Abstract
Few studies have been conducted on the reproductive biology and embryology of Kinosternon scorpioides. Determining the development of embryonic stages is essential for studies on comparative anatomy and phylogenetic relationships. The aim of this research was to examine the macroscopic embryonic development of K. scorpioides. At least three eggs were collected at incubation intervals of 5 days. After morphometry, embryos in stages 9–12 were classified in relation to the presence of pharyngeal arches, optic and otic vesicles, as well as sequential changes in both the forelimbs and hindlimbs. Embryos from stages 13 to 17 were identified through evident eye pigmentation, limb morphology, carapace appearance, and urogenital protuberance. From stages 19 to 22, the presence of digits on digital plates and pigmentation of the body were used for defining each of the stages. From stages 23 to 26, the digits and dense pigmentation on the body were used to define the stages and the disappearance of the urogenital protuberance and umbilical hernia. These results describe the ontogenetic changes that occur in this species, therefore facilitating the correct ex situ handling practices during incubation and serving as a basis for phylogenetic studies among the Kinosternidae.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The first morphological embryonic descriptions regarding the order Chelonia were performed in the nineteenth century (Rathke 1848; Agassiz 1857; Parker 1880). Subsequently, the embryonic development of semi-aquatic chelonians was standardized based on macroscopic characters in Chelydra serpentina in 27 stages (0–26) (Yntema 1968). Posteriorly, the development of Chrysemys picta was defined in 23 stages (Mahmound et al. 1973), and development of marine turtles was standardized in 31 stages with preovipositional stage characteristics included for the families Cheloniidae and Dermochelyidae (Miller 1885; Miller et al. 2017).
Species-specific features are important for understanding the development of animals (Werneburg 2009). However, this distinction between species makes it more difficult to standardize the development stages. This is the reason there are different tables for different chelonian species (Guzmán and Bonilla 1990; Greenbaum and Carr 2002; Okada et al. 2011). These tables still contain variations in numbers of development stages and incubation temperature (Santos Braga et al. 2020), which might hinder analysis among taxa. Embryonic development is standardized to understand each developmental stage of the animal, each of which are sensitive to environmental variations during incubation, since this phase is one of the most critical periods in the turtle’s life (Kobayashi et al. 2017; Koláčková et al. 2019). Embryological studies contribute to laboratory research that aims to use these animals as model organisms (Cordero and Janzen 2014). Moreover, they assist in taxonomic and phylogenetic studies, since embryonic development exhibits homologies that facilitate the understanding of phylogenetic relationships between groups of species (Boughner et al. 2007).
There is little information on the embryology of Kinosternidae, and there are few tables which explain the embryonic development of this family. The present study used C. serpentina standardized criteria (Yntema 1968) to include characteristics that allow the developmental stages to be identified (Ewert 1985). A comparison was made of Testudines families considering the developmental characteristics and incubation period, using Kinosternidae specimens (Ewert 1985). Some aspects of the development of Kinosternidae, Sternotherus odoratus, and Kinosternon subrubum morphology have been compared to other turtle species to determine the origins of skeletal innovations (Cordero and Quinteros 2015). In addition, Kinosternon scorpioides (Linnaeus 1766) embryos (21 days incubation) were used for the study of melatonin (Silva and Sampaio 2014). Smith‐Paredes et al. (2020) recently analyzed the development of S. odoratus, complementing the study with musculoskeletal observations. Recently, the skeletogenesis of the limbs of K. scorpioides was present (dos Santos Braga et al. 2020).
The present study characterizes the embryonic stages of K. scorpioides from the 10th day after oviposition through hatchings under laboratory conditions. This established the developmental stages of the embryo and the relationship between these stages. External morphological characters are essential in the criteria of differentiation of embryonic stages, and we therefore emphasize specific characteristics which facilitate comparison with other Kinosternidae and other groups of testudines.
Materials and methods
Embryonated eggs of K. scorpioides were collected between April and August from 2014 to 2017 on the experimental farm of the Brazilian Agricultural Research Corporation (Embrapa Amazônia Oriental) (LO 7310/2014-SEMAS-PA), located in Marajó Island (Salvaterra, Pará, Brazil, 0°42′26.90″ S and 48°33′34.70″ W). This study was approved by the Embrapa (Empresa Brasileira de Pesquisa Agropecuária)—Amazônia Oriental Ethics Committee for Use of Animals (n° 001/2016). Vouchers of embryonic development stages were deposited in the herpetological collection of Museu Paraense Emílio Goeldi (MPEG 1667—1683).
After being laid, the eggs were removed from the nest, washed with water, and transferred to a commercial incubator (Ecological Premium®, IP-R model) where they remained in a substrate of moisturized vermiculite under a mean temperature of 30 °C ± 0.5. The substrate was prepared using a kiln (Embrapa 1997) and dried at 95–110 °C for 72 h. The humidity level was set at 80% and checked daily. To calculate the amount of water in the substrate, the boxes were weighed twice a week and water was added, if necessary (Alvine et al. 2013).
Eggs were opened for fixation at 5 day intervals from the 10th incubation day until hatching. Morphometry of eye width, head width, crown–rump length, carapace straight length, width, plastron straight length, width, and height between carapace-plastron were measured using a manual caliper with 0.2 mm precision, to facilitate staging (Costa et al. 2017). Body volume of stage 18–26 embryos (0.90 ± 0.06 to 2.82 ± 0.07 cm, mean carapace length) was measured. After being eviscerated (n = 96), the heart, liver, and gastro-enteric tube were removed to measure humid volume, using the Archimedes’ Principle. This law of physics considers the displaced water volume as an approximate value to humid volume of the submerged organ (El Bizri et al. 2017; Andrade et al. 2018). This measurement was performed with help of hypodermic needles (containing 0.01 accuracy).
The embryos were fixed in a Bouin solution for 24 h and stored in 70% ethyl alcohol. This solution was used, because it better fixed the tissue due to its high penetrating power, and it guarantees firmness to these fragile tissue samples (Banks 1992; Soares et al. 2006; Magalhães et al. 2017). Each developmental stage was recorded with the help of a digital camera (CANON® EOS Rebel T5 coupled to a 60 mm macro-lens); sketches were made from the photographs (Supplementary S1). Criteria for embryo identification were adapted from the descriptions by Yntema (1968) and are shown in Table 1. A total of 181 embryos were obtained, with at least three embryos for each developmental stage. The number of embryos per embryonic development stage is shown in Table 2. The same amount of embryos was used for the statistical tests.
Mean values and standard error of morphometric measurements were calculated, as well as volumetric measurements, to test the existence of a relationship between measurements. To determine the embryo growth equation, a polynomial curve was performed between carapace length and incubation period using RStudio®. Then, polynomial curves were realized to determine the relationship between the increase in organ volume and the total volume of embryo bodies.
Results
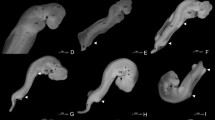
K. scorpioides embryo stages were observed from stages 10 to 26, in an incubation period lasting an average of 129.50 ± 21.73 (Fig. 1). The initial stages (0–9) were similar to those of C. serpentina, which are common among the other chelonian taxa (Greenbaum and Carr 2002); they therefore are not indicated here. New diagnostic features and the pre-hatching stage (26) were described. Pictures and schematic sketches of embryos are shown in Fig. 2. These figures show the embryonic development from the appearance of eyes until the formation of limbs. Detailed descriptions of the morphological features are presented in Table 1.
Stages 10–12 (Fig. 2) were determined by the presence of branchial arches, morphology of optic vesicle, and initial indications of limb formation (Fig. 2). Stages 13 and 14 (Fig. 2), in turn, were determined by the presence of nasal process and heart bludge, as well as the digital plate on the limbs (Fig. 2), beginning of urogenital bud, and lateral edge of the carapace. Stages 15–18 (Fig. 2) were classified by the presence of carapace, ribs, and plastron; aside from the formation of the three carapace keels and scutes, the appearance of structures such as eyelids, caruncles, and digits (Fig. 2) were reported during this period. Stages 19–22 (Fig. 2) were defined by the dispersion of body pigmentation the appearance of claws, and retraction of urogenital bud. Development stages 23–26 (Fig. 2) were determined by the closure of the vent, opening of nostrils, formation of ungueal phalanx (Fig. 2), and closing of umbilical hernia.
The maximum duration of the egg incubation period was 160 days, in which completely formed embryos were observed with total or partial absorption of the vitelline sac. Morphometric embryo measurements are shown in Table 2, with their mean values and deviation by embryo development stage.
On the other hand, the growth pattern showed that embryos developed rapidly in stages 10–17. However, their highest growth rates were observed from stage 21 onwards. The growth function (Fig. 1) was: y = − 1.770 + 8.225e−02 × x – 3.599e−04 × x2; R2 = 0.82; F = 161.6, p value = 0.000.
The curves performed on humid volumetric measures (Fig. 3) showed that the heart volume (y = 0.0043810 + 0.0146767 × x – 0.0013359 × x2; R2 = 0.16; F = 7.42, p value = 0.001) grew more slowly compared to the liver (y = 0.010252 + 0.059758 × x − 0.005288 × x2; R2 = 0.49; F = 34.85, p value = 0.000) and gastro-enteric tube (y = 0.004699 + 0.069700 × x – 0.006761 × x2; R2 = 0.55; F = 42.51; p value = 0.000), with the latter having a faster growth.
Discussion
This study describes the embryonic development of a Kinosternidae species and characterizes stages 10 to 26 of K. scorpioides (Table 1). Although the features observed here were similar to those observed in C. serpentina (Yntema 1968), stage-specific characters of K. scorpioides specie were also considered in the present study.
Table 3 shows the comparison of embryonic development stages of turtle species incubated at different temperatures. C. serpentina (Yntema 1968), sea turtles (Miller et al. 2017), and S. odoratus (Smith-Paredes et al. 2020) had one of the shortest incubation periods, even though it is the closest phylogenetically of K. scorpioides (Crawford et al. 2015; Shaffer et al. 2017). Therefore, it is evident that each stage is related to a specific expression, and that temperature affects the acceleration (or lack thereof) of the appearance of these characters, meaning that temperature determines the duration of each stage, and thus, the incubation period.
In reptiles, embryonic study is limited to egg and embryonic characteristics, but without observing their ontogenetic growth (Thompson 1989; Lourdais et al. 2015), unlike studies conducted with some mammals in which this tool has already been used (Lane and Gardner 1992; El Bizri et al. 2017; Andrade et al. 2018). Specific volume is an important aspect of the organogenesis of these animals, which can even be studied using fixed animals from zoological collections. Similar to mammals, the gastrointestinal tube and liver of K. scorpioides showed a strong relationship with body volume (El Bizri et al. 2017; Andrade et al. 2018): the liver has hematopoietic functions (Hill et al. 2019) which justifies the intense relationship with body volume, while the gastrointestinal tube must be prepared to feed and aid the digestion of hatchlings after birth (Vieira-Lopes et al. 2014).
Embryonic growth is explained by polynomial curves, which was also observed in C. picta (Cordero and Janzen 2014) and Carettochelys insculpta (Beggs et al. 2000). Although it is not a characteristic that defines development stages, a variation in embryo size can be another characteristic for criteria of stages (Miller et al. 2017).
Species-specific stage characters
Embryologic clade identification in K. scorpioides occurred from stage 15 onwards, with the formation of carapace, similar to other chelonians (Yntema 1968; Magalhães et al. 2017). In addition, species-specific identification began at stage 18, with the appearance of the three keels and carapace scutes. These features, along with body pigmentation, are patterns typical of Kinosternidae. This was similar to that observed in the present study, C. picta can be distinguished from other embryos at stage 17 (Cordero and Janzen 2014), affirming that clade-specific phenotypic differences are established at the middle one-third of incubation.
K. scorpioides has one of the longest incubation periods found in freshwater species. It is known that artificial incubation can last for up to 266 days in this species (Rocha and Molina 1990); however, this period varies in the literature, e.g., from 116 to 145 days (Guimarães et al. 2017), 129.3 ± 19.6 days (Costa et al. 2017), and 176 days (Vogt 2008). The incubation period in K. scorpioides and Kinosternon flavescens can be extended by up to 116 and 232 days, respectively (Ewert 1991) due to the synchronic hatching in periods that are favorable to juvenile feeding. This occurs during the early rainy season in tropical regions (Ewert 1991; Ewert and Wilson 1996; Rafferty and Reina 2012; Cristo et al. 2017).
Three morphological characters observed in adult animals could not be observed in embryos or in hatchlings of K. scorpioides: 1—presence of a nail at the tip of the tail; 2—smooth-like carapace, with concentric sculptures; 3—yellow plastron, with concentric and well-defined sculptures. The latter only showed dark spots in hatchlings. Therefore, hatchlings of this species might have morphological patterns on the head, carapace, and plastron (Guimarães et al. 2017) which may facilitate their camouflaging, as observed in mammal cubs (El Bizri et al. 2017) and alligators (Vieira et al. 2011).
Comparisons with other groups of Testudines
Stage patterns described in the chelonian embryonic development tables were effective in determining the development of K. scorpioides. However, studies on chelonian embryonic development have used different temperatures (seen in Table 3), making it difficult to compare the tables, as different temperatures change the metabolic rates of embryos, thus causing different responses in embryonic development, e.g., changes in duration between developmental stages (Yntema 1979; Packard and Packard 2000).
Pharyngeal slits in P. unifilis (Guzmán and Bonilla 1990) are responsible for the external facial appearance of embryos as seen at stage 10 and which disappear at stage 14. As in K. scorpioides, these are related to the initial development of the digestive, respiratory, and lymphoid systems. Maxillary and mandibular processes were described in most of the staging systems starting at stages 10 or 11. Development ends with upper and lower jaw occlusion between stages 18 and 19 (Greenbaum and Carr 2002; Okada et al. 2011; Magalhães et al. 2017), as seen in this study in stage 19. Werneburg et al. (2009) point out that Pleurodira have the most accelerated mandibular development when compared to Cryptodira, stating that this process may be related to the development of mandibular musculature and the way in which prey are captured (Werneburg et al. 2009).
The caruncle, egg tooth (Molina and Gomes 1998), was visible in K. scorpioides at stage 17, much later compared to P. expansa (Magalhães et al. 2017) in which it appeared at stage 14. This structure is linked to hatching, but also has importance on the origin of air spaces inside the egg during incubation. Besides opening cracks in extraembryonic membranes, the early appearance of the caruncle is related to the maturation that becomes keratinized (Ferguson 1985). Two days after hatching degenerates are not being present in hatchlings, as noted in Trachemys dorbignyi (Molina and Gomes 1998).
Limb pigmentation occurred from stage 16 onwards, similar to that found in C. serpentina embryos (Yntema 1968). This is an interspecies variation; the pigmentation patterns differ among species. However, due to their fragility, the application of Bouin to fix the samples of K. scorpioides could influence the pigmentation of the embryos in this work.
From their appearance at stage 10 onwards, limbs are important criteria for the differentiation and characterization of all embryonic developmental stages of K. scorpioides. Moreover, these structures provide key information regarding the evolution of tetrapod groups (Billet et al. 1985; Sheil and Portik 2008; Richardson et al. 2009; Joyce et al. 2013). Claws, seen from stage 20 onwards, were similar to what occurred in P. sinensis (Tokita and Kuratani 2001; Okada et al. 2011). Ungueal phalanx was first seen on the sheath of K. scorpioides embryos at stage 24 (Yntema 1968; Greenbaum 2002; Okada et al. 2011). This is later than in A. spinifera and T. scripta, in which it occurs during stage 22 (Beggs et al. 2000; Greenbaum and Carr 2002).
Carapace formation, which began in stage 14, was similar to C. serpentina (Yntema 1968), with the appearance of lateral and anterior carapace edges and marginal shields. On the other hand, the plastron formation pattern was similar to that found in stage 15 of M. japonica (Okada et al. 2011). However, the skin folds between the pectoral, abdominal, and femoral shields were only seen from stage 19 of incubation. The plastron of Kinosternidae is one of the main characteristics of this clade, due to the kinetic hinges that guarantee the partial or total closure of the shell (Bramble et al. 1984); in C. serpentina, this characteristic is not considered (Yntema 1968). The appearance of hinges is incomplete in newly hatched turtles (Gilbert et al. 2001; Cordero et al. 2018), as observed in this work. Hatchlings did not present total body retraction, due to the relationship between the size of the shell with the head and the limbs. This kinesis occurs in some species from the age of 3–5 (Cordero et al. 2018), however, a better understanding of the evolution of this phenotype in different turtle species is necessary.
Urogenital papillae were observed from stages 14 to 23. After stage 23, it was no longer visible, unlike the findings in other chelonian species where this structure was observed from stage 12 onwards (Greenbaum 2002; Okada et al. 2011). In this regard, urogenital papilla might contribute to a better understanding of the homologies in this group (Larkins and Cohn 2015) as they have different morphological variations among amniotes.
This study has described the embryonic development of K. scorpioides, from stage 10 to stage 26, under an incubation temperature of 30 °C for a period of 129.50 ± 21.73 days (dos Santos Braga et al. 2020). Together with characteristics common to other chelonians, embryonic development in this species has specific features that are essential for describing its stages. The results consequently contribute to understanding the ontogenetic aspects of this species and serve as a basis for comparative studies that aim to know the systematic relationships between groups of Testudines. Moreover, the proper ex situ embryo handling practices during incubation procedures were followed, allowing the determination of the time to translocate the nests to incubators that can control the incubation period and sex of the hatchlings, in addition to making it possible to identification the species and age of embryos where the spawning day is unknown (Hildebrand et al. 1997; Magalhães et al. 2017).
References
Agassiz L (1857) Contributions to the natural history of the US of America Embryology of the turtle, Part III, vol II. Little Brown and Company, Boston
Alvine T, Rhen T, Crossley DA (2013) Temperature-dependent sex determination modulates cardiovascular maturation in embryonic snapping turtles Chelydra serpentina. J Exp Biol 216:751–758. https://doi.org/10.1242/jeb.074609
Andrade RS, Monteiro FOB, El Bizri HR, Vicente WRR, Guimarães DAA, Mayor P (2018) Fetal development of the Poeppig’s woolly monkey (Lagothrix poeppigii). Therio 110:34–43. https://doi.org/10.1016/j.theriogenology.2017.12.022
Banks WJ (1992) Histologia veterinária aplicada. Manole, São Paulo
Beggs K, Young J, Georges A, West P (2000) Ageing the eggs and embryos of the pig-nosed turtle, Carettochelys insculpta (Chelonia: Carettochelydidae), from northern Australia. Can J Zoo 78:373–392. https://doi.org/10.1139/z99-214
Billet F, Gans C, Maderson PFA (1985) Why study reptilian development. In: Billet F, Maderson PFA (eds) Biology of the reptilia, vol 14. Wiley, New York, pp 1–25
Boughner JC, Buchtová M, Fu K, Diewert V, Hallgrímsson B, Richman JM (2007) Embryonic development of Python sebae—I: Staging criteria and macroscopic skeletal morphogenesis of the head and limbs. Zoology 110:212–230. https://doi.org/10.1016/j.zool.2007.01.005
Bramble DM, Hutchison JH, Legler JM (1984) Kinosternid shell kinesis: structure, function and evolution. Copeia 1984:456–475. https://doi.org/10.2307/1445203
Cordero GA, Janzen FJ (2014) An enhanced developmental staging table for the painted turtle, Chrysemys picta (Testudines: Emydidae). J Morphol 275:442–455. https://doi.org/10.1002/jmor.20226
Cordero GA, Quinteros K (2015) Skeletal remodelling suggests the turtle’s shell is not an evolutionary straitjacket. Biol Lett 11:20150022. https://doi.org/10.1098/rsbl.2015.0022
Cordero GA, Quinteros K, Janzen FJ (2018) Delayed trait development and the convergent evolution of shell kinesis in turtles. Proc R Soc B Biol Sci 285:20181585. https://doi.org/10.1098/rspb.2018.1585
Costa JS, Marques L, Matos AS, Silva CS, Figueró MR, Sales RL, Da Silva FE, Guimarães DAA, Marques JRF (2017) Características produtivas de Kinosternon scorpioides nas fases de acasalamento, postura e eclosão, criados em cativeiro na Amazônia. Arch Zootec 66:389–396
Crawford NG, Parham JF, Sellas AB, Faircloth BC, Glenn TC, Papenfuss TJ, Henderson JB, Hansen MH, Simison WB (2015) A phylogenomic analysis of turtles. Mol Phylogent Evol 83:250–257. https://doi.org/10.1016/j.ympev.2014.10.021
Cristo SS, Baía-Júnior PC, Silva JS, Marques JRF, Guimarães DAA (2017) The trade of Kinosternon scorpioides on Marajó island, Brazilian Amazon: from hunting to consumption. Herp J 27:361–367
El Bizri HR, Monteiro FOB, Andrade RS, Valsecchi J, Guimarães DAA, Mayor P (2017) Embryonic and fetal morphology in the lowland paca (Cuniculus paca): a precocial hystricomorph rodent. Therio 104:7–17. https://doi.org/10.1016/j.theriogenology.2017.08.004
Embrapa, (1997) Manual de métodos de análises de solo. Centro Nacional de Levantamento e Conservação Do Solo. Embrapa Solos 1:7–8
Ewert MA (1985) Embryology of turtles. In: Gans C, Billett F, Maderson PFA (eds) Biology of the reptilia, vol 14. Wiley, New York, pp 75–268
Ewert MA (1991) Cold torpor, diapause, delayed hatching and aestivation in reptiles and birds. In: Deeming DC, Ferguson MWJ (eds) Egg incubation: its effects on embryonic development in birds and reptiles. Cambridge University Press, Cambridge, pp 173–191
Ewert MA, Wilson DS (1996) Seasonal variation of embryonic diapause in the striped mud turtle (Kinosternon baurii) and general considerations for conservation planning. Chel Cons Biol 2:43–54
Ferguson MWJ (1985) Reproductive biology and embryology of the crocodilians. In: Cans C, Billett F, Maderson PA (eds) Biology of the reptilia, vol 14. Wiley, New York, pp 329–491
Gilbert SF, Loredo GA, Brukman A, Burke AC (2001) Morphogenesis of the turtle shell: the development of a novel structure in tetrapod evolution. Evol Dev 3:47–58. https://doi.org/10.1046/j.1525-142x.2001.003002047.x
Greenbaum E (2002) A standardized series of embryonic stages for the emydid turtle Trachemys scripta. Can J Zoo 80:1350–1370. https://doi.org/10.1139/z02-111
Greenbaum E, Carr JL (2002) Staging criteria for embryos of the spiny softshell turtle, Apalone spinifera (Testudines: Trionychidae). J Morphol 254:272–291. https://doi.org/10.1002/jmor.10036
Guimarães CDO, Silva ASL, Palha MDC (2017) Incubação de ovos e desenvolvimento pós-natal de Kinosternon scorpioides (Linnaeus, 1766) (Testudines, Kinosternidae) em cativeiro. Pubvet 11:1285–1292. https://doi.org/10.22256/pubvet.v11n12.1285-1292
Guzmán N, Bonilla H (1990) Serie normal del desarrollo morfológico embrionario de Podocnemis unifilis (Troschel, 1848) (Testudinata: Pelomedusidae). Acta Biol Colomb 2:129–150
Hildebrand PV, Bermudez N, Peñuela MC (1997) La tortuga charapa (Podocnemis expansa) em el bajo rio Caqueta Amazonas, Colombia: Aspectos de la Biologia reproductiva y Tecnicas para sua manejo. Disloque Editora, Colombia
Hill K, Stewart KM, Rajeev S, Conan A, Dennis MM (2019) Pathology of leatherback sea turtle (Dermochelys coriacea) embryos and hatchlings from nests in st. kitts, West Indies (2015–16). J Wildl Dis 55:782–793. https://doi.org/10.7589/2018-07-169
Joyce WG, Werneburg I, Lyson TR (2013) The hooked element in the pes of turtles (Testudines): a global approach to exploring primary and secondary homology. J Anat 223:421–441. https://doi.org/10.1111/joa.12103
Kobayashi S, Wada M, Fujimoto R, Kumazawa Y, Arai K, Watanabe G, Saito T (2017) The effects of nest incubation temperature on embryos and hatchlings of the loggerhead sea turtle: implications of sex difference for survival rates during early life stages. J Exp Mar Biol Ecol 486:274–281. https://doi.org/10.1016/j.jembe.2016.10.020
Koláčková M, Kreisinger J, Albrecht T, Hořák D (2019) Effect of incubation temperature on sex-dependent embryo mortality and morphological traits in Mallard. J Therm Biol 83:95–102. https://doi.org/10.1016/j.jtherbio.2019.05.007
Lane M, Gardner DK (1992) Effect of incubation volume and embryo density on the development and viability of mouse embryos in vitro. Hum Reprod 7:558–562
Larkins CE, Cohn MJ (2015) Phallus development in the turtle Trachemys scripta. Sex Dev 9:34–42. https://doi.org/10.1159/000363631
Linnaeus C (1766) Systema naturæ per regna tria naturæ, secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis. Tomus I, Laurentii Salvii, Stockholm, Holmiae.
Lourdais O, Lorioux S, Dupoué A, Wright C, DeNardo DF (2015) Embryonic water uptake during pregnancy is stage-and fecundity-dependent in the snake Vipera aspis. Comp Biochem Phys A 189:102–106. https://doi.org/10.1016/j.cbpa.2015.07.019
Magalhães MS, Vogt RC, Sebben A, Dias LC, Oliveira MF, Moura CEB (2017) Embryonic development of the Giant South American river turtle, Podocnemis expansa (Testudines: Podocnemididae). Zoomorphology 136:523–537. https://doi.org/10.1007/s00435-017-0365-8
Mahmound LY, Hess GL, Klicka J (1973) Normal embryonic Stages of the western painted Turtle Chrysemys picta belii. J Morphol 141:69–279
Miller JD (1985) Embryology of turtles. In: Gans C, Billett F, Maderson PFA (eds) Biology of the reptilia, vol 14. Wiley, New York, pp 270–317
Miller JD, Mortimer JA, Limpus CJ (2017) A field key to the developmental stages of marine turtles (Cheloniidae) with notes on the development of Dermochelys. Chel Cons Biol 16:111–122. https://doi.org/10.2744/CCB-1261.1
Molina FB, Gomes N (1998) Incubação artificial dos ovos e processo de eclosão em Trachemys dorbignyi (Duméril and Bibron) (Reptilia, Testudines, Emydidae). Rev Bras Zool 15:135–1043
Okada Y, Yabe T, Oda S-I (2011) Embryonic development of the Japanese pond turtle, Mauremys japonica (Testudines: Geoemy-didae). Curr Herpetol 30:89–102. https://doi.org/10.5358/hsj.30.89
Packard GC, Packard MJ (2000) Developmental plasticity in Painted Turtles, Chrysemys picta. Funct Ecol 14:474–483. https://doi.org/10.1046/j.1365-2435.2000.00448.x
Parker WK (1880) Report on development of the green turtle (Chelonia viridis Schneid). In: Report of the scientific results of the voyage of H. M. S. Challenger during the years 1872–1876, Green and Co, London, pp 1–57
Rafferty AR, Reina RD (2012) Arrested embryonic development: a review of strategies to delay hatching in egg-laying reptiles. Proc Roy Soc B Biol Sci 279:2299–2308. https://doi.org/10.1098/rspb.2012.0100
Rathke H (1848) Ueber die Entwickelung der Schildkroten. Freidrich Vieweg und Sohn, Braunschweig
Richardson MK, Gobes SMH, Van Leeuwen AC, Polman JAE, Pieau C, Sánchez-Villagra MR (2009) Heterochrony in limb evolution: developmental mechanisms and natural selection. J Exp Zool Part B 312:639–664. https://doi.org/10.1002/jez.b.21250
Rocha MB, Molina FB (1990) Reproductive Biology of Kinosternon scorpioides (Testudines: Kinosternidae) in Captivity. Tortoises Turt 5:8
dos Santos Braga BS, Fernandes Neto DL, Teixeira LC, Silva Costa J, Ferreira MAP, Oliveira-Bahia VR, Marques JRF, Araújo Guimarães DA (2020) Skeletal development of Kinosternon scorpioides Limbs (Chelonia: Kinosternidae). Anat Rec ar 24578:1–14. https://doi.org/10.1002/ar.24578
Shaffer HB, McCartney-Melstad E, Near TJ, Mount GG, Spinks PQ (2017) Phylogenomic analyses of 539 highly informative loci dates a fully resolved time tree for the major clades of living turtles (Testudines). Mol Phylogenet Evol 115:7–15. https://doi.org/10.1016/j.ympev.2017.07.006
Sheil CA, Portik D (2008) Formation and ossification of limb elements in Trachemys scripta and a discussion of autopodial elements in turtles. Zool Sci 25:622–641. https://doi.org/10.2108/zsj.25.622
Silva RN, Sampaio LFS (2014) Immunoreactivity of Mel1a-like melatonin receptor and NRH: quinone reductase enzyme (QR2) in testudine whole embryo and in developing whole retinas. Trends Dev Biol 8:39–46
Smith-Paredes D, Lord A, Meyer D, Bhullar BS (2020) A developmental staging system and musculoskeletal development sequence of the Common Musk turtle (Sternotherus odoratus). Developmental Dynamics dvdy. https://doi.org/10.1002/dvdy.210
Soares JM, Belletti ME, Machado ER, Silva M (2006) Histomorfometria de testículos de gatos (Felis domestica) utilizando-se três diferentes fixadores. Biosci J 22(1):175–181
Thompson MB (1989) Patterns of metabolism in embryonic reptiles. Respir Physiol 76:243–255
Tokita M, Kuratani S (2001) Normal embryonic stages of the Chinese Softshelled turtle Pelodiscus sinensis (Trionychidae). Zool Sci 18:705–715. https://doi.org/10.2108/zsj.18.705
Vieira LG, Lima FC, Santos ALQ, Mendonça SHST, Moura LR, Iasbeck JR, Sebben A (2011) Description of embryonic stages in Melanosuchus niger (Spix, 1825) (Crocodylia: Alligatoridae). J Morphol Sci 28:11–22
Vieira-Lopes DA, Nascimento AA, Sales A, Ventura A, Novelli IA, Sousa BM, Pinheiro NL (2014) Histologia e histoquímica do tubo digestório de Phrynops geoffroanus (Testudines, Chelidae). Acta Amazon 44:135–142
Vogt RC (2008) Tartarugas Da Amazonia. Gráfica Biblos, Lima
Werneburg I (2009) A standard system to study vertebrate embryos. PLoS ONE. https://doi.org/10.1371/journal.pone.0005887
Werneburg I, Hugi J, Müller J, Sánchez-Villagra MR (2009) Embryogenesis and ossification of Emydura subglobosa (Testudines, Pleurodira, Chelidae) and patterns of turtle development. Dev Dynam 238:2770–2786. https://doi.org/10.1002/dvdy.22104
Yntema CL (1968) A series of stages in the embryonic development of Chelydra serpentina. J Morphol 125:219–251. https://doi.org/10.1002/jmor.1051250207
Yntema CL (1979) Temperature levels and periods of sex determination during incubation of eggs of Chelydra serpentina. J Morphol 159:17–27. https://doi.org/10.1002/jmor.1051590103
Acknowledgements
The present study was performed with the support of Fundação Amazônia de Amparo a Estudos e Pesquisa (FAPESPA)—ICAAF 144/2014 (Thematic Projects: Agriculture and Family Farming). We extend our sincere thanks to Empresa Brasileira de Pesquisa Agropecuária (EMBRAPA Amazônia Oriental)—BAGAM, to Laboratório de Técnicas Histológicas (ICB/UFPA) and Laboratório Multidisciplinar de Morfofisiologia Animal (ICB/UFPA). We also thank Laboratório de Biologia Celular e Helmintologia (ICB/UFPA). BSSB and SRS thank the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for the Grant linked to the Graduate Degree Program in Biodiversity and Conservation of the Universidade Federal do Pará (UFPA), BSSB and DLFN thank the CAPES for the Grant linked to the Graduate Degree Program in Animal Science of the UFPA. And we are grateful for support of PROPESP/UFPA.
Funding
The research has a Grant supported by Fundação Amazônia de Amparo a Estudos e Pesquisa (FAPESPA)—ICAAF 144/2014 (Thematic Projects: Agriculture and Family Farming). Scholarships were funded by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, and supported by PROPESP/UFPA, PAPQ 01/2019.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no potential conflict of interest.
Ethical approval
This article does not contain any studies with human participants or living animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
dos Santos Braga, B.S., Fernandes-Neto, D.L., Leal, R.P. et al. Embryonic development of Kinosternon scorpioides (Testudines: Kinosternidae). Zoomorphology 140, 279–290 (2021). https://doi.org/10.1007/s00435-021-00517-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00435-021-00517-5