concentration range between 5 and 100 mM, by forming a permeability transition pore (Pfeiffer et al. 1995). Based on this finding, Yamada et al. (2005) have encapsulated mastoparan with a transferrin-modified liposome with a pH-sensitive fusogenic peptide (GALA) for selective delivery to mithocondria in K562 cells—human chronic myelogenous leukemia. The encapsulated mastoparan was able to release cytochrome C in the cell line studied, indicating its potential use as an anticancer agent. Souza et al. (2009) isolated two novel mastoparan peptides, Polybia-MP-II and Polybia-MP-III, from venom of Polybia paulista wasp, which exhibited hemolytic activity on erythrocytes. Also, Wang et al. (2008) have reported that Polybia-MPI have anti-tumor activity. Polybia-MPI is able to target non polar lipid cell membranes, forming ion permeable channels, and leading to depolarization, irreversible cytolysis, and cell death (Matsuzaki et al. 1997).
Conclusion
Insect-based medicine has been long used in traditional medicine and is coming nowadays under increasing interest in biomedicine. Even though research into the science behind using helminthes and insects as therapies is proliferating, challenges and opportunities are still enormous. Moreover, bioactive molecules found in insect’s venoms can exert important pharmacological activities and will contribute extensively in Biomedical Sciences.
References
Abdelgabar AM, Bhowmick BK (2003) The return of the leech. Int J Clin Pract 57(2):103–105
Adams SL (1988) The medicinal leech. A page from the annelids of internal medicine. Ann Intern Med 109:399–405
Altman RD, Schultz DR, Collins-Yudiskas B, Aldrich J, Arnold PI, Brown HE (1984) The effects of a partially purified fraction of an ant venom in rheumatoid arthritis. Arthritis Rheum 27(3):277–278
Alvarez Flores MP, Fritzen M, Reis CV, Chudzinski-Tavassi AM (2006) Losac, a factor X activator from Lonomia obliqua bristle extract: its role in the pathophysiological mechanisms and cell survival. Biochem Biophys Res Commun 343:1216–1223
Amendt J, Campobasso CP, Gaudry E, Reiter C, LeBlanc HN, Hall MJR (2007) Best practice in forensic entomology—standards and guidelines. Int J Legal Med 121:90–104
Amendt J, Richards CS, Campobasso CP, Zehner R, Hall MJR (2011) Forensic entomology: applications and limitations. Forensic Sci Med Pathol 7:379–392
Andereya S, Stanzel S, Maus U (2008) Assessment of leech therapy for knee osteoarthritis: a randomized study. Acta Orthop 79:235–243
Andrade E, Villanova F, Borra P, Leite K, Troncone L, Cortez I, Messina L, Paranhos M, Claro J, Srougi M (2008) Penile erection induced in vivo by a purified toxin from the Brazilian spider Phoneutria nigriventer. BJU Int 102(7):835–837
Andreu D, Merrifield RB, Steiner H, Boman HG (1985) N-Terminal analogues of cecropin A: synthesis, antibacterial activity, and conformational properties. Biochemistry 24:1683–1688
Arbiser JL, Kau T, Konar M, Narra K, Ramchandran R, Summers SA, Vlahos CJ, Ye K, Perry BN, Matter W, Fischl A, Cook J, Silver PA, Bain J, Cohen P, Whitmire D, Furness S, Govindarajan B, Bowen JP (2007) Solenopsin, the alkaloidal component of the fire ant (Solenopsis invicta), is a naturally occurring inhibitor of phosphatidylinositol-3-kinase signaling and angiogenesis. Blood 109(2):560–565
Ardehali B, Hand K, Nduka C, Holmes A, Wood S (2006) Delayed leech-borne infection with Aeromonas hydrophilia in escharotic flap wound. J Plast Reconstr Aesthet Surg 59(1):94–95
Argiolas A, Pisano JJ (1984) Isolation and characterization of two new peptides, mastoparan C and crabrolin, from the venom of the European hornet, Vespa crabro. J Biol Chem 259:10106–10111
Armstrong DG, Salas P, Short B, Martin BR, Kimbriel HR, Nixon BP, Boulton AJ (2005) Maggot therapy in “lower-extremity hospice” wound care: fewer amputations and more antibiotic-free days. J Am Podiatr Med Assoc 95(3):254–257
Arora AS, De Groen PC, Croall DE, Emori Y, Gores GJ (1996) Hepatocellular carcinoma cells resist necrosis during anoxia by preventing phospholipase-mediated calpain activation. J Cell Physiol 167(3):434–442
Baer WS (1931) The treatment of chronic osteomyelitis with the maggot (larva of the blowfly). J Bone Jt Surg 13:438–475
Balamurugan M, Parthasarathi K, Cooper EL, Ranganathan LS (2007) Earthworm paste (Lampito mauritii, Kinberg) alters inflammatory, oxidative, haematological and serum biochemical indices of inflamed rat. Eur Rev Med Pharmacol Sci 11(2):77–90
Balamurugan M, Parthasarathi K, Cooper EL (2009) Anti-inflammatory and anti-pyretic activities of earthworm extract-Lampito mauritii (Kinberg). J Ethnopharmacol 121(2):330–332
Barbaro KC, Knysak I, Martins R, Hogan C, Winkel K (2005) Enzymatic characterization, antigenic cross-reactivity and neutralization of dermonecrotic activity of five Loxosceles spider venoms of medical importance in the Americas. Toxicon 45:489–499
Barla F, Higashijima H, Funai S, Sugimoto K, Harada N, Yamaji R, Fujita T, Nakano Y, Inui H (2009) Inhibitive effects of alkyl gallates on hyaluronidase and collagenase. Biosci Biotechnol Biochem 73:2335–2337
Barnard DR (1977) Skeletal-muscular mechanisms of the larva of Lucilia sericata (Meigen) in relation to feeding habit. Pan-Pac Entomol 53:223–229
Becker S, Terlau H (2008) Toxins from cone snails: properties, applications and biotechnological production. Appl Microbiol Biotechnol 79:1–9
Belokoneva OS, Villegas E, Corzo G, Dai L, Nakajima T (2003) The hemolytic activity of six arachnid cationic peptides is affected by the phosphatidylcholine-to-sphingomyelin ratio in lipid bilayers. Biochimica et Biophysica Acta-Biomembranes 1617(1–2):22–30
Bexfield A, Nigam Y, Thomas S, Ratcliffe NA (2004) Detection and partial characterisation of two antibacterial factors from the excretions/secretions of the medicinal maggot Lucilia sericata and their activity against methicillin-resistant Staphylococcus aureus (MRSA). Microbes Infect 6(14):1297–1304
Bingham JP, Mitsunaga E, Bergeron ZL (2010) Drugs from slugs—past, present and future perspectives of omega conotoxin research. Chem Biol Interact 183(1):1–18
Boman HG, Faye I, Gudmundsson GH, Lee JY, Lidholm DA (1991) Cell-free immunity in Cecropia. A model system for antibacterial proteins. Eur J Biochem 201:23–31
Bonn D (2000) Maggot therapy: an alternative for wound infection. Lancet 356(9236):1174
Bonnemain B (2005) Helix and drugs: snail for western health care from antiquity to the present. Evid Based Complement Alternat Med 2:25–28
Borges A, Silva S, Op den Camp HJ, Velasco E, Alvarez M, Alfonzo MJ, Jorquera A, De Sousa L, Delgado O (2006) In vitro leishmanicidal activity of Tityus discrepans scorpion venom. Parasitol Res 99:167–173
Bourel B, Fleurisse L, HÃdouin V, Cailliez JC, Creusy C, Gosset D, Goff ML (2001) Immunohistochemical contribution to the study of morphine metabolism in calliphoridae larvae and implications in forensic entomotoxicology. J Forensic Sci 46(3):596–599
Bowler PG, Davies BJ (1999) The microbiology of acute and chronic wounds. Wounds 11(4):72–78
Bowling FL, Salgami EV, Boulton AJ (2007) Larval therapy: a novel treatment in eliminating methicillin-resistant Staphylococcus aureus from diabetic foot ulcers. Diabetes Care 30(2):370–371
Broadhurst MJ, Leung JM, Kashyap V, McCune JM, Mahadevan U, McKerrow JH, Loke P (2010) IL-22+ CD4+ T cells are associated with therapeutic Trichuris trichiura infection in an ulcerative colitis patient. Sci Transl Med 2(60):60ra88
Bryson RW, Riddle BR, Graham MR, Smith BT, Prendini L (2013) As old as the hills: montane scorpions in Southwestern North America reveal ancient associations between bioticvdiversification and landscape history. PLoS One 8:e52822
Bubien JK, Ji HL, Gillespie GY, Fuller CM, Markert JM, Mapstone TB, Benos DJ (2004) Cation selectivity and inhibition of malignant glioma Na+ channels by Psalmotoxin 1. Am J Physiol-Cell Physiol 287(5 56-5):C1282–C1291
Buchman J, Blair JE (1932) Maggots and their use in the treatment of chronic osteomyelitis. Surg Gynecol Obstet 55:177–190
Campobasso CP, Di Vella G, Introna F (2001) Factors affecting decomposition and Diptera colonization. Forensic Sci Int 120:18–27
Carvalho LML (2010) Toxicology and forensic entomology. In: Amendt J, Campobasso CP, Goff ML, Grassberger M (eds) Current concepts in forensic entomology. Springer, Dordrecht, pp 163–178
Castro HJ, Mendez-Inocencio JI, Omidvar B, Omidvar J, Santilli J, Nielsen HS Jr, Pavot AP, Richert JR, Bellanti JA (2005) A phase I study of the safety of honeybee venom extract as a possible treatment for patients with progressive forms of multiple sclerosis. Allergy Asthma Proc 26(6):470–476
Catts EP (1992) Problems in estimating the post-mortem interval in death investigations. J Agric Entomol 9:245–255
Chaim OM, Trevisan-Silva D, Chaves-Moreira D, Wille ACM, Ferrer VP, Matsubara FH, Mangili OC, da Silveira RB, Gremski LH, Gremski W, Senff-Ribeiro A, Veig SS (2011) Brown spider (Loxosceles genus) venom toxins: tools for biological purposes. Toxins 3:309–344
Chambers L, Woodrow S, Brown AP, Harris PD, Phillips D, Hall M, Church JCT, Pritchard DI (2003) Degradation of extracellular matrix components by defined proteinases from the green bottle larva Lucilia sericata used for the clinical debridement of non-healing wounds. Br J Dermatol 148(1):14–23
Chan SC, Hui L, Chen HM (1998) Enhancement of the cytolytic effect of anti-bacterial cecropin by the microvilli of cancer cells. Anticancer Res 18:4467–4474
Chee KG, Longmate J, Quinn DI, Chatta G, Pinski J, Twardowski P, Pan CX, Cambio A, Evans CP, Gandara DR, Lara PN Jr (2007) The AKT inhibitor perifosine in biochemically recurrent prostate cancer: a phase II California/Pittsburgh cancer consortium trial. Clin Genitourinary Cancer 5(7):433–437
Chen HM, Wang W, Smith D, Chan SC (1997) Effects of the anti-bacterial peptide cecropin B and its analogs, cecropins B-1 and B-2, on liposomes, bacteria, and cancer cells. Biochim Biophys Acta 1336:171–179
Chen YJ, Shieh CJ, Tsai TH et al (2005) Inhibitory effect of norcantharidin, a derivative compound from blister beetles, on tumor invasion and metastasis in CT26 colorectal adenocarcinoma cells. Anticancer Drugs 16:293–299
Chen CT, Lin JC, Lu TW, Tsai FJ, Huang CY, Yao CH, Chen YS (2010) Earthworm extracts facilitate pc12 cell differentiation and promote axonal sprouting in peripheral nerve injury. Am J Chinese Med 38(3):547–560
Cheng KC, Lee HM, Shum SF, Yip CP (1990) A fatality due to the use of cantharides from Mylabris phalerata as an abortifacient. Med Sci Law 30:336–340
Cho BH, Ahn HB (1999) Microsurgical replantation of a partial ear, with leech therapy. Ann Plast Surg 43(4):427–429
Cho SY, Shim SR, Rhee HY, Park HJ, Jung WS, Moon SK, Park JM, Ko CN, Cho KH, Park SU (2012) Effectiveness of acupuncture and bee venom acupuncture in idiopathic Parkinson’s disease. Parkinsonism and Related Disord 18(8):948–952
Chueng WY (1982) Calmodulin: an overview. Fed Proc 41:2253–2257
Conduit R, Sasse A, Hodgson W, Trinder J, Veasey S, Tucker A (2007) A neurotoxinological approach to the treatment of obstructive sleep apnoea. Sleep Med Rev 11:361–375
Cooke A, Tonks P, Jones FM, O’Shea H, Hutchings P, Fulford AJC, Dunne DW (1999) Infection with Schistosoma mansoni prevents insulin dependent diabetes mellitus in non-obese diabetic mice. Parasite Immunol 21(4):169–176
Cooper EL, Ru B, Weng N (2004) Earthworms: sources of antimicrobial and anticancer molecules. Adv Exp Med Biol 546:359–389
Cooper PJ, Barreto ML, Rodrigues LC (2006) Human allergy and geohelminth infections: a review of the literature and a proposed conceptual model to guide the investigation of possible causal associations. Br Med Bull 79–80(1):203–218
Cooper EL, Balamurugan M, Huang CY, Tsao CR, Heredia J, Tommaseo-Ponzetta M, Paoletti MG (2012) Earthworms dilong: ancient, inexpensive, noncontroversial models may help clarify approaches to integrated medicine emphasizing neuroimmune systems. Evidence-based Complement Altern Med 2012:164152
Croese J, O’neil J, Masson J, Cooke S, Melrose W, Pritchard D, Speare R (2006) A proof of concept study establishing Necator americanus in Crohn’s patients patients and reservoir donors. Gut 55(1):136–137
da Silveira RB, Chaim OM, Mangili OC, Gremski W, Dietrich CP, Nader HB, Veiga SS (2007) Hyaluronidases in Loxosceles intermedia (Brown spider) venom are endo-beta-N-acetyl-d-hexosaminidases hydrolases. Toxicon 49:758–768
Daeschlein G, Mumcuoglu KY, Assadian O, Hoffmeister B, Kramer A (2007) In vitro antibacterial activity of Lucilia sericata maggot secretions. Skin Pharmacol Physiol 20:112–115
Darwish SF, El-Bakly WM, Arafa HM, El-Demerdash E (2013) Targeting TNF-a and NF-kB activation by bee venom: role in suppressing adjuvant induced arthritis and methotrexate hepatotoxicity in rats. Plos One 8(11):e79284
de Castro CS, Silvestre FG, Araujo SC, Gabriel de MY, Mangili OC, Cruz I, Chavez-Olortegui C, Kalapothakis E (2004) Identification and molecular cloning of insecticidal toxins from the venom of the brown spider Loxosceles intermedia. Toxicon 44:273–280
de Chalain TM (1996) Exploring the use of the medicinal leech: a clinical risk–benefit analysis. J Reconstr Microsurg 12:165–172
Deshane J, Garner CC, Sontheimer H (2003) Chlorotoxin inhibits glioma cell invasion via matrix metalloproteinase-2. J Biol Chem 278(6):4135–4144
Dittrich AM, Erbacher A, Specht S, Diesner F, Krokowski M, Avagyan A, Stock P, Ahrens B, Hoffmann WH, Hoerauf A, Hamelmann E (2008) Helminth infection with Litomosoides sigmodontis induces regulatory T cells and inhibits allergic sensitization, airway inflammation, and hyperreactivity in a murine asthma model. J Immunol 180(3):1792–1799
Donato JL, Moreno RA, Hyslop S, Duarte A, Antunes E, Le Bonniec BF, Rendu F, de Nucci G (1998) L. obliqua caterpillar spicules trigger human blood coagulation via activation of factor X and prothrombin. Thromb Haemost 79:539–542
Dorn DC, Kou CA, Png KJ, Moore MA (2009) The effect of cantharidins on leukemic stem cells. Int J Cancer 124:2186–2199
Duke RC, Witter RZ, Nash PB, Young JDE, Ojcius DM (1994) Cytolysis mediated by ionophores and pore-forming agents: role of intracellular calcium in apoptosis. FASEB J 8(2):237–246
Efferth T (2005) Mechanistic perspectives for 1,2,4-trioxanes in anti-cancer therapy. Drug Resist Updat 8:85–97
Efferth T, Rauh R, Kahl S, Tomicic M, Böchzelt H, Tome ME, Briehl MM, Bauer R, Kaina B (2005) Molecular modes of action of cantharidin in tumor cells. Biochem Pharmacol 1; 69(5):811–888
Eldor A, Orevi M, Rigbi M (1996) Role of the leech in medical therapeutics. Blood Rev 10:201–209
Ellenhorn MJ (1997) Ellenhorn’s medical toxicology: diagnosis and treatment of human poisoning, 2nd edn. Md Williams & Wilkins, Baltimore
Escoubas P (2006) Molecular diversification in spider venoms: a web of combinatorial peptide libraries. Mol Divers 10(4):545–554
Escoubas P, De Weille JR, Lecoq A, Diochot S, Waldmann R, Champigny G, Moinier D, Menez A, Lazdunski M (2000) Isolation of a tarantula toxin specific for a class of proton-gated Na+ channels. J Biol Chem 275(33):25116–25121
Etesse B, Beaudroit L, Deleuze M, Nouvellon E, Ripart J (2009) Hyaluronidase: here we go again. Ann Fr Anesth Reanim 28:658–665
Feng L, Gao R, Gopalakrishnakone P (2008) Isolation and characterization of a hyaluronidase from the venom of Chinese red scorpion Buthus martensi. Comp Biochem Physiol C Toxicol Pharmacol 148:250–257
Fields WS (1991) The history of leeching and hirudin. Haemostasis 21:3–10
Fine A, Alexander H (1934) Maggot therapy: technique and clinical application. J Bone Joint Surg Am 16:572–582
Fiołka MJ, Lewtak K, Rzymowska J, Grzywnowicz K, Hułas-Stasiak M, Sofińska-Chmiel W, Skrzypiec K (2013) Antifungal and anticancer effects of a polysaccharide-protein complex from the gut bacterium Raoultella ornithinolytica isolated from the earthworm Dendrobaena veneta. Pathog Dis 21
Fleischmann W, Russ M, Moch D, Marquardt C (1999) Biosurgery—maggots, are they really the better surgeons? Biochirurgie-Sind Fliegenmaden wirklich die besseren Chirurgen? 70(11):1340–1346
Foucher G, Henderson HR, Maneau M, Merie M, Braun FM (1981) Microsurgery. Int J Microsurg 3:265–270
Funt TR (1961) Cantharidin treatment of molluscum contagiosum. Arch Dermatol 83:504–505
Futrell JM (1992) Loxoscelism. Am J Med Sci 304:261–267
Gantenbein B, Largiadèr CR (2003) The phylogeographic importance of the Strait of Gibraltar as a gene flow barrier in terrestrial arthropods: a case study with the scorpion Buthus occitanus as model organism. Mol Phylogenet Evol 28:119–130
Gao L, Yu S, Wu Y, Shan B (2007) Effect of spider venom on cell apoptosis and necrosis rates in MCF-7 cells. DNA Cell Biol 26(7):485–489
Gao R, Zhang Y, Gopalakrishnakone P (2008) Purification and N-terminal sequence of a serine proteinase-like protein (BMK-CBP) from the venom of the Chinese scorpion (Buthus martensii Karsch). Toxicon 52(2):348–353
Gao F, Kiesewetter D, Chang L, Ma K, Rapoport SI, Igarashi M (2009) Whole-body synthesis secretion of docosahexaenoic acid from circulating eicosapentaenoic acid in unanesthetized rats. J Lipid Res 50:2463–2470
Garg SK, Croft AM, Bager P (2014) Helminth therapy (worms) for induction of remission in inflammatory bowel disease. Cochrane Database Syst Rev 20:1
Gest JE, Salomon Y (1987) Inhibition by melittin and fluphenazine of melanotropin receptor function and adenylate cyclase in M2R melanoma cell membranes. Endocrinology 121:1766–1772
Gevod VS, Birdi KS (1984) Melittin and the 8-26 fragment. Differences in ionophoric properties as measured by monolayer method. Biophys J 45(6):1079–1083
Girish KS, Kemparaju K (2007) The magic glue hyaluronan and its eraser hyaluronidase: a biological overview. Life Sci 80:1921–1943
Glavinovic MI, Joshi A, Trifaró JM (1992) Mastoparan blockade of currents through Ca(2+)-activated K+ channels in bovine chromaffin cells. Neurosciences 50:675–684
Godekmerdan A, Arusan S, Bayar B, Saglam N (2011) Medicinal leeches and hirudotherapy. Turkiye Parazitoloji Derg 35:234–239
Goolsby TV, Lombardo FA (2006) Extravasation of chemotherapeutic agents: prevention and treatment. Semin Oncol 33:139–143
Goudet C, Chi CW, Tytgat J (2002) An overview of toxins and genes from the venom of the Asian scorpion Buthus martensi Karsch. Toxicon 40(9):1239–1258
Gouveia AICB, Silveira RB, Nader HB, Dietrich CP, Gremski W, Veiga SS (2005) Identification and partial characterization of hyaluronidades in Lonomia obliqua venom. Toxicon 45:403–410
Gremski LH, da Silveira RB, Chaim OM, Probst CM, Ferrer VP, Nowatzki J, Weinschutz HC, Madeira HM, Gremski W, Nader HB, Senff-Ribeiro A, Veiga SS (2010) A novel expression profile of the Loxosceles intermedia spider venomous gland revealed by transcriptome analysis. Mol Biosyst 6:2403–2416
Gross MP, Apesos J (1992) The use of leeches for treatment of venous congestion of the nipple following breast surgery. Aesthetic Plast Surg 16:343–348
Gruden-Movsesijan A, Ilic N, Mostarica-Stojkovic M, Stosic-Grujicic S, Milic M, Sofronic-Milosavljevic L (2008) Trichinella spiralis: modulation of experimental autoimmune encephalomyelitis in DA rats. Exp Parasitol 118(4):641–647
Gupta SD, Gomes A, Debnath A, Saha A (2010) Apoptosis induction in human leukemic cells by a novel protein Bengalin, isolated from Indian black scorpion venom: through mitochondrial pathway and inhibition of heat shock proteins. Chem Biol Interact 183(2):293–303
Gwee MCE, Nirthanan S, Khoo HE, Gopalakrishnakone P, Kini RM, Cheah LS (2002) Autonomic effects of some scorpion venoms and toxins. Clin Exp Pharmacol Physiol 29(9):795–801
Haeberli S, Kuhn-Nentwig L, Schaller J, Nentwig W (2000) Characterisation of antibacterial activity of peptides isolated from the venom of the spider Cupiennius salei (Araneae: Ctenidae). Toxicon 38:373–380
Hahn BS, Jo YY, Yang KY, Wu SJ, Pyo MK, Yun-Choi HS, Kim YS (1997) Evaluation of the in vivo antithrombotic, anticoagulant and fibrinolytic activities of Lumbricus rubellus earthworm powder. Arch Pharm Res 20:17–23
Hait WN, Cadman E, Benz C, Cole J, Weiss B (1983) Inhibition of growth of L1210 cyclic leukemic cells by inhibitors of nucleotide phosphodiesterase and calmodulin. Cancer Res 2:5–9
Hama A, Sagen J (2009) Antinociceptive effects of the marine snail peptides conantokin-G and conotoxin MVIIA alone and in combination in rat models of pain. Neuropharmacology 56:556–563
Han SM, Lee KG, Pak SC (2013) Effects of cosmetics containing purified honeybee (Apis mellifera L.) venom on acne vulgaris. J Integr Med 11(5):320–326
Harris LG, Bexfield A, Nigam Y, Rohde H, Ratcliffe NA, Mack D (2009) Disruption of Staphylococcus epidermidis biofilms by medicinal maggot Lucilia sericata excretions/secretions. Int J Artif Organs 32:555–564
Haycraft JB (1884) On the action of secretion obtained from the medicinal leech on coagulation of the blood. Proc R Soc London 36:478
Higashijima T, Uzu S, Nakajima T, Ross EM (1988) Mastoparan, a peptide toxin from wasp venom, mimics receptors by activating GTP-binding regulatory proteins (G proteins). J Biol Chem 263(14):6491–6494
Hill TA, Stewart SG, Sauer B, Gilbert J, Ackland SP, Sakoff JA, McCluskey A (2007) Heterocyclic substituted cantharidin and norcantharidin analogues—synthesis, protein phosphatase (1 and 2A) inhibition, and anti-cancer activity. Bioorg Med Chem Lett 17:3392–3397
Hirai Y, Kuwada M, Yasuhara T, Yoshida H, Nakajima T (1979) A new mast cell degranulating peptide homologous to Mastoparan in the venom of Japanese hornet (Vespa xanthoptera). Chem Pharm Bull 27(8):1945–1946
Holle L, Song W, Holle E, Wei Y, Wagner T, Yu X (2003) A matrix metalloproteinase 2 cleavable melittin/avidin conjugate specifically targets tumor cells in vitro and in vivo. Int J Oncol 22:93–98
Horobin AJ, Shakesheff KM, Pritchard DI (2005) Maggots and wound healing: an investigation of the effects of secretions from Lucilia sericata larvae upon the migration of human dermal fibroblasts over a fibronectin-coated surface. Wound Repair Regen 13(4):422–433
Hossler EW (2009) Caterpillars and moths. Dermatol Ther 22(4):353–366
Hsieh CH, Chao KSC, Liao HF, Chen YJ (2013) Norcantharidin, derivative of cantharidin, for cancer stem cells. Evidence-based Complementary Altern Med 2013:838651
Hu H, Chen D, Li Y, Zhang X (2006) Effect of polypeptides in bee venom on growth inhibition and apoptosis induction of the human hepatoma cell line SMMC-7721 in-vitro and Balb/c nude mice in-vivo. J Pharm Pharmacol 58:83–89
Hunter MM, Wang A, Hirota CL, McKay DM (2005) Neutralizing anti-IL-10 antibody blocks the protective effect of tapeworm infection in a murine model of chemically induced colitis. J Immunol 174(11):7368–7375
Ikizceli I, Avsarogullari L, Sözüer E, Yürümez Y, Akdur O (2005) Bleeding due to a medicinal leech bite. Emerg Med J 22(6):458–460
Introna F, Campobasso CP, Goff ML (2001) Entomotoxicology. Forensic Sci Int 120:42–47
Ip SW, Liao SS, Lin SY, Lin JP, Yang JS, Lin ML, Chen GW, Lu HF, Lin MW, Han SM, Chung JG (2008a) The role of mitochondria in bee venom-induced apoptosis in human breast cancer MCF7 cells. In Vivo 22:237–245
Ip SW, Wei HC, Lin JP, Kuo HM, Liu KC, Hsu SC, Yang JS, Mei Dueyang Chiu TH, Han SM, Chung JG (2008b) Bee venom induced cell cycle arrest and apoptosis in human cervical epidermoid carcinoma Ca Ski cells. Anticancer Res 28:833–842
Jang MH, Shin MC, Lim S, Han SM, Park HJ, Shin I, Lee JS, Kim K, Kim EH, Kim CJ (2003) Bee venom induces apoptosis and inhibits expression of cyclooxygenase-2 mRNA in human lung cancer cell line NCI-H1299. J Pharmacol Sci 91:95–104
Jang HS, Chung HS, Ko E, Shin JS, Shin MK, Hong MC, Kim Y, Min BI, Bae H (2009) Microarray analysis of gene expression profiles in response to treatment with bee venom in lipopolysaccharide activated RAW 264.7 cells. J Ethnopharmacol 121(2):213–220
Joo SS, Won TJ, Kim JS, Yoo YM, Tak ES, Park SY, Park HY, Hwang KW, Park SC, Lee do I (2009) Inhibition of coagulation activation and inflammation by a novel Factor Xa inhibitor synthesized from the earthworm Eisenia andrei. Biol Pharm Bull 32:253–258
Joyce-Brady M, Rubins JB, Panchenko MP, Bernardo J, Steele MP, Kolm L, Simons ER, Dickey BF (1991) Mechanisms of mastoparan-stimulated surfactant secretion from isolated pulmonary alveolar type 2 cells. J Biol Chem 266:6859–6865
Kadioglu O, Kermani NS, Kelter G, Schumacher U, Fiebig HH, Greten HJ, Efferth T (2014) Pharmacogenomics of cantharidin in tumor cells. Biochem Pharmacol 87(3):399–409
Kalapothakis E, Chatzaki M, Goncalves-Dornelas H, de Castro CS, Silvestre FG, Laborne FV, de Moura JF, Veiga SS, Chavez-Olortegui C, Granier C, Barbaro KC (2007) The Loxtox protein family in Loxosceles intermedia (Mello-Leitao) venom. Toxicon 50:938–946
Kalender ME, Comez G, Sevinc A, Dirier A, Camci C (2010) Leech therapy for symptomatic relief of cancer pain. Pain Med 11:443–444
Karimzadeh L, Nabiuni M, Kouchesfehani HM, Adham H, Bagheri A, Sheikholeslami A (2013) Effect of bee venom on IL-6, COX-2 and VEGF levels in polycystic ovarian syndrome induced in Wistar rats by estradiol valerate. J Venomous Animals Toxins Including Tropical Dis 19:32
Kekre N, Griffin C, McNulty J, Pandey S (2005) Pancratistatin causes early activation of caspase-3 and the flipping of phosphatidyl serine followed by rapid apoptosis specifically in human lymphoma cells. Cancer Chemother Pharmacol 56(1):29–38
Kelen EMA, Picarelli ZP, Duarte AC (1995) Hemorrhagic syndrome induced by contact with caterpillars of the genus Lonomia (Saturniidae, Hemileucinae). J Toxicol Toxin Rev 14:283–308
Kerridge A, Lappin-Scott H, Stevens JR (2005) Antibacterial properties of larval secretions of the blowfly, Lucilia sericata. Med Vet Entomol 19:333–337
Khan WI, Blennerhasset PA, Varghese AK, Chowdhury SK, Omsted P, Deng Y, Collins SM (2002) Intestinal nematode infection ameliorates experimental colitis in mice. Infect Immun 70(11):5931–5937
Kim YS, Pyo MK, Park KM, Hahn BS, Yang KY, Yun-Choi HS (1998) Dose dependency of earthworm powder on antithrombotic and fibrinolytic effects. Arch Pharm Res 21:374–377
Kim JE, Koo KH, Kim YH, Sohn J, Park YG (2008) Identification of potential lung cancer biomarkers using an in vitro carcinogenesis model. Exp Mol Med 40:709–720
Kim YM, Ku MJ, Son YJ, Yun JM, Kim SH, Lee SY (2013) Anti-metastatic effect of cantharidin in A549 human lung cancer cells. Arch Pharm Res 36(4):479–484
Kini RM (2006) Anticoagulant proteins from snake venoms: structure, function and mechanism. Biochem J 397(3):377–387
Kintz P, Godelar A, Tracqui A, Mangin P, Lugnier AA, Chaumont AJ (1990) Fly larvae: a new toxicological method of investigation in forensic medicine. J Forensic Sci 35:204–207
Kollipara PS, Kim JH, Won D, Lee SM, Sung HC, Chang HS, Lee KT, Lee KS, Park MH, Song MJ, Song HS, Hong JT (2014) Co-culture with NK-92MI cells enhanced the anti-cancer effect of bee venom on NSCLC cells by inactivation of NF-κB. Arch Pharm Res 1:1
Kuhn-Nentwig L (2003) Antimicrobial and cytolytic peptides of venomous arthropods. Cell Mol Life Sci 60:2651–2668
Kuijk LM, Klaver EJ, Kooij G, van der Pol SMA, Heijnen P, Bruijns SCM, Kringel H, Pinelli E, Kraal G, de Vries HE, Dijkstra CD, Bouma G, van Die I (2012) Soluble helminth products suppress clinical signs in murine experimental autoimmune encephalomyelitis and differentially modulate human dendritic cell activation. Mol Immunol 51(2):210–218
Lee GL, Hait WN (1985) Inhibition of growth of C6 astrocytoma cells by inhibitors of calmodulin. Life Sci 36(4):347–354
Lee JD, Park HJ, Chae Y, Lim S (2005) An overview of bee venom acupuncture in the treatment of arthritis. Evidence-based Complement Altern Med 2(1):79–84
Lee YJ, Kang SJ, Kim BM, Kim YJ, Woo HD, Chung HW (2007) Cytotoxicity of honeybee (Apis mellifera) venom in normal human lymphocytes and HL-60 Cells. Chem Biol Interact 169:189–197
Lee YC, Lee LM, Yang CH, Lin AMY, Huang YC, Hsu CC, Chen MS, Chi CW, Yin PH, Kuo CD, Liao JF, Lee HC (2013) Norcantharidin suppresses cell growth and migration with enhanced anticancer activity of gefitinib and cisplatin in human non-small cell lung cancer cells. Oncol Rep 29(1):237–243
Li XH, Zhang DX, Xu JF, Wang WY, Du YM, Zhang Y, Lei YW, Zhang YX (2007) Effect of earthworm decoction on airway inflammation of bronchial asthma in guinea pigs. Zhongguo Zhong Yao Za Zhi 32(14):1445–1448
Li W, Li S, Zhong J, Zhu Z, Liu J, Wang W (2011) A novel antimicrobial peptide from skin secretions of the earthworm, Pheretima guillelmi (Michaelsen). Peptides 32(6):1146–1150
Lippens G (1995) NMR sequential assignments and solution structure of chlorotoxin, a small scorpion toxin that blocks chloride channels 4,5. Biochemistry 34(1):13–21
Liu D, Chen Z (2009) The effects of cantharidin and cantharidin derivates on tumour cells. Anticancer Agents Med Chem 9:392–396
Liu YF, Ma RL, Wang SL, Duan ZY, Zhang JH, Wu LJ, Wu CF (2003) Expression of an antitumor-analgesic peptide from the venom of Chinese scorpion Buthus martensii karsch in Escherichia coli. Protein Expr Purif 27:253–258
Liu XD, Zhang JL, Zheng HG, Liu FY, Chen Y (2008) Clinical randomized study of bee-sting therapy for rheumatoid arthritis. Zhen Ci Yan Jiu 33(3):197–200
Liu CH, Lin YM, Tang NY, Liu HJ, Huang CY, Hsieh CL (2013) Effect of oral administration of Pheretima Aspergillum (Earthworm) in rats with cerebral infarction induced by middle-cerebral artery occlusion. Afr J Tradis Complement Altern Med 10:66–82
Lokeshwar VB, Selzer MG (2008) Hyalurondiase: both a tumor promoter and suppressor. Semin Cancer Biol 18:281–287
Lubbers NL, Campbell TJ, Polakowski JS, Bulaj G, Layer RT, Moore J, Gross GJ, Cox BF (2005) Postischemic administration of CGX-1051, a peptide from cone snail venom, reduces infarct size in both rat and dog models of myocardial ischemia and reperfusion. J Cardiovasc Pharmacol 46:141–146
Luo C, Chen J, Li HL, Li JS (1998) Spatial and temporal expression of c-Fos protein in the spinal cord of anesthetized rat induced by subcutaneous bee venom injection. Brain Res 806:175–185
Ma Q, Geng Y, Xu W, Wu Y, He F, Shu W, Huang M, Du H, Li M (2009) The role of translationally controlled tumor protein in tumor growth and metastasis of colon adenocarcinoma cells. J Proteome Res 9:40–49
Mangan NE, Fallon RE, Smith P, Van Rooijen N, McKenzie AN, Fallon PG (2004) Helminth infection protects mice from anaphylaxis via IL-10-producing B cells. J Immunol 173(10):6346–6356
Mangan NE, Van Rooijen N, McKenzie ANJ, Fallon PG (2006) Helminth-modified pulmonary immune response protects mice from allergen-induced airway hyperresponsiveness. J Immunol 176(1):138–147
Marineau ML, Herrington MT, Swenor KM, Eron LJ (2011) Maggot debridement therapy in the treatment of complex diabetic wounds. Hawaii Med J 70(6):121–124
Markwardt F (1956) Studies on the mechanism of the anticoagulant effect of hirudin. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol 229:389–399
Markwardt F (2002) Hirudin as alternative anticoagulant—a historical review. Semin Tromb Hemost 28:405–414
Marsh N, Williams V (2005) Practical applications of snake venom toxins in haemostasis. Toxicon 45:1171–1181
Matausic-Pisl M, Tomicic M, Micek V, Grdisa M (2011) Influences of earthworm extract G-90 on haematological and haemostatic parameters in Wistar rats. Eur Rev Med Pharmacol Sci 15(1):71–78
Matsushita O, Okabi A (2001) Clostridial hydrolytic enzymes degrading extracellular components. Toxicon 39:1769–1780
Matsuzaki K, Sugishita K, Harada M, Fujii N, Miyajima K (1997) Interactions of an antimicrobial peptide, magainin 2, with outer and inner membranes of gram-negative bacteria. Biochim Biophys Acta 1327:119–130
McInnes IB, Leung BP, Harnett M, Gracie JA, Liew FY, Harnett W (2003) A novel therapeutic approach targeting articular inflammation using the filarial nematode-derived phosphorylcholine-containing glycoprotein ES-62. J Immunol 171(4):2127–2133
McKay DM (2008) The therapeutic helminth? Trends Parasitol 25:109–114
McKay DM (2009) The therapeutic helminth? Trends Parasitol 25(3):109–114
McLachlan A, Kekre N, McNulty J, Pandey (2005) Pancratistatin: a natural anti-cancer compound that targets mitochondria specifically in cancer cells to induce apoptosis. Apoptosis 10(3):619–630
Michalsen A, Klotz S, Ludtke R (2003) Effectiveness of leech therapy in osteoarthritis of the knee: a randomized, controlled trial. Ann Intern Med 139:724–730
Mihara H, Sumi H, Mizumoto H, Ikeda R, Seiki M, Maruyama M (1991) A novel fibrinolytic enzyme extracted from the earthworm, Lumbricus rubellus. Jpn J Physiol 41(3):461–472
Miljanich GP (2004) Ziconotide: neuronal calcium channel blocker for treating severe chronic pain. Curr Med Chem 11(23):3029–3040
Miller ML, Lord WD, Goff ML, Donnelly B, McDonough ET, Alexis JC (1994) Isolation of amitrptyline and nortriptyline from fly puparia (Phoridae) and beetle exuviae (Dermestidae) associated with mummified human remains. J Forensic Sci 39:1305–1313
Moller M, Gravenor MB, Roberts SE, Sun D, Gao P, Hopkin JM (2007) Genetic haplotypes of Th-2 immune signalling link allergy to enhanced protection to parasitic worms. Hum Mol Genet 16(15):1828–1836
Moon DO, Park SY, Heo MS, Kim KC, Park C, Ko WS (2006) Key regulators in bee venom-induced apoptosis are Bcl-2 and caspase-3 in human leukemic U937 cells through downregulation of ERK and Akt. Int Immunopharmacol 6(12):1796–1807
Moore AJ, Devine DA, Bibby MC (1994) Preliminary experimental anticancer activity of cecropins. Pept Res 7:265–269
Moreels TG, Nieuwendijk RJ, De Man JG, De Winter BY, Herman AG, Van Marck EA, Pelckmans PA (2004) Concurrent infection with Schistosoma mansoni attenuates inflammation induced changes in colonic morphology, cytokine levels, and smooth muscle contractility of trinitrobenzene sulphonic acid induced colitis in rats. Gut 53(1):99–107
Mortenson BW, Dawson KH, Murakami C (1998) Medicinal leeches used to salvage a traumatic nasal flap. Br J Oral Maxillofac Surg 36(6):462–464
Mortimer K, Brown A, Feary J, Jagger C, Lewis S, Antoniak M, Pritchard D, Britton J (2006) Dose-ranging study for trials of therapeutic infection with Necator americanus in humans. AmJTrop Med Hyg 75(5):914–920
Mousavi SM, Imani S, Haghighi S, Mousavi SE, Karimi A (2012) Effect of Iranian honey bee (apis mellifera) venom on blood glucose and insulin in diabetic rats. J Arthropod-Borne Dis 6(2):136–143
Moye VA, Cathcart S, Morrell DS (2014) Safety of cantharidin: a retrospective review of cantharidin treatment in 405 children with molluscum contagosum. Pediatr Dermatol 31(4):450–454
Muchmore DB, Vaughn DE (2010) Review of the mechanism of action and clinical efficacy of recombinant human hyaluronidase coadministration with current prandial insulin formulations. J Diabetes Sci Technol 4:419–428
Mumcuoglu KY (2001) Clinical applications for maggots in wound care. Am J Derm 2:219–227
Nakajima T, Uzu S, Wakamatsu K, Saito K, Miyazawa T, Yasuhara T, Tsukamoto Y, Fujino M (1986) Amphiphilic peptides in wasp venom. Biopolymers 25:115–121
Nam KW, Je KH, Lee JH, Han HJ, Lee HJ, Kang SK, Mar W (2003) Inhibition of COX-2 activity and proinflammatory cytokines (TNF-alpha and IL-1beta) production by water-soluble sub-fractionated parts from bee (Apis mellifera) venom. Arch Pharm Res 26(5):383–388
Namias N, Varela JE, Varas RP, Quintana O, Ward CG (2000) Biodebridement: a case report of maggot therapy for limb salvage after fourth-degree burns. J Burn Care Rehabil 21:254–257
Nolte KB, Pinder RD, Lord WD (1992) Insect larvae used to detect cocaine poisoning in a decomposed body. J Forensic Sci 37(4):1179–1185
Ori M, Ikeda H (1998) Spider venoms and spider toxins. J Toxicol Toxin Rev 17(3):405–426
Oršolić N (2012) Bee venom in cancer therapy. Cancer Metastasis Rev 31(1–2):173–194
Orsolić N, Sver L, Verstovsek S, Terzic S, Basic I (2003) Inhibition of mammary carcinoma cell proliferation in vitro and tumor growth in vivo by bee venom. Toxicon 41:861–870
Osada Y, Shimizu S, Kumagai T, Yamada S, Kanazawa T (2009) Schistosoma mansoni infection reduces severity of collagen-induced arthritis via down-regulation of pro-inflammatory mediators. Int J Parasitol 39(4):457–464
Ownby CL, Powell JR, Jiang MS, Fletcher JE (1997) Melittin and phospholipase A2 from bee (Apis mellifera) venom cause necrosis of murine skeletal muscle in vivo. Toxicon 35(1):67–80
Ozaki Y, Matsumoto Y, Yatomi Y, Higashirhara M, Kariya T, Kume S (1990) Mastoparan, a wasp venom, activates platelets via pertussis toxin-sensitive GTP-binding proteins. Biochem Biophys Res Commun 170:779–785
Pan R, Zhou Y, He HJ, He RQ (2011) An enzyme from the earthworm Eisenia fetida is not only a protease but also a deoxyribonuclease. Biochem Biophys Res Commun 407(1):113–117
Park SP, Kim BM, Koo JY, Cho H, Lee CH, Kim M, Na HS, Oh U (2008) A tarantula spider toxin, GsMTx4, reduces mechanical and neuropathic pain. PAIN 137(1):208–217
Pechter EA, Sherman RA (1983) Maggot therapy: the surgical metamorphosis. Plast Reconstr Surg 72(4):567–570
Perianin A, Snyderman R (1989) Mastoparan, a wasp venom peptide, identifies two discrete mechanisms for elevating cytosolic calcium and inositol trisphosphates in human polymorphonuclear leukocytes. J Immunol 143:1669–1673
Pfeiffer DR, Gudz TI, Novgorodov SA, Erdahl WL (1995) The peptide mastoparan is a potent facilitator of the mitochondrial permeability transition. J Biol Chem 270:4923–4932
Pien K, Laloup M, Pipeleers-Marichal M (2004) Toxicological data and growth characteristics of single post-feeding larvae and puparia of Calliphora vicina (Diptera: Calliphoridae) obtained from a controlled nordiazepam study. Int J Leg Med 118:190–193
Pinto AF, Silva KR, Guimarães JA (2006) Proteases from Lonomia obliqua venomous secretions: comparison of procoagulant, fibrin (ogen) olytic and amidolytic activities. Toxicon 47(1):113–121
Possani LD, Merino E, Corona M, Bolivar F, Becerril B (2000) Peptides and genes coding for scorpion toxins that affect ion-channels. Biochimie 82(9–10):861–868
Prakash M, Gunasekaran G (2010) Gastroprotective effect of earthworm paste (Lampito mauritii, Kinberg) on experimental gastric ulcer in rats. Eur Rev Med Pharmacol Sci 14(3):171–176
Prezoto BC, Maffei FHA, Mattar L, Chudzinski-Tavassi AM, Curi PR (2002) Antithrombotic effect of Lonomia obliqua caterpillar bristle extract on experimental venous thrombosis. Braz J Med Biol Res 35(6):703–712
Quintero-Hernández V, Jiménez-Vargas JM, Gurrola GB, Valdivia HH, Possani LD (2013) Scorpion venom components that affect ion-channels function. Toxicon 76:328–342
Rash LD, Hodgson WC (2002) Pharmacology and biochemistry of spider venoms. Toxicon 40:225–254
Rauh R, Kahl S, Boechzelt H (2007) Molecular biology of cantharidin in cancer cells. Chin Med 2:8
Reddy A, Fried B (2007) The use of Trichuris suis and other helminth therapies to treat Crohn’s disease. Parasitol Res 100(5):921–927
Reddy A, Fried B (2009) An update on the use of helminths to treat Crohn’s and other autoimmunune diseases. Parasitol Res 104(2):217–221
Reibe S, Doetinchem PV, Madea B (2010) A new simulation-based model for calculating post-mortem intervals using developmental data for Lucilia sericata (Dipt.: Calliphoridae). Parasitol Res 107(1):9–16
Reis CV, Kelen EMA, Farsky SHP, Portaro FCV, Sampaio CAM, Fernandes BL, Camargo ACM, Chudzinski-Tavassi AM (1999) A Ca2+ activated serine protease (LOPAP) could be responsible for the haemorrhagic syndrome caused by the caterpillar L. obliqua. Lancet 353:1942
Reis CV, Portaro FC, Andrade SA, Fritzen M, Fernandes BL, Sampaio CA, Camargo AC, Chudzinski-Tavassi AM (2001) A prothrombin activator serine protease from the Lonomia oblique caterpillar venom (Lopap) biochemical characterization. Thromb Res 102:427–436
Riede F, Koenen W, Goerdt S, Ehmke H, Faulhaber J (2010) Medicinal leeches for the treatment of venous congestion and hematoma after plastic reconstructive surgery. J Dtsch Dermatol Ges 8(11):881–888
Robinson W (1935) Stimulation of healing in non-healing wounds by allantoin occurring in maggot secretions and of wide biological distribution. J Bone Joint Surg 17:267–271
Robinson W (1940) Ammonium bicarbonate secreted by surgical maggots stimulates healing in purulent wounds. Am J Surg 47:111–115
Robinson W, Norwood VH (1933) The role of surgical maggots in the disinfection of osteomyelitis and other infected wounds. J Bone Joint Surg 15:409–412
Rocha FA, Leite AK, Pompeu MM, Cunha TM, Verri WA Jr, Soares FM, Castro RR, Cunha FQ (2008) Protective effect of an extract from Ascaris suum in experimental arthritis models. Infect Immun 76(6):2736–2745
Rodrigues EG, Dobroff AS, Cavarsan CF, Paschoalin T, Nimrichter L, Mortara RA, Santos EL, Fázio MA, Miranda A, Daffre S, Travassos LR (2008) Effective topical treatment of subcutaneous murine B16F10-Nex2 melanoma by the antimicrobial peptide gomesin. Neoplasia 10(1):61–68
Rook GAW (2009) Review series on helminths, immune modulation and the hygiene hypothesis: the broader implications of the hygiene hypothesis. Immunology 126(1):3–11
Ruyssers NE, De Winter BY, De Man JG, Ruyssers ND, Van Gils AJ, Loukas A, Pearson MS, Weinstock JV, Pelckmans PA, Moreels TG (2010) Schistosoma mansoni proteins attenuate gastrointestinal motility disturbances during experimental colitis in mice. World J Gastroenterol 16(6):703–712
Sales PB, Santoro ML (2008) Nucleotidase and DNase activities in Brazilian snake venoms. Comp Biochem Physiol C Toxicol Pharmacol 147:85–95
Schopf L, Luccioli S, Bundoc V, Justice P, Chan C-C, Wetzel BJ, Norris HH, Urban JF, Keane-Myers A (2005) Differential modulation of allergic eye disease by chronic and acute ascaris infection. Invest Ophthalmol Vis Sci 46(8):2772–2780
Schroeder FC, Taggi AE, Gronquist M, Malik RU, Grant JB, Eisner T, Meinwald J (2008) NMR spectroscopic screening of spider venom reveals sulfated nucleosides as major components for the brown recluse and related species. Proc Natl Acad Sci U S A 105:14283–14287
Seibert CS, Tanaka-Azevedo AM, Santoro ML, Mackessy SP, Soares Torquato RJ, Lebrun I, Tanaka AS, Sano-Martins IS (2006) Purification of a phospholypase A2 from Lonomia obliqua caterpillar. Biochem Biophys Res Commun 342(4):1027–1033
Senff-Ribeiro A, Henrique da Silva P, Chaim OM, Gremski LH, Paludo KS, Bertoni da Silveira R, Gremski W, Mangili OC, Veiga SS (2008) Biotechnological applications of brown spider (Loxosceles genus) venom toxins. Biotechnol Adv 26:210–218
Seo BK, Lee JH, Sung WS, Song EM, Jo DJ (2013) Bee venom acupuncture for the treatment of chronic low back pain: study protocol for a randomized, double-blinded, sham-controlled trial. Trials 14:16
Sewell D, Qing Z, Reinke E, Elliot D, Weinstock J, Sandor M, Fabry Z (2003) Immunomodulation of experimental autoimmune encephalomyelitis by helminth ova immunization. Int Immunol 15(1):59–69
Shaposhnikova VV, Egorova MV, Kudryavtsev AA, Levitman MK, Korystov YN (1997) The effect of melittin on proliferation and death of thymocytes. FEBS Lett 410(2–3):285–288
Sharma SV (1992) Melittin resistance: a counterselection for ras transformation. Oncogene 7(2):193–201
Sherman RA (2003) Maggot therapy for treating diabetic foot ulcers unresponsive to conventional therapy. Diabetes Care 26(2):446–451
Sherman RA (2009) Maggot therapy takes us back to the future of wound care: new and improved maggot therapy for the 21st century. J Diabetes Sci Technol 3(2):336–344
Sherman RA, Shimoda KJ (2004) Presurgical maggot debridement of soft tissue wounds is associated with decreased rates of postoperative infection. Clin Infect Dis 39(7):1067–1070
Sherman RA, Wyle FA, Vulpe M (1995) Maggot therapy for treating pressure ulcers in spinal cord injury patients. J Spinal Cord Med 18(2):71–74
Sherman RA, Hall MJR, Thomas S (2000) Medicinal maggots: an ancient remedy for some contemporary afflictions. Annu Rev Entomol 45(1):55–81
Sherman RA, Shapiro CE, Yang RM (2007) Maggot therapy for problematic wounds: uncommon and off-label applications. Adv Skin Wound Care 20(11):602–610
Shin SY, Lee MK, Kim KL, Hahm KS (1997) Structure-antitumor and hemolytic activity relationships of synthetic peptides derived from cecropin A-magainin 2 and cecropin A-melittin hybrid peptides. J Pept Res 50:279–285
Siedlakowski P, McLachlan-Burgess A, Griffin C, Tirumalai SS, McNulty J, Pandey S (2008) Synergy of pancratistatin and tamoxifen on breast cancer cells in inducing apoptosis by targeting mitochondria. Cancer Biol Ther 7(3):376–384
Slaby O, Sobkova K, Svoboda M, Garajova I, Fabian P, Hrstka R, Nenutil R, Sachlova M, Kocakova I, Michalek J, Smerdova T, Knoflickova D, Vyzula R (2009) Significant overexpression of Hsp110 gene during colorectal cancer progression. Oncol Rep 21:1235–1241
Smith KJ, Skelton HG, Turiansky G, Wagner KF (1997) Hyaluronidase enhances the therapeutic effect of vinblastine in intralesional treatment of Kaposis’s sarcoma. J Am Acad Dermatol 36:239–242
Smith P, Mangan NE, Walsh CM, Fallon RE, McKenzie ANJ, van Rooijen N, Fallon PG (2007) Infection with a helminth parasite prevents experimental colitis via a macrophage-mediated mechanism. J Immunol 178(7):4557–4566
Smits H, Everts B, Hartgers F, Yazdanbakhsh M (2010) Chronic helminth infections protect against allergic diseases by active regulatory processes. Curr Allergy Asthma Rep 10(1):3–12
Son DJ, Park MH, Chae SJ, Moon SO, Lee JW, Song HS, Moon DC, Kang SS, Kwon YE, Hong JT (2007) Inhibitory effect of snake venom toxin from Vipera lebetina turanica on hormone-refractory human prostate cancer cell growth: induction of apoptosis through inactivation of nuclear factor kappaB. Mol Cancer Ther 6(2):675–683
Sonoda Y, Hada N, Kaneda T, Suzuki T, Ohshio T, Takeda T, Kasahara T (2008) A synthetic glycosphingolipid-induced antiproliferative effect in melanoma cells is associated with suppression of FAK, Akt, and Erk activation. Biol Pharm Bull 31(6):1279–1283
Soroceanu L, Manning TJ Jr, Sontheimer H (1999) Modulation of glioma cell migration and invasion using Cl- and K+ ion channel blockers. J Neurosci 19(14):5942–5954
Souza AP, Peixoto CC, Maranga L, Carvalhal AV, Moraes RH, Mendonça RM, Pereira CA, Carrondo MJ, Mendonça RZ (2005) Purification and characterization of an anti-apoptotic protein isolated from Lonomia obliqua hemolymph. Biotechnol Prog 21:99–105
Souza BM, da Silva AV, Resende VM, Arcuri HA, Dos Santos Cabrera MP, Ruggiero Neto J, Palma MS (2009) Characterization of two novel polyfunctional mastoparan peptides from the venom of the social wasp Polybia paulista. Peptides 30:387–1395
Staats PS, Yearwood T, Charapata SG, Presley RW, Wallace MS, Byas-Smith M, Fisher R, Bryce DA, Mangieri EA, Luther RR, Mayo M, McGuire D, Ellis D (2004) Intrathecal ziconotide in the treatment of refractory pain in patients with cancer or AIDS: a randomized controlled trial. Jama 291:63–70
Stamenova PK, Marchetti T, Simeonov I (2001) Efficacy and safety of topical hirudin (Hirudex): a doubleblind, placebo-controlled study. Eur Rev Med Pharmacol Sci 5:37–42
Stix G (2005) A toxin against pain. Sci Am 292:88–93
Strigenz T (2014) Ziconotide. J Pain Palliat Care Pharmacother 28(1):73–74
Sukontason K, Narongchai P, Kanchai C, Vichairat K, Sribanditmongkol P, Bhoopat T, Kurahashi H, Chockjamsai M (2007) Forensic entomology cases in Thailand: a review of cases from 2000 to 2006. Parasitol Res 101:1417–1423
Summers RW, Elliott DE, Urban JF Jr, Thompson R, Weinstock JV (2005) Trichuris suis therapy for active ulcerative colitis: a randomized controlled trial. Gastroenterology 128(4):825–832
Sun ZJ, Liu XC, Sun LH, Song C (1997) Earthworm as a potential protein resource. Ecology of Food Nutr 36(2–4):221–236
Suttmann H, Retz M, Paulsen F, Harder J, Zwergel U, Kamradt J, Wullich B, Unteregger G, Stöckle M, Lehmann J (2008) Antimicrobial peptides of the Cecropin-family show potent antitumor activity against bladder cancer cells. BMC Urol 3:8
Thyssen P, Mara de Souza C, Shimamoto PM, de Britto Salewski T, Moretti TC (2014) Rates of development of immatures of three species of Chrysomya (Diptera: Calliphoridae) reared in different types of animal tissues: implications for estimating the postmortem interval. Parasitol Res 113(9):3373–3380
Tuncali D, Terzioglu A, Cigsar B, Aslan G (2004) The value of medical leeches in the treatment of class IIC ring avulsion injuries: report of 2 cases. J Hand Surg [Am] 29:943–946
Tuynder M, Fiucci G, Prieur S, Lespagnol A, GÃant A, Beaucourt S, Duflaut D, Besse S, Susini L, Cavarelli J, Moras D, Amson R, Telerman A (2004) Translationally controlled tumor protein is a target of tumor reversion. Proc Natl Acad Sci U S A 101(43):15364–15369
van de Loo A, Bode C (2002) Hirudin in acute coronary syndromes. Semin Tromb Hemost 28:459–466
van de Sande WWJ, Janse D-J, Hira V, Goedhart H, van der Zee R, Ahmed AOA, Ott A, Verbrugh H, van Belkum A (2006) Translationally controlled tumor protein from Madurella mycetomatis, a marker for tumorous mycetoma progression. J Immunol 177(3):1997–2005
van den Biggelaar AHJ, van Ree R, Rodrigues LC, Lell B, Deelder AM, Kremsner PG, Yazdanbakhsh M (2000) Decreased atopy in children infected with Schistosoma haematobium: a role for parasite-induced interleukin-10. Lancet 356(9243):1723–1727
van der Plas MJ, Jukema GN, Wai SW, Dogterom-Ballering HC, Lagendijk EL, van Gulpen C, van Dissel JT, Bloemberg GV, Nibbering PH (2008) Maggot excretions/secretions are differentially effective against biofilms of Staphylococcus aureus and Pseudomonas aeruginosa. J Antimicrob Chemother 61:117–122
Van Kruiningen HJMD, West BAMDF (2005) Potential danger in the medical use of Trichuris suis for the treatment of inflammatory bowel disease. (Letter). Inflamm Bowel Dis 11(5):515
Varanda EA, Tavares DC (1998) Radioprotection: mechanism and radioprotective agents including honey bee venom. Venom Anim Toxins 4(1):5–21
Varanda EA, Monti R, Tavares DC (1999) Inhibitory effect of propolis and bee venom on the mutagenicity of some direct- and indirect-acting mutagens. Teratog Carcinog Mutagen 19(6):403–413
Veiseh M, Gabikian P, Bahrami SB, Veiseh O, Zhang M, Hackman RC, Ravanpay AC, Stroud MR, Kusuma Y, Hansen SJ, Kwok D, Munoz NM, Sze RW, Grady WM, Greenberg NM, Ellenbogen RG, Olson JM (2007) Tumor paint: a chlorotoxin: Cy5.5 bioconjugate for intraoperative visualization of cancer foci. Cancer Res 67:6882–6888
Villanova FE, Andrade E, Ãl L, Andrade PM, Borra RC, Troncone LRP, Magalhaes L, Leite KRM, Paranhos M, Claro J, Srougi M (2009) Erection induced by Tx2-6 toxin of Phoneutria nigriventer spider: expression profile of genes in the nitric oxide pathway of penile tissue of mice. Toxicon 54(6):793–801
Vistnes LM, Lee R, Ksander GA (1981) Proteolytic activity of blowfly larvae secretions in experimental burns. Surgery 90(5):835–841
Vitale V, Battelli D, Gasperoni E, Monachese N (2008) Intrathecal therapy with ziconotide: clinical experience and considerations on its use. Minerva Anestesiol 74(12):727–733
von Rheinbaben F, Riebe O, Koehnlein J, Werner S (2014) Viral infection risks for patients using the finished product Hirudo verbana (medicinal leech). Parasitol Res 113(11):4199–4205
Wallace MA, Carter HR (1989) Effects of the wasp venom peptide, mastoparan, on a phosphoinositide-specific phospholipase C purified from rabbit brain membranes. Biochim Biophys Acta 1006:311–316
Walsh KP, Brady MT, Finlay CM, Boon L, Mills KH (2009) Infection with a helminth parasite attenuates autoimmunity through TGF-beta-mediated suppression of Th17 and Th1 responses. J Immunol 183(3):1577–1586
Walton RL, Beahm EK, Brown RE, Upton J, Reinke K, Fudem G, Banis J, Davidson J, Dabb R, Kalimuthu R, Kitzmiller WJ, Gottlieb LJ, Buncke HJ (1998) Microsurgical replantation of the lip: a multi-institutional experience. Plast Reconstr Surg 102(2):358–368
Wang GS (1989) Medical uses of mylabris in ancient China and recent studies. J Ethnopharmacol 26(2):147–162
Wang KR, Zhang BZ, Zhang W, Yan JX, Li J, Wang R (2008) Antitumor effects, cell selectivity and structure–activity relationship of a novel antimicrobial peptide polybia-MPI. Peptides 29:963–968
Wang Z, Wang W, Shao Z, Gao B, Li J, Ma J, Li J, Che H, Zhang W (2009) Eukaryotic expression and purification of anti-epilepsy peptide of Buthus martensii Karsch and its protein interactions. Mol Cell Biochem 330(1–2):97–104
Wayman J, Nirojogi V, Walker A, Sowinski A, Walker MA (2000) The cost effectiveness of larval therapy in venous ulcers. J Tissue Viability 10(3):91–94
Weil G, Simon R, Sweadner WR (1933) A biological, bacteriological and clinical study of larval or maggot therapy in the treatment of acute and chronic pyogenic infections. Am J Surg 19:36–48
Wheeler-Jones CPD, Saermark T, Kakkar V, Authi KS (1992) Mastoparan promotes exocytosis and increases intracellular cyclic AMP in human platelets. Evidence for the existence of a Ge-like mechanism of secretion. Biochem J 281:465–472
Whitaker IS, Elmiyeh B, Wright DJ (2003) Hirudo medicinalis: the need for prophylactic antibiotics. Plast Reconstr Surg 112(4):1185–1186
Whitaker IS, Rao J, Izadi D, Butler PE (2004) Historical Article: Hirudo medicinalis: ancient origins of, and trends in the use of medicinal leeches throughout history. Br J Oral Maxillofac Surg 42(2):133–137
Wilasrusmee C, Marjareonrungrung M, Eamkong S, Attia J, Poprom N, Jirasisrithum S, Thakkinstian A (2014) Maggot therapy for chronic ulcer: a retrospective cohort and a meta-analysis. Asian J Surg 37(3):138–147
Winder D, Gunzburg WH, Erfle V, Salmons B (1998) Expression of antimicrobial peptides has an antitumour effect in human cells. Biochem Biophys Res Commun 242:608–612
Wohlleben G, Trujillo C, MÃller J, Ritze Y, Grunewald S, Tatsch U, Erb KJ (2004) Helminth infection modulates the development of allergen-induced airway inflammation. Int Immunol 16(4):585–596
Yamada Y, Shinohara Y, Kakudo T, Chaki S, Futaki S, Kamiya H, Harashima H (2005) Mitochondrial delivery of mastoparan with transferring liposomes equipped with a pH-sensitive fusogenic peptide for selective cancer therapy. Int J Pharm 303:1–7
Yang S, Xiao Y, Kang D, Liu J, Li Y, Undheim EA, Klint JK, Rong M, Lai R, King GF (2013) Discovery of a selective NaV1.7 inhibitor from centipede venom with analgesic efficacy exceeding morphine in rodent pain models. Proc Natl Acad Sci U S A 110(43):17534–17539
Yoon SY, Kwon YB, Kim HW, Roh DH, Seo HS, Han HJ, Lee HJ, Beitz AJ, Lee JH (2008) Bee venom injection produces a peripheral anti-inflammatory effect by activation of a nitric oxide-dependent spinocoeruleus pathway. Neurosci Lett 430:163–168
Zaidi SM, Jameel SS, Zaman F, Jilani S, Sultana A, Khan SA (2011) A systematic overview of the medicinal importance of sanguivorous leeches. Altern Med Rev 16(1):59–65
Zhang SJ, Yang XM, Liu GS, Cohen MV, Pemberton K, Downey JM (2003) CGX-1051, a peptide from conus snail venom, attenuates infarction in rabbit hearts when administered at reperfusion. J Cardiovasc Pharmacol 42:764–771
Zheng LH, Bao YL, Wu Y, Yu CL, Meng X, Li YX (2008) Cantharidin reverses multidrug resistance of human hepatoma HepG2/ADM cells via down-regulation of P-glycoprotein expression. Cancer Lett 272:102–109
Zhu W-L, Cheng H-X, Han NA, Liu D-L, Zhu W-X, Fan B-L, Duan F-L (2008) Messenger RNA Expression of translationally controlled tumor protein (TCTP) in liver regeneration and cancer. Anticancer Res 28(3A):1575–1580
Ziffren SE, Heist HE, May SC, Womack NA (1953) The secretion of collagenase by maggots and its implication. Ann Surg 138(6):932–934
Zimmerberg J, Parsegian VA (1986) Polymer inaccessible volume changes during opening and closing of a voltage-dependent ionic channel. Nature 323(6083):36–39
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
El-Tantawy, N.L. Helminthes and insects: maladies or therapies. Parasitol Res 114, 359–377 (2015). https://doi.org/10.1007/s00436-014-4260-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-014-4260-7