Abstract
The prevalences of heteroxenous parasites are influenced by the interplay of three main actors: hosts, vectors, and the parasites themselves. We studied blood protists in the nesting populations of raptors in two different areas of the Czech Republic. Altogether, 788 nestlings and 258 adult Eurasian sparrowhawks (Accipiter nisus) and 321 nestlings and 86 adult common buzzards (Buteo buteo) were screened for parasites by the microscopic examination of blood smears and by cultivation. We examined the role of shared vectors and parasite phylogenetic relationships on the occurrence of parasites. In different years and hosts, trypanosome prevalence ranged between 1.9 and 87.2 %, that of Leucocytozoon between 1.9 and 100 %, and Haemoproteus between 0 and 72.7 %. Coinfections with Leucocytozoon and Trypanosoma, phylogenetically distant parasites but both transmitted by blackflies (Simuliidae), were more frequent than coinfections with Leucocytozoon and Haemoproteus, phylogenetically closely related parasites transmitted by different vectors (blackflies and biting midges (Ceratopogonidae), respectively). For example, 16.6 % buzzard nestlings were coinfected with Trypanosoma and Leucocytozoon, while only 4.8 % with Leucocytozoon and Haemoproteus and 0.3 % with Trypanosoma and Haemoproteus. Nestlings in the same nest tended to have the same infection status. Furthermore, prevalence increased with the age of nestlings and with Julian date, while brood size had only a weak negative/positive effect on prevalence at the individual/brood level. Prevalences in a particular avian host species also varied between study sites and years. All these factors should thus be considered while comparing prevalences from different studies, the impact of vectors being the most important. We conclude that phylogenetically unrelated parasites that share the same vectors tend to have similar distributions within the host populations of two different raptor species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Trypanosomatids and haemosporidians are relatively common parasites of birds. While haemosporidians (Haemospororida: Plasmodium, Leucocytozoon, and Haemoproteus) are well studied due to their relatively simple diagnosis, avian trypanosomes (Trypanosoma; Kinetoplastea, Trypanosomatida) are rarely investigated, or inappropriate diagnostic methods are used, despite the fact that their prevalences are comparable to haemosporidia. Although haemosporidians (Alveolata) and trypanosomes (Excavata) are phylogenetically unrelated, their dixenous life cycles share some similarities. These parasites multiply mainly in avian inner organs, whereas developmental stages predisposed for transmission to vectors are found in the peripheral blood. After ingestion by dipteran blood-sucking vectors, namely blackflies (Simuliidae), biting midges (Ceratopogonidae), mosquitoes (Culicidae), or hippoboscid flies (Hippoboscidae), parasites develop infective stages that are transmitted to a subsequent bird host during the next blood feeding.
In addition to parasite taxonomic bias, studies on blood parasites are also biased toward passerines, due to their simple trapping and handling. Studies on raptors (Falconiformes) have been mostly performed on migrating, captive, or injured birds (Krone et al. 2001, 2008; Valkiunas 2005; Sehgal et al. 2006; Outlaw and Ricklefs 2009), and generally less attention has been paid to breeding populations (Ashford et al. 1990, 1991). The studies of factors influencing the prevalences of trypanosomes and haemosporidians in the breeding populations of raptors have several advantages. Newly hatched nestlings are parasite free, and therefore susceptible to infection, parasites are acquired at the study site, the acute phase of infection has no effect on sampling since nestlings are immobile, and seasonal parasitemias are high (Valkiunas 2005).
Detailed studies aimed at the identification of avian trypanosome species are rather limited; most trypanosomes isolated from raptors to date belong to the Trypanosoma avium group, which is transmitted by blackflies (Diptera, Simuliidae; (Bennett 1961; Votypka and Svobodova 2004)). More rarely, raptors can also harbor Trypanosoma corvi, which is transmitted by hippoboscid flies (Diptera, Hippoboscidae), and Trypanosoma bennetti, whose vector is unknown (Baker 1956a; Votýpka et al. 2004; Kirkpatrick et al. 1986; Zídková et al. 2012).
Leucocytozoon species are transmitted to raptors by blackflies. Leucocytozoon toddi is the only species that has been reported from raptors; however, these likely actually belong to at least two species: Leucocytozoon mathisi infecting sparrowhawks and Leucocytozoon buteonis infecting buzzards (Valkiunas et al. 2010). Haemoproteus (Parahaemoproteus) species are transmitted by biting midges (Diptera: Ceratopogonidae) (Valkiunas 2005; Martinsen et al. 2008). Five species of Haemoproteus have been reported from holarctic raptors, among which Haemoproteus nisi and Haemoproteus buteonis are widespread, Haemoproteus elani is rare, and two species occur in falconids only (Valkiunas 2005). Molecular typing reveals a higher diversity of avian haemosporidia. According to the MalAvi database, out of 29 different Leucocytozoon haplotypes from the order Falconiformes, two have been found in Buteo buteo and 4 in Accipiter nisus (plus 16 and 6 in other members of the genera). None of 11 Haemoproteus haplotypes detected in raptors (Falconiformes) originate from B. buteo or A. nisus, and just one from the genus Buteo.
The co-occurrence of parasites of different taxa within the same host raises the important question whether the prevalences are affected (i) by the vectors involved in parasite transmission or (ii) by the phylogenetic relationship of parasites. Furthermore, other factors such as nestling age, sampling date, number of siblings, home range of avian host, year, and locality may all influence parasite prevalences.
We expect that due to prolonged exposure, the prevalence of blood parasites in nestlings increases with age and that due to the seasonally increasing occurrence of (infected) vectors, later broods have a greater probability of infection. We also expect that bigger broods should have higher parasite prevalences, because the higher biomass increases the attractiveness for vectors. We evaluated whether these factors similarly influence the prevalences of blood parasites that are either phylogenetically related but transmitted by different vectors (Haemoproteus transmitted by ceratopogonids vs. Leucocytozoon transmitted by blackflies) or unrelated but transmitted by the same vectors (trypanosomes and Leucocytozoon, both transmitted by blackflies).
Material and methods
Statement of animal rights
This study was carried out in accordance with the Czech national law. The protocol was approved by the Committee on the Ethics of Animal Experiments of the Faculty of Science, Charles University in Prague. Experiments were performed by licensed workers. Licensed ringers had permission to visit nests and to catch wild birds, including the sparrowhawk (specially protected species, Act N. 114/1992). Four of the authors (MS, LP, PV, PV) were licensed ringers during the study period, and at least one of them took part in each field sampling. The license was issued by the Ringing Centre of the National Museum in Prague.
Sampling
Blood samples from raptors (125 μl) were taken from the brachial vein using a tuberculin syringe. Adult raptors were trapped close to their active nests using mist nets and a stuffed eagle owl (Bubo bubo) as a decoy. Nestlings were sampled directly at the nests. Authors MS and P. Volf had licenses to perform and control experiments on animals, issued by the Central Committee for Animal protection, at the time of the study.
Study area and field work
The breeding populations of raptors and the prevalence of their blood parasites were studied in the following areas of the Czech Republic:
-
1.
Prague, Eurasian sparrowhawk (A. nisus)
An urban sparrowhawk population in the City of Prague (49.99–50.14 N, 14.30–14.62 E). The total area is ca. 500 km2 and covers the area of the city and its suburbs. Habitats include built-up areas, parks, gardens, and orchards as well as small tracks of woodland. Sparrowhawks breed in trees, usually in parks and gardens, across the whole area including the city center. The population size has been estimated at 90 to 120 breeding pairs (Peške 1990). The population was sampled during the breeding seasons 1996–2001 (mid-June to July). Ages of sparrowhawk nestlings were assessed according to the development of feathers (Peške 1987). The average age of sampled sparrowhawk nestlings was 22.3 days (SD = 4.6, range 9–32, n = 774).
-
2.
Prague suburbs, common buzzard (B. buteo)
A common buzzard population in the eastern suburbs of the City of Prague (49.94–50.00 N, 14.57–14.65 E). The total area is 60 km2 and is characterized by extensive farmland with scattered small tracks of woodland, tree lines, and solitary trees, with dense human settlement and a dense network of roads. Buzzards breed in trees, usually in small tracks of woodland but also in tree lines along streams and sometimes also in solitary trees. The population size has been estimated at 15–20 breeding pairs (J. Matouš, pers. comm.) The population was sampled during the breeding seasons 2000–2002 (early May to June). Ages of common buzzard nestlings were estimated using measurements of the forearm or wing length (Voříšek and Lacina 1998). The average age of buzzard nestlings was 26.4 days (SD = 5.3, range 16–35, n = 61).
-
3.
South Moravia, common buzzard (B. buteo)
A common buzzard population in the forest complex “Milovický les” located in the Protected Landscape Area and Biosphere Reserve Pálava (48.48–48.51 N, 16.39–16.44 E). The study area is a 22-km2 oak-hornbeam forest complex surrounded by intensively managed farmland. The population density of common buzzard is high in the area, with 32 to 51 breeding pairs confirmed in 1993 to 1995 (Voříšek 2000). Two game preserves for red and fallow deer (Cervus elaphus, Dama dama), and fallow deer and mouflon (Ovis orientalis), respectively, comprise the majority of the forest. Nearly 20 % of the total forest area is open habitats (e.g., clear cuts, fields). The population was sampled during the breeding seasons 1996–2001 (early May to June). The average age of buzzard nestlings was 24.1 days (SD = 6.3, range 10–39, n = 245).
The active nests of both raptor species were searched for by a combination of systematic searches for nests (complete census) and observations of adults attending the nests with nest material or food. The nests were directly inspected, in most cases, several times during the breeding season. All nests found were marked on detailed maps allowing a determination of individual home ranges.
Since different raptor species have different habitat preferences, their populations were sampled in different habitats, and any between-species comparison of prevalence would be misleading. Hence, we focus on variation in the prevalence within host species.
Diagnostic methods
Blood samples from raptors were acquired, and trypanosome cultivation was done as described elsewhere (Votýpka et al. 2002). Briefly, 125 μl of blood was taken from the brachial vein using a tuberculin syringe. Blood smears were air dried, fixed with methanol, and stained with Giemsa (Sigma). Microscopic examination was performed at ×1000 magnification for 10 min, which in our hands corresponds to approximately 30,000 erythrocytes. Additionally, slides were checked at ×160 magnification for 5 min, to find Leucocytozoon infections of low intensity. We have identified parasites to the generic level, since the splitting of the data to genetical lineages would decrease the statistical power of our analyses.
Data analysis
As this is an exploratory study, we focused on detecting patterns in the data and estimating effect sizes, rather than on formal hypothesis testing. Raw prevalence was calculated as the proportion of positive sampling units, either individuals (nestlings, adults) or broods. A whole brood was classified as positive if ≥1 nestling in the brood was positive.
The frequency of coinfections with different parasite genera was evaluated by constructing a 23 contingency table (Trypanosoma × Leucocytozoon × Haemoproteus). The eight-cell frequencies (with an added constant of 0.5) represented the response variable in a Poisson regression model (GLM with log link and Poisson error distribution) fitted separately to each host category. We first fitted the full model to check for a three-way interaction (interpreted as triple associations among parasites). Next, we removed the three-way interaction and examined the reduced model with all two-way interactions (interpreted as double associations between parasites). All parameters were estimated by the maximum likelihood method implemented in Proc Genmod (SAS Institute, Inc. 2013).
The effects of multiple predictor variables on the infection status of individual nestlings or broods (coded 1 = positive, 0 = negative) were evaluated by fitting a logistic mixed model (GLMM with logit link and binomial error distribution) to each host-parasite combination. The prevalence of Haemoproteus in sparrowhawk nestlings was too low for analysis. We present regression coefficients (on logit scale) estimated by the full model containing all predictor variables that were a priori deemed important. The fixed effect predictors were brood size (1–6 nestlings), nestling age (9–37 days), sampling date entered as a Julian date (130–202), and sampling locality (S. Moravia vs. Prague; analyzed only for buzzards). The random effects were year and brood identity (in models for individual nestlings) or year and home range identity (in models for whole broods). For random effects, we modeled random intercepts and assumed variance component variance-covariance structure. All parameters were estimated by the residual pseudo-likelihood method implemented in Proc Glimmix (SAS Institute, Inc. 2013). To visualize model predictions, we plotted prevalence against nestling age/Julian date while keeping the other variables fixed at a modal value (brood size) or at about the midpoint of the observed range of values (age/date).
Results
Prevalence of blood parasite genera
We detected three genera of blood parasites in the studied raptor populations: Trypanosoma, Leucocytozoon, and Haemoproteus (Table 1). Plasmodium and microfilariae were not found. Trypanosome infections were first detected in a 14-day-old sparrowhawk and 19-day-old buzzard; Leucocytozoon was first detected in a 16-day-old sparrowhawk and 17-day-old buzzard and Haemoproteus in a 23-day-old sparrowhawk and 22-day-old buzzard.
Coinfections with different parasite genera
Combined infections were checked in individual nestlings, whole broods, and adults (Table 2). We did not find any triple association among all three parasite genera in any host category (Table 3). Of the three possible double parasite combinations, we found a consistent positive association between trypanosome and Leucocytozoon in all host categories except in adult sparrowhawks, where the effect was still positive, but weaker. A positive association between Haemoproteus and Leucocytozoon was less consistent (Table 3).
Factors affecting prevalence in nestlings of buzzards and sparrowhawks
Brood size, nestling age, Julian date, year, and study locality were evaluated for their effects on Trypanosoma, Leucocytozoon, and Haemoproteus prevalences. The effects were evaluated for individual nestlings (Table 4) and whole broods (Table 5).
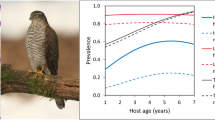
We found only weak, yet consistent, effects of brood size—prevalences at the level of individual nestlings tended to decrease with increasing brood size (Table 4), while prevalences at the level of whole broods tended to increase with brood size (Table 5). The prevalences of all parasite genera consistently increased with host age (Fig. 1) and with Julian date (Fig. 2) at both the nestling (Table 4) and brood (Table 5) levels. Prevalences differed markedly between the two studied buzzard populations, Haemoproteus being more prevalent in S. Moravia, while Leucocytozoon and trypanosomes were more prevalent in Prague.
Age-dependent variation in parasite prevalence in raptor nestlings. Prevalence was modeled using logistic mixed models fitted separately for each host-parasite combination (see Table 4 for model parameter estimates). The data unit was an individual nestling. The fixed effect predictors were brood size, nestling age, Julian date, and locality (only in buzzard); the random effects were year and brood identity. The prevalence predicted by the models for an observed range of nestling ages is plotted, with brood size set to the modal value (2 and 4 nestlings in buzzard and sparrowhawk, respectively) and Julian date set to the midpoint of observed range of dates (June 4 in buzzard, June 24 in sparrowhawk), separately for both localities; a buzzard in Prague, b buzzard in South Moravia, and c sparrowhawk in Prague. The prevalence of Haemoproteus in sparrowhawk nestlings was too low for analysis (see Table 1 for overall prevalence values)
Seasonal variation in parasite prevalence in raptor nestlings. Prevalence was modeled using logistic mixed models fitted separately for each host-parasite combination (see Table 4 for model parameter estimates). The data unit was an individual nestling. The fixed effect predictors were brood size, nestling age, Julian date, and locality (only in buzzard); the random effects were year and brood identity. The prevalence predicted by the models for an observed range of Julian dates is plotted, with brood size set to the modal value (2 and 4 nestlings in buzzard and sparrowhawk, respectively) and nestling age set to the midpoint of observed range of ages (25 days in both host species), separately for both localities; a buzzard in Prague, b buzzard in South Moravia, and c sparrowhawk in Prague. The prevalence of Haemoproteus in sparrowhawk nestlings was too low for analysis (see Table 1 for overall prevalence values)
After accounting for the variation due to the above fixed effects, there was still a detectable component of variation in prevalences among the annual samples in some host-parasite combinations (Table 1) when the year was treated as a random effect (Tables 4 and 5). The consistent variation in prevalences among broods (except for Haemoproteus, possibly due to low prevalence; Table 4) indicates that the infection status of individual nestlings within a brood was correlated (i.e., not independent). On the contrary, we did not find clear support for a consistent variation in prevalences among individual home ranges (Table 5), suggesting that the infection status of individual broods within a home-range (across years) was not correlated.
Discussion
Prevalence of blood parasite genera
The prevalences of all three parasite genera (Trypanosoma, Leucocytozoon, and Haemoproteus) detected in the studied raptor populations were consistently higher in adults than in nestlings. This seems expectable, since the exposure to vectors increases with age. Moreover, some infections might remain undetected at the time of sampling, due to the prepatent period (the time from infection to the demonstration of the parasite in the blood). This will cause higher bias in nestling samples due to their low age, sometimes even comparable to the prepatent period. On the other hand, some infections in adults may be cleared, or there may be parasite-related mortality. The higher prevalence in adults might therefore suggest that the infections are lifelong, chronically patent, and no significant parasite-related mortality occurs. However, the repeated sampling of adult sparrowhawks revealed that while Trypanosoma infections seem to be lifelong, Haemoproteus and Leucocytozoon infection status varies in both directions, although the overall prevalences in the studied population remains stable (Votýpka et al., unpublished observations; see also Synek et al. 2013a). The majority of numerous studies comparing young and adult hosts detected the higher prevalences of haemosporidians in adults (Valkiunas 2005). Studies on trypanosomes are scarce, and no differences between passerine age groups have been detected; however, trypanosomes have typically been diagnosed on slides (Merino and Potti 1995; Deviche et al. 2010), and thus probably underestimated (Apanius 1991).
The prevalence of Leucocytozoon in nestlings was higher than that of Haemoproteus. While comparing the prevalences of different parasites, one has to bear in mind that the detectability of the infection is influenced, among other factors, by the prepatent period. The youngest sparrowhawks reported to be infected with Leucocytozoon were about 12 days old (Ashford et al. 1991). We detected Leucocytozoon in a 16-day-old sparrowhawk and a 17-day-old buzzard. While data on the prepatent period of Haemoproteus in raptors are lacking, in other bird species, it ranges from 11 days to 3 weeks (Valkiunas 2005; Atkinson 2008). For Haemoproteus, the youngest birds in which we detected infection were a 22-day-old buzzard and 23-day-old sparrowhawk. Although sparrowhawks were sampled later in the season, the host age at sampling was similar for buzzards and sparrowhawks. Interestingly, in both avian hosts, Haemoproteus is detected at a more advanced age than that of Leucocytozoon, suggesting that the prepatent period is longer for Haemoproteus than Leucocytozoon species in raptors.
The youngest bird with a trypanosome infection detected in this study was a 14-day-old sparrowhawk and a 19-day-old buzzard. The prepatent period of avian trypanosomes is possibly only 18–24 h, at least for T. corvi (Baker 1956b). However, buzzards were sampled earlier in the season, and their more advanced age at first detection may relate to the seasonal availability of infected vectors. The development of infective forms in vectors is affected by temperature, lasting about 5 days at 24 °C and longer at lower temperatures (Bennett 1961).
We used microscopy to diagnose haemosporidia infections. It has been shown that microscopy is just as sensitive as PCR diagnosis (e.g., Krone et al. 2008); moreover, PCR underestimates mixed infections (Valkiunas et al. 2006) and can give false-positive results due to the detection of sporozoites in the blood that may not represent specific parasites (Valkiunas et al. 2009). Generally, we do not expect any significant bias in the results due to the diagnostic methods used.
We have identified the parasites to the generic level, since identification into a level of species or strain was not required given the purpose of our study. Our main aim was to show that phylogenetically unrelated parasites that share the same vectors (Trypanosoma and Leucocytozoon) show similar prevalence patterns while closely related Haemoproteus and Leucocytozoon (which have different vectors) have different prevalences. Splitting of the data set would also decrease the statistical power of our analyses to detect patterns in prevalences.
Coinfection with different parasite genera
As expected, there was a strong positive association between Trypanosoma and Leucocytozoon in both nestlings and whole broods of buzzards and sparrowhawks and in adult buzzards. We explain this pattern by their transmission by the same vectors. Most raptors (over 90 %) in our studied populations were infected with trypanosomes of the “T. avium” group (Zídková et al. 2012), which are transmitted by blackflies of the subgenus Eusimulium (Votýpka et al. 2002; Votýpka and Svobodová 2004; Zídková et al. 2012). Haemosporidian sporozoites were repeatedly found in blackflies attacking raptor nestlings sampled in this study (Svobodová et al., unpublished). Since the majority of Leucocytozoon species are transmitted by blackflies, while Haemoproteus (Parahaemoproteus) is transmitted by biting midges, we suppose that these were Leucocytozoon sporozoites. Moreover, Leucocytozoon was detected by PCR exclusively in blackflies while Haemoproteus in biting midges (Synek et al. 2013b; Bobeva et al. 2014). This, together with the proven occurrence of midges and blackflies in the nests of these studied raptors (Votýpka et al. 2002; Votýpka and Svobodová 2004), supports the respective roles of these vectors in the transmission of haemosporidians.
The lack of a positive association between trypanosomes and Leucocytozoon in adult sparrowhawks is unexpected but might be caused by a higher diversity of infecting trypanosomes in adults. We have isolated a trypanosome strain belonging to the T. bennetti clade (Zídková et al. 2012), a species with an unknown vector. Moreover, predation has been suggested as a mode of transmission of avian trypanosomes in sparrowhawks (Dirie et al. 1990). Alternatively, a patent infection with Leucocytozoon might affect the probability of catching adult sparrowhawks, while in nestlings, there is not such a bias (Valkiunas 2005). Moreover, the repeated sampling of adult sparrowhawks has revealed that some Leucocytozoon infections are lost, while trypanosome infection seems to be lifelong (Votýpka et al., unpublished; see above).
Haemoproteus and Leucocytozoon infections in nestlings (but not in broods) were also positively associated, yet the evidence is generally weaker than those for the association between Trypanosoma and Leucocytozoon. This association of parasites transmitted by different vectors might be a by-product of the age of nestlings at sampling, in concordance with the low overall prevalence of Haemoproteus.
Wiehn et al. (1999) failed to find a significant dependence of coinfections between Haemoproteus, Leucocytozoon, and trypanosomes in adult Eurasian kestrels (Falco tinnunculus). However, the prevalence of Leucocytozoon in that study was very low (0–1 %). On the other hand, Kirkpatrick and Lauer (1985) found sharp-shinned hawks (Accipiter striatus) infected with trypanosomes to be more likely infected with Leucocytozoon, but not with Haemoproteus, and they suggested this is due to a shared vector.
In the white-winged crossbill (Loxia leucoptera), a positive association of trypanosome infection was found both with Leucocytozoon and Haemoproteus (Deviche et al. 2010). If we suppose the same vector groups as in raptors, this might be explained by the age of the birds as a cofactor of infection, since the study was done on adults. Then, if the trypanosome infection is lifelong as in the case of sparrowhawks, the observed pattern is a function of age.
Factors influencing prevalences in nestlings
Two opposing mechanisms may have influenced the relationship between prevalence and brood size. First, prevalences at the level of individual nestlings tended to decrease with increasing brood size (Table 4), which is to be expected if more nestlings in a brood cause a dilution of vectors (Ratti et al. 2006). Second, prevalences at the level of entire broods tended to increase with brood size (Table 5), which is to be expected if nests with more nestlings have higher biomass, and are supposed to be more attractive for vectors. For instance, blackflies and midges are known to be more abundant in nests with more flycatcher (Ficedula hypoleuca) and tit (Parus spp.) nestlings (Martinez-de la Puente et al. 2009a, b). The probability of infection of an entire brood with Leucocytozoon was not shown to increase with increasing sparrowhawk brood size (Ashford et al. 1991). Although none of the brood size effects was formally statistically significant in our case, the change from a negative effect at the individual level (Table 4) to a positive effect at the brood level (Table 5) was consistent (the effect for Haemoproteus in buzzard was positive in both cases, but its slope increased) and in accordance with the above explanation.
The prevalences of all parasites increased with the age of nestlings/broods (Tables 4 and 5, Fig. 1). Prolonged exposure to vectors is thus positively correlated with prevalence. This suggests that there is no parasite-related mortality or that parasite-related mortality is overcompensated by new infections.
The prevalence of all parasites generally increased with date (Tables 4 and 5), being the most pronounced for Haemoproteus in buzzard. Since the increase of prevalence due to nestling age was statistically controlled for, the effect of date may be explained by the increasing occurrence of infected vectors throughout the season. Blackfly and midge abundance increases along with delayed hatching date in flycatchers and tits in Southern Europe (Martinez-de la Puente et al. 2009a, b; Tomas et al. 2008). In Central Europe, ornithophilic Culicoides species usually culminate in June (Chvála et al. 1980; Votýpka, unpublished); however, detailed data on ornithophilic species are lacking. The strong seasonal increase in the prevalence of Haemoproteus could be also affected by the low overall prevalence of this parasite in the population.
The duration of this study was not sufficient to evaluate long-term trends in prevalences. Nevertheless, variation in prevalence among annual samples (year treated as random effect) suggests caution in the comparison of results obtained in different years without taking this variation into account. Similarly, the markedly different prevalences of all three parasite genera in two buzzard populations demonstrate that prevalences may vary considerably among sampling locations within host species. On a smaller spatial scale, within a host population, we found little support for the effect of home range on infection probability. This is likely due to the change of nest location in subsequent years within a home range (obligately for sparrowhawk, commonly for buzzard) (Hudec and Šťastný 2013). It seems that nest detectability is greatly influenced by its fine-scale location within the home range, which is the most important factor in visually orientating vectors such as blackflies.
Clustering of infection in broods
As expected, infection with all three genera of blood parasites was not independent for nestlings in the same nest, i.e., a sibling of an infected nestling has a greater probability of being infected with the same parasite genus, and vice versa. Similarly, Ashford et al. (1991) found that broods tend to be either entirely uninfected or almost entirely infected with Leucocytozoon. This suggests that some nests are either easier to locate for vectors or, once a vector finds a nest, it may move between siblings while feeding, thus increasing the number of exposed individuals. Alternatively, the offspring of certain parents may have similar genetic predisposition for being infected, which differs between broods. Since it is unlikely that genetic predisposition for infection with haemoproteids and trypanosomes is similar due to their phylogenetic distance and consequently different physiology, we suppose that different exposure to vectors is the cause of infection clustering.
Conclusion and future directions
Our data on the prevalences of blood parasites in nestling and adult raptors revealed that phylogenetically unrelated parasites that share the same vectors tend to have similar distributions within host populations. This emphasizes the importance of future vector studies for our knowledge of wildlife parasites. Such studies are recently under way for passerine trypanosomes and their vectors.
References
Apanius V (1991) Avian trypanosomes as models of hemoflagellate evolution. Parasitol Today 7:87–90
Ashford RW, Wyllie I, Newton I (1990) Leucocytozoon toddi in British sparrowhawks Accipiter nisus—observations on the dynamics of infection. J Nat Hist 24:1101–1107
Ashford RW, Green EE, Holmes PR, Lucas AJ (1991) Leucocytozoon toddi in British sparrowhawks Accipiter nisus: patterns of infection in nestlings. J Nat Hist 25:269–277
Atkinson CT (2008) Haemoproteus. Atkinson, CT, Thomas, NJ, Hunter, DB. Parasitic diseases of wild birds. Wiley-Blackwell. pp 13–34
Baker JR (1956a) Studies on Trypanosoma avium Danilewsky 1885. 2. Transmission by Ornithomyia avicularia L. Parasitology 46:331–334
Baker JR (1956b) Studies on Trypanosoma avium Danilewsky 1885. 3. Life cycle in vertebrate and invertebrate hosts. Parasitology 46:335–352
Bennett GF (1961) On specificity and transmission of some avian trypanosomes. Can J Zool 39:17–33
Bobeva A, Ilieva M, Dimitrov D, Zehtindjiev P (2014) Degree of associations among vectors of the genus Culicoides (Diptera: Ceratopogonidae) and host bird species with respect to haemosporidian parasites in NE Bulgaria. Parasitol Res 113:4505–4511
Chvála M, Hůrka K, Chalupský J, Knoz J, Minář J et al (1980) Bloodsucking flies and botflies 1. Diptera. Fauna ČSSR, Vol. 22. Academia Prague
Deviche P, Fokidis HB, Lerbour B, Greiner E (2010) Blood parasitaemia in a high latitude flexible breeder, the white-winged crossbill, Loxia leucoptera: contribution of seasonal relapse versus new inoculations. Parasitology 137:261–273
Dirie MF, Ashford RW, Mungomba LM, Molyneux DH (1990) Avian trypanosomes in Simulium and sparrowhawks (Accipiter nisus). Parasitology 101:243–247
Hudec K, Šťastný K, editors (2013) Fauna of the Czech Republic, Birds 2/1. Academia Prague
Kirkpatrick CE, Lauer DM (1985) Hematozoa of raptors from Southern New Jersey and adjacent areas. J Wildl Dis 21:1–6
Kirkpatrick CE, Terwaythompson CA, Iyengar MR (1986) Biochemical characterization of some raptor trypanosomes. 2. Enzyme studies, with a description of Trypanosoma bennetti n. sp. Can J Zool 64:195–203
Krone O, Priemer J, Streich J, Sommer P, Langgemach T et al (2001) Haemosporida of birds of prey and owls from Germany. Acta Protozool 40:281–289
Krone O, Waldenstrom J, Valkiunas G, Lessow O, Muller K et al (2008) Haemosporidian blood parasites in European birds of prey and owls. J Parasitol 94:709–715
Martinez-de la Puente J, Merino S, Lobato E, Rivero-De Aguilar J, Del Cerro S et al (2009a) Does weather affect biting fly abundance in avian nests? J Avian Biol 40:653–657
Martinez-de la Puente J, Merino S, Tomas G, Moreno J, Morales J et al (2009b) Factors affecting Culicoides species composition and abundance in avian nests. Parasitology 136:1033–1041
Martinsen ES, Perkins SL, Schall JJ (2008) A three-genome phylogeny of malaria parasites (Plasmodium and closely related genera): evolution of life-history traits and host switches. Mol Phylogenet Evol 47:261–273
Merino S, Potti J (1995) High prevalence of hematozoa in nestlings of a passerine species, the pied flycatcher (Ficedula hypoleuca). Auk 112:1041–1043
Outlaw DC, Ricklefs RE (2009) On the phylogenetic relationships of haemosporidian parasites from raptorial birds (Falconiformes and Strigiformes). J Parasitol 95:1171–7116
Peške L (1987) Aging nestling raptors for the ringing and the reconstruction of breeding data. Buteo 2:67–72
Peške L (1990) Study of the sparrowhawk (Accipiter nisus) population in Prague: the possibility to compare the results of bird breeding distribution mapping and the actual situation. Bird census and atlas studies:99–102
Ratti O, Ojanen U, Helle P (2006) Increasing group size dilutes black fly attack rate in Black Grouse. Ornis Fennica 83:86–90
Sehgal RNM, Hull AC, Anderson NL, Valkiūnas G, Markovets MJ, Kawamura S, Tell LA (2006) Evidence for cryptic speciation of Leucocytozoon spp. (Haemosporida, Leucocytozoidae) in diurnal raptors. J Parasitol 92:375–379
Synek P, Albrecht T, Vinkler M, Schnitzer J, Votypka J (2013a) Haemosporidian parasites of a European passerine wintering in South Asia: diversity, mixed infections and effect on host condition. Parasitol Res 112:1667–1677
Synek P, Munclinger P, Albrecht T, Votypka J (2013b) Avian haemosporidians in haematophagous insects in the Czech Republic. Parasitol Res 112:839–845
Tomas G, Merino S, Martinez-de la Puente J, Moreno J, Morales J et al (2008) Determinants of abundance and effects of blood-sucking flying insects in the nest of a hole-nesting bird. Oecologia 156:305–312
Valkiunas G (2005) Avian malaria parasites and other haemosporidia. CRC Press, Boca Raton
Valkiunas G, Bensch S, Iezhova TA, Krizanauskiene A, Hellgren O et al (2006) Nested cytochrome B polymerase chain reaction diagnostics underestimate mixed infections of avian blood haemosporidian parasites: microscopy is still essential. J Parasitol 92:418–422
Valkiunas G, Iezhova TA, Loiseau C, Sehgal RNM (2009) Nested cytochrome B polymerase chain reaction diagnostics detect sporozoites of hemosporidian parasites in peripheral blood of naturally infected birds. J Parasitol 95:1512–1515
Valkiunas G, Santiago-Alarcon D, Levin II, Iezhova TA, Parker PG (2010) A New Haemoproteus species (Haemosporida: Haemoproteidae) from the endemic Galapagos dove Zenaida galapagoensis, with remarks on the parasite distribution, vectors, and molecular diagnostics. J Parasitol 96:783–792
Voříšek P (2000) An extremely high population density of common buzzard (Buteo buteo) in Biosphere Reserve Pálava (Czech Republic) and its possible causes. Buteo 11:51–56
Voříšek P, Lacina D (1998) Age estimation of young common buzzards (Buteo buteo) and European kestrels (Falco tinnunculus) with the use of biometric data. Buteo 10:35–50
Votypka J, Svobodova M (2004) Trypanosoma avium: experimental transmission from black flies to canaries. Parasitol Res 92:147–151
Votypka J, Obornik M, Volf P, Svobodova M, Lukes J (2002) Trypanosoma avium of raptors (Falconiformes): phylogeny and identification of vectors. Parasitology 125:253–263
Votypka J, Lukes J, Obornik M (2004) Phylogenetic relationship of Trypanosoma corvi with other avian trypanosomes. Acta Protozool 43:225–231
Wiehn J, Korpimaki E, Pen I (1999) Haematozoan infections in the Eurasian kestrel: effects of fluctuating food supply and experimental manipulation of parental effort. Oikos 84:87–98
Zidkova L, Cepicka I, Szabova J, Svobodova M (2012) Biodiversity of avian trypanosomes. Inf Gen Evol 12:102–112
Acknowledgments
The idea of the project came from discussions with Richard W. Ashford. We thank Jan Matouš for the help during the field work on Prague Buzzards, and Petra Černá, Dušan Eremiáš, and David Lacina for checking some of the blood smears. David Hardekopf edited the manuscript.
Funded by Czech Science Foundation 14-02482S and 206/00/1094, Grant Agency of Charles University 282/96.
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Svobodová, M., Weidinger, K., Peške, L. et al. Trypanosomes and haemosporidia in the buzzard (Buteo buteo) and sparrowhawk (Accipiter nisus): factors affecting the prevalence of parasites. Parasitol Res 114, 551–560 (2015). https://doi.org/10.1007/s00436-014-4217-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-014-4217-x