Abstract
Acoel statocysts are fluid-filled chambers formed by two parietal cells underlying basal lamina as a capsule and contain a movable statolith cell called a lithocyte. The statocyst is needed for geotaxis; however, the mechanism of the gravity receptor system has not been elucidated. We focused on the geotactic ability of the acoel Praesagittifera naikaiensis, acquired during the development of the statocyst and its nervous system after hatching, and examined the three-dimensional relationship between the statocyst and its nervous system. Acoel geotactic ability was acquired between 0 and 7 days after hatching. No major changes in neural structures, namely a commissural brain, nerve cords, and commissures, were observed between juveniles and adults. The statocyst-associated commissure (stc), a commissural brain component, was circular and was located ventral to the statocyst but was not observed in neural connections to the capsule’s lumen. Fine structures of the statocyst revealed that the statolith developed after hatching. We hypothesized that geotactic ability needs the following conditions: (1) a sufficient concentration of calcium salt in the statolith; (2) stc must work as afferent neurons; and (3) a ventral polar cell, which is present outside the capsule, must be a sensory cell stimulated by the lithocyte.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
All living animals are subjected to various mechanical forces in their environments. Among these forces, gravity sensing is fundamental for appropriate behavior and orientation in space. In aquatic invertebrates, sensory organs for gravity sensing are known as statocysts. The most common type of statocyst consists of a fluid-filled chamber containing a solid granule called a statolith (Budelmann 1988; Brusca et al. 2016). The chamber’s inner lining includes touch-sensitive epithelium, such as hair cells (Budelmann 1988). The structural and functional diversity of statocysts has been discovered during evolution (Horridge 1969; Budelmann 1988; Bezares-Calderón et al. 2020). Among aquatic invertebrates, acoel flatworms are simple bilaterians lacking gut epithelium, an anus, and coelomic cavity. They also possess statocysts. Acoela belongs to the phylum Xenacoelomorpha (Philippe et al. 2011; Cannon et al. 2016); however, the phylogenetic position of Xenacoelomorpha is still unclear. Several phylogenetic analyses have suggested that Xenacoelomorpha is a sister group to Nephrozoa or Ambulacraria (Cannon et al. 2016; Marlétaz et al. 2019; Philippe et al. 2019). Thus, it is suggested that the simplicity of Acoela represents ancient bilaterian characteristics or secondary losses from a complex ancestor.
The acoel statocyst is a fluid-filled chamber formed by two parietal cells underlying basal lamina as a capsule. The chamber contains a movable statolith cell called a lithocyte (Ferrero 1973; Ehlers 1991). Acoel statocysts are surrounded by anterior conglomerations of neurons described by various terms such as the commissural brain (Raikova et al. 1998), statocyst ganglion (Bery et al. 2010), and cerebral ganglion (Ferrero 1973). Therefore, parietal cells or other sensory cells might be mechanically stimulated by lithocytes when the worms move and transmit the stimulus to afferent neurons. Although fine structural analyses of acoel statocysts have been conducted (Ferrero 1973; Ehlers 1991; Achatz and Martínez 2012), a clearly defined relation between sensory cells and those afferent neurons has not yet been determined. An acoel flatworm of Symsagittifera roscoffensis shows positive geotaxis, but just-hatched S. roscoffensis juveniles are occasionally observed failing to respond to gravity (Gamble and Keeble 1904; Keeble 1912). These findings suggest that acoel geotactic abilities should be acquired during the development of the statocyst and its nervous system after hatching.
In this study, we used Praesagittifera naikaiensis specimens that were collected in the Seto Inland Sea, Okayama, Japan. P. naikaiensis belongs to the family Convolutidae (Jondelius et al. 2011). The genus Praesagittifera is defined by the lack of sagittocysts, in contrast with the genus Symsagittifera (Gschwentner et al. 2002). P. naikaiensis has a cylindrical and flattened body that is green due to the presence of symbiotic algae. At the anterior part of worms, one statocyst and paired eyes are present (Yamasu 1991). These sensory organs are required for behavioral repertoires, including geotaxis and phototaxis. So far, the fine structures of the eyes and phototactic behavior have been investigated (Yamasu 1991). However, other morphological studies have not been conducted, despite the abundant availability of worms that could easily be collected in nature, maintained, and reproduced under laboratory conditions. Thus, we focused on geotaxis, which should be acquired after hatching, and examined the possibility that juveniles lack properly developed statocysts or nervous systems. This study compared the structure of the statocysts and nervous systems of juveniles and adults.
Additionally, to examine the three-dimensional relationship between the statocyst and nervous system of P. naikaiensis and consider gravity receptor systems in Acoela, we developed a new immunohistochemical strategy to simultaneously visualize both the nervous system and the statocyst. Although Drosophila synaptic protein 47 (dSap47) antibody can be used as a neural marker that selectively labels nerve terminals (Reichmuth et al. 1995; Sprecher et al. 2015), any appropriate markers for the statocyst have not been reported until now. Thus, we focused on underlying basal lamina of the epithelial parietal cells, which is a component of the statocyst. We used the type-IV collagen antibody to label the statocyst’s basal lamina, and the dSap47 antibody to label the nervous system. To the best of our knowledge, this study is the first report to visualize the relationship between acoel statocysts and nervous systems using confocal microscopy.
Materials and methods
Animals
P. naikaiensis adults were collected in the upper part of the intertidal zone on the Seto Inland Sea coasts in Japan. They were kept in aquariums with filtered seawater at 18 °C with a 12:12 h light–dark cycle. When spawning was observed, the eggs containing embryos were isolated and cultured in artificial seawater. The filtered and artificial seawater were changed weekly or according to needs. Adults were harvested within one month of collection. All worms were immersed in 7.14% MgCl2 hexahydrate to avoid muscle contraction before fixation.
Morphological analysis
Light microscopy images were acquired using a differential interference contrast microscope (IX71, Olympus, Tokyo, Japan) equipped with an Olympus DP21 microscope camera. Using ImageJ software (National Institutes of Health, Bethesda, Maryland, USA), we measured the lengths and widths of worm specimens. All data were expressed as means ± SE (n, number of worms).
Behavioral geotaxis assay
We followed a procedure described by Sprecher et al. (2015) to test the geotactic ability of worms during development after hatching. We used juveniles at 0 and 7 days after hatching (DAH) and adults. The geotactic ability index was defined as time spent descending a given slope. For the experiment, a 100 µl pipette tip was marked by a black line 1.7 cm from its tip. Each worm was drawn into the 100 µl pipette tip and allowed to settle behind the marked line. Then, the pipette tip was held at a 45° angle as the slope, and the time spent crossing the black line to the end of the pipette tip was measured. The maximum observation time was set to 120 s in case the worms did not show positive geotaxis. All data were expressed as means ± SE (n, number of worms).
Immunohistochemistry and confocal laser microscopy
Worms were immersed in a fixative containing 4% paraformaldehyde and 0.1 M phosphate buffer (pH 7.4) for 30 min for adults and 10 min for juveniles at room temperature. The specimens were then washed with a buffer solution: phosphate-buffered saline (PBS) containing 1% TritonX-100 was used for adults, and PBS containing 0.1% TritonX-100 was used for juveniles. After blocking with 3% normal goat serum, the worms were incubated with primary antibodies overnight at 4 °C and were then incubated with secondary antibodies for 2 h at room temperature. After washing with PBS, the worms were mounted in Fluoromount-G (Southern Biotechnology Associates, Alabama, USA). The following primary antibodies were used: mouse anti-dSap47 antibody at a 1:20 dilution (DSAP47-1, Developmental Studies Hybridoma Bank, Iowa, USA), and rabbit anti-mouse type-IV collagen antibody at a 1:150 dilution (LB1403, LSL, Tokyo, Japan). The following secondary antibodies were used: goat anti-mouse IgG antibody, Alexa Fluor 488 (A11001, Thermos Fisher Scientific, Tokyo, Japan), and goat anti-rabbit IgG antibody, Alexa Fluor 546 (A11010, Thermos Fisher Scientific, Tokyo, Japan), both at a 1:200 dilution.
Fluorescent images were taken using a confocal laser scanning microscope system (FV1200, Olympus, Tokyo, Japan). Optical sections ranged from 0.1 to 0.5 µm. Depth coding and stereo pair images were reconstructed from confocal image stacks using LSM 510 software (version 3.2; Carl Zeiss, Germany).
Transmission electron microscopy
Worms were immersed in a fixative containing 2.5% glutaraldehyde and 0.1 M cacodylate buffer (CB) containing 6% sucrose (pH 7.4) for 90 min for adults and 45 min for juveniles at room temperature. After washing with 0.1 M CB, the worms were post-fixed in 1% OsO4 and 0.1 M CB for 90 min for adults and 45 min for juveniles at room temperature. After washing with 0.1 M CB, the worms were dehydrated in a graded series of ethanol concentrations, transferred through propylene oxide, and then embedded in Spurr’s resin. Using a transmission electron microscope (H-7650, Hitachi, Tokyo, Japan) at 80 kV, thin sections were made and stained with uranyl acetate and lead citrate and examined.
Results
Ecological and morphological characteristics
P. naikaiensis was collected throughout 2019 in the upper part of the intertidal zone on the Seto Inland Sea coasts in Japan. Adults spawned eggs containing embryos (Fig. 1). Embryonic development, from spawning to hatching, took approximately 4 days under laboratory conditions. Embryos showed duet–spiral cleavage. Oval-shaped embryos began to rotate by ciliary movement within eggshells, 2 days after spawning. Those embryos flattened and rotated rapidly, 3 days after spawning. Flattened embryos pushed against the interior wall of eggshells, 4 days after spawning. After eggshells were broken, juveniles hatched and swam freely in culture dishes. In their natural habitat, juveniles may ingest symbiotic algae, similar to other acoel flatworms (Achatz et al. 2010; Bailly et al. 2014). Newly hatched juveniles that were reared in artificial seawater without algae could not survive for more than two months. Juveniles were 0.37 ± 0.03 mm long and 0.13 ± 0.01 mm wide at 0 DAH (n = 20) and 0.43 ± 0.03 mm long and 0.14 ± 0.01 mm wide at 7 DAH (n = 20). Adults were 2.26 ± 0.21 mm long and 0.44 ± 0.11 mm wide (n = 17). The brownish color of juveniles was due to the presence of protrusions, including pigments (Figs. 1, 2a, b). These protrusions extended toward the epidermal ciliary layer and were also present in adults (Fig. 2c, d), and may be glands with dark secretory products (Bery et al. 2010; Gschwentner et al. 2002). The greenish color of adults was due to the presence of symbiotic algae (Figs. 1, 2c, d). One statocyst and paired eyes were observed in the anterior region of both adult and juvenile bodies (Fig. 2a, c). The worms’ body surfaces were covered with epidermal cilia and sensory cilia (Fig. 2b, d). Sensory cilia were composed of microtubules (data not shown).
Life cycle of P. naikaiensis. Adults spawned eggs containing embryos. Embryonic development, from spawning to hatching, took approximately 4 days under laboratory conditions. After hatching, juveniles ingested symbiotic algae from their original habitat. Note color changes from brown to green during development after hatching. Scale bar 200 µm
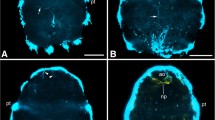
Sensory organs showing the statocyst, eyes, and sensory cilia. Each image consists of a light micrograph of the anterior (left) and lateral (right) regions of a worm body in juveniles (a, b) and adults (c, d). Note protrusions, including brown pigments, extending toward the ciliated epidermal cells. Arrowheads sensory cilia, e eyes, s statocyst, cl ciliary layer. Scale bars 30 µm (a, c), 20 µm (b, d)
Geotactic ability
The geotactic ability of worms was evaluated by the index defined as time spent descending a given slope in juveniles at 0 and 7 DAH, and adults (Fig. 3). Most of juveniles at 0 DAH did not show positive geotaxis (n = 27) since the maximum observation time was set to 120 s. A part of those showed positive geotaxis with an average time of 64.0 ± 16.5 s (n = 3). In contrast, all of juveniles at 7 DAH and adults showed positive geotaxis with an average time of 17.2 ± 1.0 s (n = 31) and 22.2 ± 2.5 s (n = 30), respectively. There was no significant difference in the average time between adults and juveniles at 7 DAH (P = 0.073). The geotactic ability of worms should be acquired between 0 and 7 DAH.
Comparison of geotactic ability among juveniles at 0 and 7 DAH and adults. The geotactic ability index was defined as time spent descending a given slope as described previously (Sprecher et al. 2015). The maximum observation time was set to 120 s. Most of juveniles at 0 DAH (n = 27) did not show positive geotaxis (open bar), although a part of them (n = 3) did (hatched bar). In contrast, the juveniles at 7 DAH (n = 30) and adults (n = 31) showed positive geotaxis (hatched bar). Data are expressed as mean ± SE. n.s. no significant difference
Nervous system
Immunoreactive patterns of dSap47 revealed nervous systems of juveniles at 0 and 7 DAH, and adults. In all stages, a commissural brain was observed at the anterior region, and three pairs of longitudinal nerve cords extended from the worm’s brain along the entire length of the worm body (Fig. 4). The nerve cords were dorsomedial nerve cords (dmc), dorsolateral nerve cords (dlc), and ventrolateral nerve cords (vlc). At the posterior region, the nerve cords were fused. Transverse commissures were interconnected among those nerve cords. The number of transverse commissures increased with worm age.
Three-dimensional observations of entire nervous systems of juveniles at 0 DAH (a), 7 DAH (b), and adults (c). Depth coding images were reconstructed from 140 optical sections taken at 0.2 µm for juveniles and at 100 optical sections taken at 0.5 µm for adults. A commissural brain was observed in the anterior region and connected to three pairs of longitudinal nerve cords in all stages (a, b, c). Note transverse commissures interconnecting among longitudinal nerve cords. Color-code: blue (ventral) to red (dorsal). dmc dorsomedial nerve cord, dlc dorsolateral nerve cord, vlc ventrolateral nerve cord. Scale bars 200 µm
The commissural brain was a bilateral symmetry and comprised an anterior aggregate of transverse commissures and three pairs of longitudinal nerve cords. No changes in its general arrangement were observed from the young to adult stages (Fig. 5). On the dorsal sides of the commissural brain, its longitudinal axis was derived from dmc and dlc. The pair of dmc was interconnected by three transverse commissures: a dorsal frontal commissure (dfc), a dorsal anterior commissure (dac), and a dorsal posterior commissure (dpc). The anterior tip of dlc was connected to a contact point between dmc and dac. On the ventral sides of the commissural brain, its longitudinal axis was derived from vlc. The pair of vlc was interconnected by two transverse commissures: a ventral anterior commissure (vac), and a statocyst-associated commissure (stc) that was circular.
Three-dimensional observations of commissural brains of juveniles at 0 DAH (a) and 7 DAH (b), and adults (c). Stereo pairs of confocal images were reconstructed from 70 optical sections taken at 0.2 µm. To obtain stereo images, view the left panel with the left eye and the right panel with the right eye at a distance of 30–35 cm. Each image was viewed from the ventral side. The commissural brain comprised an anterior aggregate of transverse commissures and longitudinal nerve cords (a, b, c). Note no changes in the general arrangement among all stages. dac dorsal anterior commissure, dfc dorsal frontal commissure, dpc dorsal posterior commissure, stc statocyst-associated commissure, vac ventral anterior commissure. Scale bars 20 µm
The statocyst’s basal lamina was labeled by the type-IV collagen antibody to elucidate the three-dimensional relationship between the statocyst and its nervous system (Fig. 6). In adults, the statocyst’s basal lamina was visualized as a spherical shape (Fig. 6a, arrowhead). Other parts stained with this antibody might show basal laminae surrounding smooth muscles (Gay et al. 1981; Jacob 2003). The statocyst was surrounded by the commissural brain (Fig. 6b, c); dfc, dac, and vac were located in the anterior region of the statocyst; dpc was located in the posterior region of the statocyst. A circular stc was located ventral to the statocyst (Fig. 6c). Neural connections to the lumen of the statocyst could not be observed. In juveniles at 0 and 7 DAH, the statocysts’ basal laminae were not visualized clearly (data not shown).
Three-dimensional relationship between statocyst and commissural brain in adults. Stereo pairs of confocal fluorescence images were reconstructed from 70 optical sections taken at 0.2 µm. To obtain stereo images, view the left panel with the left eye and the right panel with the right eye at a distance of 30–35 cm. Each image is viewed from the ventral side. The basal lamina of the statocyst was labeled by the type-IV collagen antibody (a). A commissural brain was labeled by the dSap47 antibody (b). Merged image showing the statocyst surrounded by the commissural brain (c). Note stc locating ventral to the statocyst. Arrowheads the basal lamina of the statocyst, stc statocyst-associated commissure. Scale bars 20 µm
Statocyst
We measured the diameter of lithocyte using a light microscope (Fig. 7). Juvenile lithocytes were 60.4 ± 2.6 µm2 at 0 DAH (n = 5) and 65.7 ± 7.1 µm2 at 7 DAH (n = 5). Adult lithocytes were 130.0 ± 7.7 µm2 (n = 5). The diameter of the lithocyte expanded with worm age. In all stages, the statocyst was a chamber that contained a lithocyte, including a statolith (Fig. 8). The inner lining of the chamber included parietal cells underlying basal lamina. The parietal cells were not ciliated. The cytoplasm of parietal cells was thinner at the ventral polar region of the statocyst. During thin-sectioning, the juvenile statoliths at 0 and 7 DAH were retained in those sections (Fig. 8a, b); in contrast, the adult statolith dropped off, and there was a hole (Fig. 8c).
Electron micrographs of transverse sections through statocyst of juveniles at 0 DAH (a) and 7 DAH (b), and adults (c). Dorsal sides face upwards. Note statocyst consists of three cells: two parietal cells and a movable statolith cell called a lithocyte. lc lithocyte, p parietal cell, sl statolith. Scale bars 10 µm
Discussion
P. naikaiensis is a Japanese endemic species and belongs to the Convolutidae family (Jondelius et al. 2011). The fine structures of the eyes and phototactic behavior has been previously reported (Yamasu 1991). However, other morphological and behavioral studies have not been conducted until now. In this study, we focused on positive geotaxis, and our findings revealed that the geotactic ability was acquired after hatching. Here, we discuss the gravity receptor system by comparing statocyst structures and nervous systems of juveniles and adults.
We compared our findings to the data available on two other acoel species, namely the Convolutidae S. roscoffensis and Symsagittifera psammophila. The nervous systems of S. roscoffensis are well-studied (Bery et al. 2010; Semmler et al. 2010; Bery and Martínez 2011; Sprecher et al. 2015; Gavilán et al. 2016), and it is endemic to the North Atlantic coasts (Bailly et al. 2014). S. roscoffensis adults are approximately 3–4 mm long and 500 µm wide (Semmler et al. 2008; Bailly et al. 2014) and are larger than P. naikaiensis adults. Moreover, the behavioral repertoires of S. roscoffensis have been investigated for over a century (Gamble and Keeble 1904; Keeble 1912; Bailly et al. 2014). Conversely, S. psammophila is similar to S. roscoffensis and can be found on the Black Sea coast near Feodosija, in the Gulf of Naples, and on the seashore near Pisa (Sarfatti and Bedini 1965). Among members of Convolutidae, S. psammophila is the only species in which the relationship between the statocyst and its nervous system is described at an electron microscopical level (Ferrero 1973; Ferrero and Bedini 1991).
Acquired geotactic ability during development after hatching
An acoel flatworm shows positive geotaxis (i.e., a vertical retreat) when a mechanical disturbance occurs (Sprecher et al. 2015; Arboleda et al. 2018). Sprecher et al. (2015) developed a behavioral geotaxis assay to evaluate the geotactic ability of S. roscoffensis during head regeneration. We applied this assay to P. naikaiensis to evaluate the worms’ geotactic ability during development after hatching. Our behavioral findings revealed that the geotactic ability of P. naikaiensis was acquired between 0 and 7 DAH. In S. roscoffensis, just-hatched juveniles were occasionally observed failing to respond to gravity, and such juveniles lacked properly developed statocyst (Gamble and Keeble 1904; Keeble 1912). In S. psammophila, by the time of hatching, the statolith has not formed crystalline nature, which is typical of the adult statolith (Ferrero and Bedini 1991). Such an immature statolith was observed in P. naikaiensis at 0 and 7 DAH at an electron microscopic level, but these results were inconsistent with our behavioral findings.
To explain this inconsistency, we hypothesized that the statolith’s chemical composition is involved in the gravity receptor system. The statolith in S. psammophila consists of calcium salt, and its adult statolith drops off during thin-sectioning (Ferrero 1973), as in adults of P. naikaiensis. The statolith in the ctenophore statocyst also consists of calcium salt, and such a dropping occurs in the adult but not in the juvenile statolith (Tamm 1982, 2014). These findings suggested that the juvenile statolith contains a lower concentration of calcium salt than the adult statolith (Tamm 1982, 2014). Thus, the P. naikaiensis statolith at 0 DAH was suggested to be more immature than at 7 DAH in terms of its calcium precipitation. To demonstrate this hypothesis, future studies are required to investigate changes in chemical compositions and the concentrations of the statolith during development after hatching.
Relationship between statocyst and its nervous system
Our results demonstrate that the statocyst of P. naikaiensis is surrounded by the commissural brain, which mainly consists of longitudinal nerve cords and transverse commissures. Brain arrangement is diverse among Acoela (Raikova et al. 1998; Bery et al. 2010; Semmler et al. 2010; Bery and Martínez 2011; Achatz and Martínez 2012; Sprecher et al. 2015; Dittmann et al. 2018). stc was only reported in S. roscoffensis (Sprecher et al. 2015), but the three-dimensional relationship between stc and the statocyst was not discussed because no appropriate markers for the statocyst have been reported until now. To visualize the statocyst, we focused on its basal lamina and tested an antibody against type-IV collagen. To the best of our knowledge, this is the first report to describe the three-dimensional relationship between the statocyst and nervous systems using an immunohistochemical method.
In Acoela, parietal cells or other sensory cells might be mechanically stimulated by a lithocyte when worms move and transmit the stimuli to afferent neurons. Our findings revealed that stc was located ventral to the statocyst and was circular. Moreover, it has been reported that outer neurons are closer to the ventral polar region, where its basal lamina is thinner than other regions of the capsule (Ferrero 1973; Ehlers 1991). In the case of parietal cells working as sensory cells, stc or other neurons must penetrate a basal lamina of the statocyst to connect to parietal cells. In Catenulida, which belongs to the phylum Platyhelminthes, its statocyst is a fluid-filled chamber formed by three parietal cells underlying basal lamina as a capsule and contains a movable statolith (Ehlers 1991). The parietal cells are sensory cells connected to outer neurons that penetrate the capsule (Ehlers and Sopott-Ehlers 1990; Ehlers 1991). However, in acoel statocysts, such penetrations and connections are not observed at the electron microscopic level (Ferrero 1973; Ehlers 1991; Achatz and Martínez 2012). It is challenging to find the neural connections on the thin sections since there are only a few connections on the spherical statocyst’s surface. Our developed method is useful for labeling both the basal lamina of a statocyst and specific nerve terminal markers to overcome this difficulty. However, further studies are needed because neural connections between the statocyst and stc could not be observed in this study.
Additionally, another hypothesis is that the sensory cells of the statocyst are present outside the capsule. In this case, sensory cells are stimulated indirectly by a lithocyte through parietal cells and basal lamina of the statocyst and transmit the stimuli to stc. In S. psammophila, it is suggested that the sensory cell is a ventral polar cell that faces the ventral pole outside the statocyst (Ferrero 1973). This cell is surrounded by a nerve cushion formed by several plasma membranes with light cytoplasm, which contains synapse vesicles (Ferrero 1973). Its cushion structure seems to correspond to the stc of P. naikaiensis. Moreover, parietal cells’ cytoplasm was thinner at the ventral polar region of the statocyst, which is better for transmitting indirect stimuli to the ventral polar cell.
To demonstrate this hypothesis, we must identify the sensory cells of acoel statocysts. The sensory cells convert mechanical stimuli into electrical signals, which is known as mechanotransduction. We should focus on transient receptor potential (TRP) channels, which are transduction ion channels. TRP channels are expressed in various animals, including hydra (Peng et al. 2015), planaria (Inoue et al. 2014), and mammals (Clapham 2003; Moran et al. 2004), and are activated by various stimuli such as touch, light, sound, chemicals, and temperature (Venkatachalam and Montell 2007). Presently, we are applying transcriptome analysis to P. naikaiensis to identify candidate trp genes and conducting expression analyses of some genes by in situ hybridization.
References
Achatz JG, Martínez P (2012) The nervous system of Isodiametra pulchra (Acoela) with a discussion on the neuroanatomy of the Xenacoelomorpha and its evolutionary implications. Front Zool 9:27. https://doi.org/10.1186/1742-9994-9-27
Achatz JG, Hooge M, Wallberg A, Jondelius U, Tyler S (2010) Systematic revision of acoels with 9 + 0 sperm ultrastructure (Convolutida) and the influence of sexual conflict on morphology. J Zool Syst Evol Res 48:9–32. https://doi.org/10.1111/j.1439-0469.2009.00555.x
Arboleda E, Hartenstein V, Martínez P, Reichert H, Sen S, Sprecher S, Bailly X (2018) An emerging system to study photosymbiosis, brain regeneration, chronobiology, and behavior: the marine Acoel Symsagittifera roscoffensis. BioEssays 40:1800107. https://doi.org/10.1002/bies.201800107
Bailly X, Laguerre L, Correc G et al (2014) The chimerical and multifaceted marine acoel Symsagittifera roscoffensis: from photosymbiosis to brain regeneration. Front Microbiol 5:498. https://doi.org/10.3389/fmicb.2014.00498
Bery A, Martínez P (2011) Acetylcholinesterase activity in the developing and regenerating nervous system of the acoel Symsagittifera roscoffensis. Acta Zool 92:383–392. https://doi.org/10.1111/j.1463-6395.2010.00472.x
Bery A, Cardona A, Martínez P, Hartenstein V (2010) Structure of the central nervous system of a juvenile acoel, Symsagittifera roscoffensis. Dev Genes Evol 220:61–76. https://doi.org/10.1007/s00427-010-0328-2
Bezares-Calderón LA, Berger J, Jékely G (2020) Diversity of cilia-based mechanosensory systems and their functions in marine animal behaviour. Phil Trans R Soc B 375:20190376. https://doi.org/10.1098/rstb.2019.0376
Brusca RC, Moore W, Shuster SM (2016) Invertebrates, 3rd edn. Sinauer Associates, Massachusetts
Budelmann BU (1988) Morphological diversity of equilibrium receptor systems in aquatic invertebrates. In: Atema J, Fay RR, Popper AN, Tavolga WN (eds) Sensory biology of aquatic animals. Springer, New York, pp 757–782
Cannon JT, Vellutini BC, Smith J, Ronquist F, Jondelius U, Hejnol A (2016) Xenacoelomorpha is the sister group to Nephrozoa. Nature 530:89–93. https://doi.org/10.1038/nature16520
Clapham DE (2003) TRP channels as cellular sensors. Nature 426:517–524. https://doi.org/10.1038/nature02196
Dittmann IL, Zauchner T, Nevard LM, Telford MJ, Egger B (2018) SALMFamide2 and serotonin immunoreactivity in the nervous system of some acoels (Xenacoelomorpha). J Morphol 279:589–597. https://doi.org/10.1002/jmor.20794
Ehlers U (1991) Comparative morphology of statocysts in the Plathelminthes and the Xenoturbellida. In: Tyler S (ed) Turbellarian biology. Springer, Dordrecht, pp 263–271
Ehlers U, Sopott-Ehlers B (1990) Organization of statocysts in the Otoplanidae (Plathelminthes): an ultrastructural analysis with implications for the phylogeny of the Proseriata. Zoomorphology 109:309–318. https://doi.org/10.1007/BF00803571
Ferrero E (1973) A fine structural analysis of the statocyst in Turbellaria Acoela. Zool Scr 2:5–16. https://doi.org/10.1111/j.1463-6409.1973.tb00793.x
Ferrero EA, Bedini C (1991) Ultrastructural aspects of nervous-system and statocyst morphogenesis during embryonic development of Convoluta psammophila (Turbellaria, Acoela). Hydrobiol 227:131–137. https://doi.org/10.1007/BF00027592
Gamble FW, Keeble F (1904) The bionomics of Convoluta roscoffensis, with special reference to its green cells. Proc R Soc Lond 72:93–98. https://doi.org/10.1098/rspl.1903.0022
Gavilán B, Perea-Atienza E, Martínez P (2016) Xenacoelomorpha: a case of independent nervous system centralization? Phil Trans R Soc B 371:20150039. https://doi.org/10.1098/rstb.2015.0039
Gay S, Martinez-Hernandez A, Rhodes RK, Miller EJ (1981) The collagenous exocytoskeleton of smooth muscle cells. Collagen Relat Res 1:377–384. https://doi.org/10.1016/S0174-173X(81)80014-3
Gschwentner R, Baric S, Rieger R (2002) New model for the formation and function of sagittocysts: Symsagittifera corsicae n. sp. (Acoela). Invertebr Biol 121:95–103. https://doi.org/10.1111/j.1744-7410.2002.tb00050.x
Horridge GA (1969) Statocysts of medusae and evolution of stereocilia. Tissue Cell 1:341–353. https://doi.org/10.1016/S0040-8166(69)80029-7
Inoue T, Yamashita T, Agata K (2014) Thermosensory signaling by TRPM is processed by brain serotonergic neurons to produce planarian thermotaxis. J Neurosc 34:15701–15714. https://doi.org/10.1523/JNEUROSCI.5379-13.2014
Jacob MP (2003) Extracellular matrix remodeling and matrix metalloproteinases in the vascular wall during aging and in pathological conditions. Biomed Pharmacother 57:195–202. https://doi.org/10.1016/S0753-3322(03)00065-9
Jondelius U, Wallberg A, Hooge M, Raikova OI (2011) How the worm got its pharynx: phylogeny, classification and Bayesian assessment of character evolution in Acoela. Syst Biol 60:845–871. https://doi.org/10.1093/sysbio/syr073
Keeble F (1912) Plant-animals: a study in symbiosis. Cambridge University Press, London
Marlétaz F, Peijnenburg KT, Goto T et al (2019) A new spiralian phylogeny places the enigmatic arrow worms among gnathiferans. Curr Biol 29:312–318. https://doi.org/10.1016/j.cub.2018.11.042
Moran MM, Xu H, Clapham DE (2004) TRP ion channels in the nervous system. Curr Opin Neurobiol 14:362–369. https://doi.org/10.1016/j.conb.2004.05.003
Peng G, Shi X, Kadowaki T (2015) Evolution of TRP channels inferred by their classification in diverse animal species. Mol Phylogenet Evol 84:145–157. https://doi.org/10.1016/j.ympev.2014.06.016
Philippe H, Brinkmann H, Copley R et al (2011) Acoelomorph flatworms are deuterostomes related to Xenoturbella. Nature 470:255–258. https://doi.org/10.1038/nature09676
Philippe H, Poustka AJ, Chiodin M et al (2019) Mitigating anticipated effects of systematic errors supports sister-group relationship between Xenacoelomorpha and Ambulacraria. Curr Biol 29:1818–1826. https://doi.org/10.1016/j.cub.2019.04.009
Raikova OI, Reuter M, Kotikova EA, Gustafsson MK (1998) A commissural brain! The pattern of 5-HT immunoreactivity in Acoela (Plathelminthes). Zoomorphology 118:69–77. https://doi.org/10.1007/s004350050058
Reichmuth C, Becker S, Benz M et al (1995) The sap47 gene of Drosophila melanogaster codes for a novel conserved neuronal protein associated with synaptic terminals. Mol Brain Res 32:45–54. https://doi.org/10.1016/0169-328X(95)00058-Z
Sarfatti G, Bedini C (1965) The symbiont alga of the flatworm Convoluta psammophila Bekl. observed at the electron microscope. Caryologia 18:207–223. https://doi.org/10.1080/00087114.1965.10796166
Semmler H, Bailly X, Wanninger A (2008) Myogenesis in the basal bilaterian Symsagittifera roscoffensis (Acoela). Front Zool 5:14. https://doi.org/10.1186/1742-9994-5-14
Semmler H, Chiodin M, Bailly X, Martínez P, Wanninger A (2010) Steps towards a centralized nervous system in basal bilaterians: insights from neurogenesis of the acoel Symsagittifera roscoffensis. Dev Growth Differ 52:701–713. https://doi.org/10.1111/j.1440-169X.2010.01207.x
Sprecher SG, Bernardo-Garcia FJ, van Giesen L et al (2015) Functional brain regeneration in the acoel worm Symsagittifera roscoffensis. Biol Open 4:1688–1695. https://doi.org/10.1242/bio.014266
Tamm SL (1982) Ctenophora. In: Shelton GAB (ed) Electrical conduction and behaviour in ‘simple’ invertebrates. Oxford University Press, pp 266–358
Tamm SL (2014) Formation of the statolith in the ctenophore Mnemiopsis leidyi. Biol Bull 227:7–18. https://doi.org/10.1086/BBLv227n1p7
Venkatachalam K, Montell C (2007) TRP channels. Annu Rev Biochem 76:387–417. https://doi.org/10.1146/annurev.biochem.75.103004.142819
Yamasu T (1991) Fine structure and function of ocelli and sagittocysts of acoel flatworms. Hydrobiol 227:273–282. https://doi.org/10.1007/BF00027612
Funding
Not applicable.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sakagami, T., Watanabe, K., Ikeda, R. et al. Structural analysis of the statocyst and nervous system of Praesagittifera naikaiensis, an acoel flatworm, during development after hatching. Zoomorphology 140, 183–192 (2021). https://doi.org/10.1007/s00435-021-00521-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00435-021-00521-9