Abstract
Morphological sexual dimorphism occurs in most insect species. Caves are relatively independent habitats with high levels of endemic insect species. The cave crickets are one of the most common insects in many caves and play an important role in the nutrient cycling of cave ecosystems. Sexual difference in sensilla has rarely been studied in cave crickets. We explore the types, number, and distribution of sensilla on the labial palps of both sexes of the cave cricket Tachycines plumiopedella Li, Feng & Luo, 2021 for the first time. Seven sensilla types were recorded on the labial palps in both sexes, including sensilla chaetica (Sc. 1–2), sensilla trichodea (St. 1–3), sensilla palmatum (Sp), Böhm bristles (Bb), sensilla campaniformia (Sca), sensilla basiconica (Sb. 1–3), and sensilla coeloconica (Sco. 1–2). The sensilla are mostly situated on the third palpomere of the labial palps, particularly on its middle to end part. Of sensilla on the labial palps, types and distribution were similar in males and females, but different in length, diameter, and number. The potential functional roles of sensilla were discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Morphological sexual dimorphism has been the subject of interest for decades, being present in most insects (Mori et al. 2017). Morphological sexual dimorphism of insects shows a remarkable diversity, which ranges from indistinguishable to the enormous differences in size and shape (Stubblefield and Seger 1994; Mori et al. 2017). For example in Hemideina crassidens (Blanchard, 1851), males have larger mandibles (Kelly 2005). In many grasshopper species, the types of antennal sensilla have no difference between males and females, but the number of antennal sensilla of males was significantly greater than females (Li et al. 2007; Nakano et al. 2022a). The reason for sexual dimorphism is complex. One of the views is that morphological sexual dimorphism may evolve for ecological causes (e.g., habitats and nutritional needs for reproduction) (Shine 1989).
Caves are a kind of relatively independent habitats, and the overall environmental characteristics are depleted nutrients, minimal changes in temperature and humidity, and weak light or darkness (Poulson and White 1969; Prous et al. 2004; Ran and Yang 2015). As a dominant species of the cave biodiversity, cave crickets play an important role in maintaining the stability of the cave ecosystem (Lavoie et al. 2007). In Southern China, the genus Tachycines Adelung, 1902 is a major component of cave crickets (Feng et al. 2020; Li et al. 2021; Cigliano et al. 2022). The genus Tachycines belongs to the tribe Aemodogryllini Jacobson, 1905 of the subfamily Aemodogryllinae Jacobson, 1905, and to date, contains a total of 98 species (Karny 1934; Cigliano et al. 2022). In the genus Tachycines, at least 43 species are known to inhabit cave habitats, and these cave species occur mainly in Southeast and East Asia (Feng et al. 2019; Cigliano et al. 2022). These cave crickets have evolved some morphological adaptations to life in caves: the reduction of both eyes and coloration, and the development of long appendages (Deharveng and Bedos 2018; Feng et al. 2020). The sensory systems of Tachycines species are very interesting to study comparatively with respect to other Orthoptera. The reason is not only the absence of useful visual signals for cave species, but also the absence of stridulatory structures and hearing, and these cave species have been traditionally regarded to strongly rely on chemosensory abilities and sense of touch (Peljhan 2018).
Insects rely mainly on sensory organs known as sensilla, which are distributed on appendages to perceive and recognize stimuli and play a vital role in various biological activities (e.g., mate recognition and food discrimination) of the insects (Chapman 1982; Byers 1995; Schrader 2000; Shi et al. 2021). The sensilla are sensory organs protruding from the exoskeleton and consist of cuticular structure, sensory neurons, and enveloping cells (Zacharuk 1980; Nakano et al. 2022b). The sensilla of crickets are classified into different types, and the most common types are sensilla chaetica, sensilla trichodea, sensilla campaniformia, sensilla basiconica, sensilla coeloconica, and Böhm bristles (Schneider and Römer 2016; Faucheux 2017). The function of each sensillum is extrapolated from its shape, size, porosity, and socket type. For example, sensilla without pores and a flexible socket are typically mechanoreceptors (Altner and Prillinger 1980; Nakano et al. 2022a). Sensilla without pores can be also responsible for thermoreception and hygroreception (Steinbrecht 1997; Hallberg et al 2003; Nowińska and Brożek 2017). Sensilla with pores are chemoreceptors. These sensilla can have a terminal pore (uniporous) or have many pores (multiporous or wall-pored), and they are considered gustatory receptors (contact chemoreception) or olfactory receptors (distance chemoreception) respectively (Klein 1981; Ring et al. 2008; Schneider and Römer 2016; Fea et al. 2019; Nakano et al. 2022a). The diversity of the sensory systems is linked to the ecological causes, and the specific ecology (e.g., habitats and sex roles) may influence the number and distribution of sensilla (Nakano et al. 2022b).
Labial palps are appendages of the insect mouthparts, and abundant sensilla on the labial palps are important for insect feeding behavior and host selection (Hashimoto 1992; Byers 1995; Guerenstein et al. 2004; Zhou et al. 2008). Previous studies have shown that labial palps are used as contact receptions during the foraging process and stay with food during the chewing process (Hao et al. 2019). In Gryllus bimaculatus De Geer, 1773, Klein (1981) described the distal segment of the labial palps through scanning electron microscopy and transmission electron microscopy, and there is a multimodal receptor field that is sensitive to different stimuli, which is one of the main sensory systems of the mouthparts.
Although there are a large number of studies on insect sensilla, the sensilla of insects that inhabit caves have received relatively little attention so far. In this study, we investigate the external morphology and the types and distribution of sensilla associated with the labial palps of male and female adults of the cave species Tachycines plumiopedella Li, Feng & Luo, 2021 using scanning electron microscopy. Our results can provide information to further study the significance of the labial palps in the behavior and chemical ecology of T. plumiopedella in cave habitats.
Materials and methods
Insects
The specimens of the T. plumiopedella were collected from Shui Cave (28°04.84139’N, 107°37.65916’E, elev. 1155.1 m) which is located in Yongan Town, Fenggang County, Zunyi City, Guizhou Province, China. Microscopy studies were conducted on both male and female adults. Ten pairs of adults of both genders were collected and stored in 75% ethanol until preparation for microscopy.
Observation and measurement of specimens
The labial palps of ten individuals per sex were removed under a stereomicroscope (Nikon SMZ1270, Nikon Corporation, Japan) using microscissors and fixed immediately in glutaraldehyde (2.5%) at 4℃ for 20 h. After washing with phosphate buffer solution (0.1 mol/L, PH = 7.4) three times (15 min per wash), an ultrasonic cleaner (JP-030S, Shenzhen Jiemeng Cleaning Equipment Co., LTD, China) was used to clean them for 30 s. The labial palps were dehydrated through an ascending ethanol series of 75%, 80%, 85%, 90%, 95% and 100% with 15 min intervals, and in 100% ethanol solutions for 12 h. The labial palps were dried for 12 h in an electric blast drying oven (101-ISB, Shaoxing Supo Instrument Co., LTD, China) at 40℃. The labial palps were then glued onto aluminum pin mounts with double-sided carbon adhesive tape and sputtered with gold for 2 min in a high-resolution sputter coater (Smart Coater, Hitachi, Japan) and finally observed under scanning electron microscopy (JCM 6000, Hitachi, Japan) operated at an electron accelerating voltage of 15 kV.
We used the outer morphology (shape, cuticular features, porosity and socket features), size and distribution to identify each distinct type of sensillum in T. plumiopedella, and the classification of sensilla was based on the studies of Schneider (1964), Zacharuk (1980), Schneider and Römer (2016), Faucheux (2017), Nowińska and Brożek (2017) and Fea et al. (2019).
The body length was taken using a digital vernier caliper (Prokit’s Inc., Shanghai, China), and the morphological data of labial palps and sensilla were collected by the “Scaler” function from the SEM measurement system. The length of body and labial palps were measured for ten individuals of each sex. The length, basal width and the number of each type of sensillum were measured based on ten replications, and we measured a maximum of two sensilla from each specimen.
Statistical analysis
Statistical analysis was undertaken with SPSS 26.0 software. Data are reported as means ± standard deviation (S.D.). Prior to conducting parametric tests, we used Levene's test to determine data for homogeneity of variances and Kolmogorov–Smirnov tests to test whether data were distributed normally. Student’s t test was used to determine any significant differences between sexes. The significance level was set at 0.05.
Results
General morphology of the labial palps
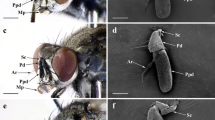
There was no significant difference in the body length of T. plumiopedella between the sexes (Student’s t test: t = 1.970, P = 0.064; Table 1). No obvious differences in the general morphology of the labial palps could be found between the sexes. Figure 1A, B shows labial palps with three palpomeres. The first palpomere is short and thick. The second palpomere is slightly longer than the first palpomere. The third palpomere possesses a bulbous protuberance on the apex (Fig. 1C). No significant sexual difference in the length of labial palps was observed (Student’s t test: t = 0.609, P = 0.550; Table 1).
Types and distribution of sensilla
The labial palps of female and male adults have the same sensilla types. According to their external morphology, size and distribution, we observed seven types of labial palps in T. plumiopedella: sensilla chaetica (subtypes 1–2), sensilla trichodea (subtypes 1–3), sensilla palmatum, Böhm bristles, sensilla campaniformia, sensilla basiconica (subtypes 1–3) and sensilla coeloconica (subtypes 1–2).
Sensilla chaetica (Sc) are stiff hairlike sensilla that are straight. Their stem may be spiral grooved or herringbone grooved and usually tapers from the base to the tip. Sensilla chaetica could be further classified into two subtypes based on shape. Sensilla chaetica subtype 1 (Sc.1) are aporous and straight, with a spiral grooved cuticular wall, a sharp tip and flexible sockets. They are at nearly 60° angle to the surface of the labial palps (Fig. 2A, B). These sensilla are numerous and densely distributed on the whole labial palps. Sensilla chaetica subtype 2 (Sc.2) are aporous and straight, with a herringbone grooved cuticular wall, a sharp tip and flexible sockets (Fig. 2A, C). These sensilla are distributed on the third palpomere.
Sensilla chaetica of subtypes 1–2 and sensilla trichodea subtypes 1 on T. plumiopedella. A Distribution of sensilla chaetica subtypes 1 (Sc.1), sensilla chaetica subtypes 2 (Sc.2), and sensilla trichodea subtypes 1 (St.1). B Sensilla chaetica subtypes 1 (Sc.1). C Sensilla chaetica subtypes 2 (Sc.2). D Sensilla trichodea subtypes 1 (St.1)
Sensilla trichoidea (St) are flexible hairlike structures that are narrowed at the tip. These sensilla are at a nearly 90° angle to the surface of the labial palps. Sensilla trichodea could be further classified into three subtypes based on shape. Sensilla trichodea subtype 1 (St.1) are aporous and straight, with a spiral grooved cuticular wall, curved at the tip and flexible sockets (Fig. 2A, D). These sensilla are numerous and are distributed on the whole labial palps. Sensilla trichodea subtype 2 (St.2) are curved and uniporous, with a spiral grooved cuticular wall and flexible sockets (Fig. 3A, B, C). Sensilla trichodea subtype 3 (St.3) are aporous and slightly curved, with a spiral grooved cuticular wall, a blunt tip and flexible sockets (Fig. 3A, D). St.2 and St.3 are distributed on the terminal bulbous protuberance of the third palpomere.
Sensilla trichodea of subtypes 2–3, sensilla palmatum, and Böhm bristles on T. plumiopedella. A Distribution of sensilla trichodea subtypes 2 (St.2), sensilla trichodea subtypes 3 (St.3), and sensilla palmatum (Sp). B Sensilla trichodea subtypes 2 (St.2). C Pore on the end of sensilla trichodea subtype 2; TP: terminal pore. D Sensilla trichodea subtypes 3 (St.3). E Sensilla palmatum (Sp). F Distribution of Böhm bristles (Bb). G Böhm bristles (Bb)
Sensilla palmatum (Sp) are straight, claw-like and aporous, with several blunt tips, a longitudinal grooved cuticular wall and inflexible sockets (Fig. 3A, E). These sensilla are distributed on the terminal bulbous protuberance of the third palpomere.
Böhm bristles (Bb) are aporous and short, with a longitudinal grooved cuticular wall, a blunt tip and flexible sockets (Fig. 3F, G). These sensilla are only distributed on the base of the second and third palpomeres in the cluster.
Sensilla campaniformia (Sca) are round or oval and button-like, with a smooth empty thin wall on the surface and smooth and clear thick-wall edges around it (Fig. 4A, B). These sensilla are sparsely distributed on the whole labial palps.
Sensilla Basiconica (Sb) are cones that are embedded in flexible sockets. The cuticular wall of the sensilla may be porous or aporous. Sensilla basiconica could be further classified into three subtypes based on shape. Sensilla basiconica subtype 1 (Sb.1) are nearly straight or slightly curved and aporous, with a longitudinal grooved cuticular wall and a sharp tip. The stem tapers from the base to the tip (Fig. 4A,C). These sensilla are sparsely distributed on the whole labial palps. Sensilla basiconica subtype 2 (Sb.2) are curved and aporous, ended in a blunt tip, wide in basal width and significantly reduced at one-third from the base. Sb.2 has a longitudinal grooved cuticular wall (Fig. 5A, B). Sensilla basiconica subtype 3 (Sb.3) are slightly curved, with a multiporous cuticular wall and a blunt tip (Fig. 5A, C, D). Sb.2 and Sb.3 are mainly distributed on the middle to end part of the third palpomere.
Sensilla basiconica of subtypes 2–3 and sensilla coeloconica of subtypes 1–2 on T. plumiopedella. A Distribution of sensilla basiconica subtype 2 (Sb.2), sensilla basiconica subtype 3 (Sb.3), sensilla coeloconica subtype 1 (Sco.1), and sensilla coeloconica subtype 2 (Sco.2). B Sensilla basiconica subtype 2 (Sb.2). C Sensilla basiconica subtype 3 (Sb.3). D Proximal portion of sensilla basiconica subtype 3; PO: pore. E Sensilla coeloconica subtype 1 (Sco.1). F Sensilla coeloconica subtype 2 (Sco.2)
Sensilla coeloconica (Sco) are tiny pegs that arise from inflexible sockets. Each peg is situated in a pit. Sensilla coeloconica could be further classified into two subtypes based on shape. Sensilla coeloconica subtype 1 (Sco.1) are smooth in the cuticular wall, and features a longitudinal grooved near the tip. The pegs arise from bulgy sockets (Fig. 5A, E). Sensilla coeloconica subtype 2 (Sco.2) are morphologically similar to Sco.1, but the sockets are sunken and the pegs are slightly out of the cavity (Fig. 5A, F). Sco.1 and Sco.2 are mainly distributed on the middle to end part of the third palpomere.
Comparison of sensilla between sexes
In the cricket species T. plumiopedella, the two sexes exhibit differences in the size of some sensilla (Table 2). The size of some sensilla was longer in females than males. They contain the length and basal width of Sc.2 (Student’s t test: t = 3.425, P = 0.003 for length; Student’s t test: t = 3.454, P = 0.003 for basal width), the length and basal width of Sco.2 (Student’s t test: t = 2.930, P = 0.009 for length; Student’s t test: t = 3.779, P = 0.001 for basal width), the length of Sp (Student’s t-test: t = 2.149, P = 0.049), the basal width of Sb.1 (Student’s t test: t = 2.682, P = 0.018), and the basal width of Sb.3 (Student’s t test: t = 5.450, P < 0.001). The size of some sensilla were shorter in females than males. They include the basal width of Sc.1 (Student’s t test: t = 5.678, P < 0.001; Table 2), the basal width of Sp (Student’s t test: t = 2.730, P = 0.016), and the length of Sco.1 (Student’s t test: t = 2.807, P = 0.012).
The number of sensilla of labial palps in T. plumiopedella differed between sexes (Table 3). The total numbers of St.1, St.2, St.3, and Sp were significantly greater in females than in males (Student’s t test: t = 3.961, P = 0.001 for St.1; Student’s t test: t = 10.255, P < 0.001 for St.2; Student’s t test: t = 8.881, P < 0.001 for St.3; Student’s t test: t = 8.165, P < 0.001 for Sp). Some types of sensilla types are more abundant on a particular palpomere. The number of St.1 on the first palpomere of labial palps in females was significantly greater than in males (Student’s t test: t = 7.415, P < 0.001). The number of Sca on the second palpomere of labial palps in males was significantly greater than in females (Student’s t test: t = 4.392, P < 0.001). The number of Sb.1 on the third palpomere of labial palps in females was significantly greater than males (Student’s t test: t = 3.539, P = 0.002).
Discussion
In this study, we investigated the external morphology, and the types and distribution of different types of sensilla associated with the labial palps of female and male adults of the cave species T. plumiopedella. The labial palps of this cave species is similar to those of other insects in consisting three palpomeres (Chapman 1998). Thirteen types of sensilla are present on the labial palps of T. plumiopedella. These sensilla have been described as being specialized for perceive particular types of stimuli and are known as mechanoreceptors, chemosensors and hygro-thermoreceptors.
Mechanoreception may be the important source of sensory information for T. plumiopedella considering that vision can be very challenging in a dark cave (Peljhan 2018). In T. plumiopedella, Sc.1, Sc.2, St.1, St.3, Sb.1, Sb.2, Bb, and Sca are mechanoreceptors. Klein (1982) described that the cricket uses its labial palps to grasp the antenna during the antennal cleaning process, and after amputation of the labial palps, it becomes difficult to position the antenna. The reason may be that crickets lack the mechanoreceptors of the labial palps and hence cannot receive the mechanical stimuli caused by the touch of antennae. Moreover, Bb and Sca are proprioceptors, which can detect the position and movement of the labial palps (Toh 1981; Faucheux 2017; Fea et al. 2019).
Chemoreception plays a vital role in the survival of T. plumiopedella, which often depend on their chemosensors to find suitable habitats and food sources (Nakano et al. 2022b). The chemoreception function is conducted by St.2 and Sb.3. St.2 and have been described in the studies as having a contact-chemosensory function (Blaney 1974; Yu et al. 2011; Fea et al. 2019). Klein (1982) observed crickets touching food with the distal end on the labial palps, and St.2 are distributed on the distal end of the labial palps, suggesting that St.2 are used for food selection. Sb.3 are typical olfactory sensilla and have the function of sensing volatile chemicals (Ring et al. 2008; Schneider and Römer 2016; Fea et al. 2019).
Thermoreception and hygroreception are very important for the cave species T. plumiopedella, which need to cope with moisture loss by evaporation, and rely on the temperature of the environment as insect populations are poikilotherms (Lavoie et al. 2007; Rebora et al. 2019). Sco.1 and Sco.2 are responsible for thermoreception and hygroreception. The cave species T. plumiopedella may detect humidity and temperature gradient by Sco.1 and Sco.2, in air to search for adequate habitats (Rebora et al. 2019).
In the cricket species T. plumiopedella, the types of sensilla of the male and female adults are similar, but the two sexes exhibit differences in the number, basal width, or length of some sensilla. There was no significant difference in the length of labial palps between sexes, but the number of St.1, St.2, St.3, and Sp of female adults was significantly higher than that of males. In addition, the size of Sb.1, Sb.3, Sc.2, and Sco.2 of female adults tends to be greater than that of males. Therefore, the labial palps of females have stronger ability of perception of environmental stimuli than those of males.
As well known, females need more energy than males to produce eggs (Lease and Wolf 2011). In some species of Orthoptera, the histolysis of flight muscles on females can contribute as the cost for oogenesis (Lorenz and Gäde 2009). However, T. plumiopedella is wingless, and the two sexes have similar body size. Females need other strategies to ensure nutritional supplementation, and a more developed sensory system may be one of the strategies to cope with the nutritional deficiencies caused by the reproductive behavior. In addition, previous studies have shown that the compounds can influence where Orthopterans produce their eggs (Tanaka et al. 2019). Therefore, females require more chemosensors than males to detect oviposition sites or recognize compounds, but the sensitivity of each sensilla type to particular compounds is not clear. Further behavioral and electrophysiological studies are needed to determine the significance of sexual dimorphism in this species.
Data availability
All data supporting our fndings are presented in the paper. The raw data can be made available on reasonable request.
References
Altner H, Prillinger L (1980) Ultrastructure of invertebrate chemo-, thermo-, and hygroreceptors and its functional significance. Int Rev Cytol 67:69–139. https://doi.org/10.1016/S0074-7696(08)62427-4
Blaney WM (1974) Electrophysiological responses of the terminal sensilla on the maxillary palps of Locusta migratoria (L.) to some electrolytes and non-electrolytes. J Exp Biol 60:275–293
Byers JA (1995) Host-tree chemistry affecting colonization in bark beetles. In: Cardé RT, Bell WJ (Eds) Chemical ecology of insects. Springer, Boston, pp 154–213. Doi: https://doi.org/10.1007/978-1-4615-1765-8_5
Chapman RF (1982) Chemoreception: the significance of receptor numbers. Adv Insect Physiol 16:247–356. https://doi.org/10.1016/S0065-2806(08)60155-1
Chapman RF (1998) The Insects: structure and function, 5th edn. Cambridge University Press, New York
Cigliano MM, Braun H, Eades DC, Otte D (2022) Orthoptera species file. Version 5.0/5.0. http://Orthoptera.SpeciesFile.org. Accessed 29 Dec 2022
Deharveng L, Bedos A (2018) Diversity of terrestrial invertebrates in subterranean habitats. In: Moldovan O, Kováč Ľ, Halse S (Eds) Cave ecology, vol 235. Springer, Cham, pp 107–172. https://doi.org/10.1007/978-3-319-98852-8_7
Faucheux MJ (2017) Antennal sensilla of the armoured ground cricket, Eugaster powysi Kirby, 1891 (Orthoptera, Tettigoniidae, Hetrodinae). Bull Inst Sci 39:1–17
Faucheux MJ, Kundrata R (2017) Comparative antennal morphology of male Drilini with special reference to the sensilla (Coleoptera: Elateridae: Agrypninae). Zool Anz 266:105–119. https://doi.org/10.1016/j.jcz.2016.11.002
Fea MP, Mark CJ, Holwell GI (2019) Sexually dimorphic antennal structures of New Zealand cave wētā (Orthoptera: Rhaphidophoridae). New Zeal J Zool 46:124–148. https://doi.org/10.1080/03014223.2018.1520266
Feng X, Huang S, Luo C (2019) A new species of the subgenus Tachycines (Gymnaeta) (Orthoptera: Rhaphidophoridae) from karst caves of southern Guizhou, China. Zootaxa 4674:491–495. https://doi.org/10.11646/zootaxa.4674.4.8
Feng X, Huang S, Luo C (2020) Three new cave species of the subgenus Tachycines (Gymnaeta) (Orthoptera: Rhaphidophoridae: Aemodogryllinae) from northern Guizhou, China. Zootaxa 4820:563–571. https://doi.org/10.11646/zootaxa.4820.3.9
Guerenstein PG, Yepez EA, van Haren J, Williams DG, Hildebrand JG (2004) Floral CO2 emission may indicate food abundance to nectar-feeding moths. Naturwissenschaften 91:329–333. https://doi.org/10.1007/s00114-004-0532-x
Hao YN, Sun YX, Liu CZ (2019) Functional morphology of the mouthparts of lady beetle Coccinella transversoguttata (Coccinellidae, Coleoptera), with reference to their feeding mechanism. J Morphol 280:701–711. https://doi.org/10.1002/jmor.20976
Hashimoto Y (1992) Unique sensillum structure of the Formicid labial palpi (Hymenoptera). Hum Nat 1:57–62. https://doi.org/10.24713/hitotoshizen.1.0_57
Hallberg E, Hansson BS, Löfstedt C (2003) Sensilla and proprioreceptors. In: Kristensen NP (ed) Lepidoptera, moths and butterflies: morphology, physiology and development, vol 2. De Gruyter, Berlin, pp 267–288
Huang J, Ma JW, Liu XR, Li MM, Hua BZ (2011) Ultrastructure of the mouthpart sensilla in adult Bittacus sinensis Walker (Mecoptera: Bittacidae). Acta Entomol Sin 54:110–116. https://doi.org/10.16380/j.kcxb.2011.01.008
Hustert R (1985) Multisegmental integration and divergence of afferent information from single tactile hairs in a cricket. J Exp Biol 118:209–227. https://doi.org/10.1242/jeb.118.1.209
Karny HH (1934) Zur Kenntnis der ostasiatischen Rhaphidophorinen (Orth. Salt. Gryllacrididae). Konowia Ztschr f Syst Insektenkd 13:216–218
Klein U (1981) Sensilla of the cricket palp. Cell Tissue Res 219:229–252. https://doi.org/10.1007/BF00210145
Klein U (1982) The articulation of cricket palps: morphology and movement patterns in behaviour. Physiol Entomol 7:297–314. https://doi.org/10.1111/j.1365-3032.1982.tb00303.x
Kelly CD (2005) Allometry and sexual selection of male weaponry in Wellington tree weta, Hemideina crassidens. Behav Ecol 16:145–152. https://doi.org/10.1093/beheco/arh141
Lavoie KH, Helf KL, Poulson TL (2007) The biology and ecology of North American cave crickets. J Cave Karst Stud 69:114–134
Lease HM, Wolf BO (2011) Lipid content of terrestrial arthropods in relation to body size, phylogeny, ontogeny and sex. Physiol Entomol 36:29–38. https://doi.org/10.1111/j.1365-3032.2010.00767.x
Li B (2022) Comparative morphology of the appendage sensilla and compound eyes of three species of genus Tachycines (Othoptera: Rhaphidophoridae) inhabiting different habitats. Dissertation, Guizhou University. https://doi.org/10.27047/d.cnki.ggudu.2022.000372
Li B, Feng X, Luo C (2021) Four new species of the subgenus Tachycines (Gymnaeta) (Rhaphidophoridae: Aemodogryllinae: Aemodogryllini) from caves in northern Guizhou, China. Zootaxa 4991:150‒160. https://doi.org/10.11646/zootaxa.4991.1.7
Li N, Ren BZ, Liu M (2007) The study on antennal sensilla of eight Acrididae species (Orthoptera: Acridoidea) in Northeast China. Zootaxa 1544:59–68. https://doi.org/10.11646/zootaxa.1544.1.3
Lorenz MW, Gäde G (2009) Hormonal regulation of energy metabolism in insects as a driving force for performance. Integr Comp Biol 49:380–392. https://doi.org/10.1093/icb/icp019
Mori E, Mazza G, Lovari S (2017). Sexual Dimorphism. In: Vonk J, Shackelford T (Eds) Encyclopedia of animal cognition and behavior. Springer, Cham, pp 1–7. https://doi.org/10.1007/978-3-319-47829-6_433-1
Nakano M, Morgan-Richards M, Clavijo-McCormick A, Trewick S (2022a) Abundance and distribution of antennal sensilla on males and females of three sympatric species of alpine grasshopper (Orthoptera: Acrididae: Catantopinae) in Aotearoa New Zealand. Zoomorphology. https://doi.org/10.1007/s00435-022-00579-z
Nakano M, Morgan-Richards M, Trewick SA, Clavijo-McCormick A (2022b) Chemical ecology and olfaction in short-horned grasshoppers (Orthoptera: Acrididae). J Chem Ecol 48:121–140. https://doi.org/10.1007/s10886-021-01333-3
Nishikawa M, Yokohari F, Ishibashi T (1985) The antennal thermoreceptor of the camel cricket, Tachycines asynamorus. J Insect Physiol 31:517–524. https://doi.org/10.1016/0022-1910(85)90107-6
Nowińska A, Brożek J (2017) Morphological study of the antennal sensilla in Gerromorpha (Insecta: Hemiptera: Heteroptera). Zoomorphology 136:327–347. https://doi.org/10.1007/s00435-017-0354-y
Peljhan NS (2018) Cave cricket genus Troglophilus as a model for studying function and evolution of sensory systems and behaviour. Acta Entomol Slov 26:131–150
Pérez-González S, Zaballos JP (2013) Antennal morphology of the endogean carabid genus typhlocharis (Coleoptera: Carabidae: Anillini): description of sensilla and taxonomic implications. J Morph 7:809–823. https://doi.org/10.1002/jmor.20140
Pervez A, Yadav M, Bozdoğan H (2022) Functional morphology of mouthparts and antennal sensillae of two co-generic aphidophagous ladybirds. Int J Trop Insect Sci 42:2531–2546. https://doi.org/10.1007/s42690-022-00780-z
Poulson TL, White WB (1969) The cave environment: limestone caves provide unique natural laboratories for studying biological and geological processes. Science 165:971–981. https://doi.org/10.1126/science.165.3897.971
Prous X, Ferreira RL, Martins RP (2004) Ecotone delimitation: Epigean–hypogean transition in cave ecosystems. Austral Ecol 29:374–382. https://doi.org/10.1111/j.1442-9993.2004.01373.x
Ran J, Yang W (2015) A review of progress in Chinese troglofauna research. J Resour Ecol 6:237–246. https://doi.org/10.5814/j.issn.1674-764x.2015.04.007
Rebora M, Salerno G, Piersanti S (2019). Aquatic Insect Sensilla: Morphology and Function. In: Del-Claro K, Guillermo R (Eds) Aquatic insects. Springer, Cham, pp 139–166. https://doi.org/10.1007/978-3-030-16327-3_7
Ring JR, Prusti RK, Mohanty S (2008) Chemical communication: a visit with insects. Curr Chem Biol 2:83–96. https://doi.org/10.2174/187231308783334153
Schneider D (1964) Insect antennae. Annu Rev Entomol 9:103–122. https://doi.org/10.1146/annurev.en.09.010164.000535
Schneider ES, Römer H (2016) Sensory structures on the antennal flagella of two katydid species of the genus Mecopoda (Orthoptera, Tettigonidae). Micron 90:43–58. https://doi.org/10.1016/j.micron.2016.08.001
Schrader Š (2000) The function of the cercal sensory system in escape behavior of the cave cricket Troglophilus neglectus Krauss. Pflügers Arch Eur J Physiol 439:R187–R189. https://doi.org/10.1007/BF03376567
Shine R (1989) Ecological causes for the evolution of sexual dimorphism: a review of the evidence. Q Rev Biol 64:419–461. https://doi.org/10.1086/416458
Shi X, Zhang SF, Liu F, Xu FY, Zhang FB, Guo XB, Zhang Z, Kong XB (2021) SEM analysis of sensilla on the mouthparts and antennae of Asian larch bark beetle Ips subelongatus. Micron 140:102976. https://doi.org/10.1016/j.micron.2020.102976
Steinbrecht RA (1997) Pore structures in insect olfactory sensilla: a review of data and concepts. Int J Insect Morphol Embryol 26:229–245. https://doi.org/10.1016/S0020-7322(97)00024-X
Stubblefield JW, Seger J (1994) Sexual dimorphism in the Hymenoptera. In: Short RV, Balaban E (eds) The Differences Between the Sexes. Cambridge University Press, Cambridge, pp 71–103
Tanaka S, Kotaki T, Nishide Y, Ben-hamouda A, Abdellaoui K, Ebbe MAB, Ely SO (2019) Effects of water extracts of frass from three locust species and various plants on oviposition and embryonic development in the desert locust, Schistocerca gregaria. J Orthop Res 28:195–204. https://doi.org/10.3897/jor28.34665
Toh Y (1981) Fine structure of sense organs on the antennal pedicel and scape of the male cockroach, Periplaneta americana. J Ultrastruct Res 77:119–132. https://doi.org/10.1016/S0022-5320(81)80036-6
Yu Y, Zhou S, Zhang S, Zhang L (2011) Fine structure of the sensilla and immunolocalisation of odorant binding proteins in the cerci of the migratory locust, Locusta migratoria. J Insect Sci 11:1–10. https://doi.org/10.1673/031.011.5001
Zacharuk RY (1980) Ultrastructure and function of insect chemosensilla. Annu Rev Entomol 25:27–47. https://doi.org/10.1146/annurev.en.25.010180.000331
Zhou SH, Zhang J, Zhang SG, Zhang L (2008) Expression of chemosensory proteins in hairs on wings of Locusta migratoria (Orthoptera: Acrididae). J Appl Entomol 132:439–450. https://doi.org/10.1111/j.1439-0418.2007.01255.x
Zhu Q, Wu N, Brozek J, Dai W (2019) Antennal morphology and sexual dimorphism of antennal sensilla in Callitettix versicolor (Fabricius) (Hemiptera: Cercopidae). InSects 10:1–16. https://doi.org/10.3390/insects10020056
Acknowledgements
We sincerely thank Ben Hong and Bing Li (Guizhou University) for their valuable help in collecting insects. We are especially grateful to Xueli Feng (Guizhou University) for her help in the identification of insects.
Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 32060110, No. 31702045).
Author information
Authors and Affiliations
Contributions
Conceptualization and methodology: CL, SH; software: CL, KY; validation: CL, KY; formal analysis: KY; investigation: KY and SH; resources: CL; data curation: KY; writing—original draft preparation: KY; writing—review and editing: CL, SH; visualization: KY; supervision: CL; project administration: CL; funding acquisition: CL. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Ethics approval
No approval of the research ethics committees was required to accomplish the goals of this study, because experimental work was conducted with an unregulated invertebrate species.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yin, K., Huang, S. & Luo, C. Sensilla on the labial palps of the cave species Tachycines plumiopedella Li, Feng & Luo, 2021 (Orthoptera: Rhaphidophoridae). Zoomorphology 142, 169–179 (2023). https://doi.org/10.1007/s00435-023-00594-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00435-023-00594-8