Abstract
Insects adapt commonly to seasonally changing habitats and reproductive contexts. Individuals that mature at different times during the year can show patterns of life cycle or morphological variation, possibly associated with changes in reproductive behaviour. Concerning mating strategies of flying insects, wing morphology may be related both to the outcome of male–male contests and to the ability in acquiring females. Therefore, different mating strategies (territorial vs. non-territorial) may have different flight morphology optima that increase fitness in their context. Males of Calopteryx splendens are mainly territorial early in the season, but with the advancing season and with increasing competition, more and more males adopt a non-territorial pursuing strategy. Given that different mating tactics have different wing morphologies, here we test whether the wing shape of males shifts from a “territorial” to a “non-territorial morphology” during the season. So, early in the season males show highly sexually dimorphic wings, which allow for high manoeuvrability and larger spots, while late in the season wing shapes of males become less sexually dimorphic and more suitable when pursuing females. Additionally, we studied the seasonal variation of other flight related traits, specifically wing lengths, abdomen length and weight. We found that these latter traits decreased along the season in both sexes without altering sexual dimorphism. However, wing shape, which resulted sexually dimorphic, showed a seasonal variation, decreasing the level of sexual dimorphism. The most probable determinant of this change is phenotypic plasticity triggered by environmental cues, but other explications of the observed pattern are discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In seasonal habitats, many environmental variables change predictably during the season, such as temperatures and photoperiod, that signal the time available before the onset of winter. As a consequence, insects have to adapt to these environmental changes through life cycle regulations and/or phenotypic plasticity, in order to maximize fitness (Nylin 1994; Gotthard and Nylin 1995; Nylin and Gotthard 1998). Phenotypic plasticity could promote adaptive changes in morphology (Nylin and Gotthard 1998) or adaptive variation in development time or growth rate (Gotthard et al. 1994), and these two aspects of adaptation often correlate (Nylin 1994; Nylin and Gotthard 1998). Life cycle regulations determined by environmental changes may trigger morphological adaptations, generating the most common kind of insect “polymorphism”, seasonal polyphenism, in which different phenotypes predominate at different times of the season (Shapiro 1976; Nijhout 2003).
Most studies investigating the effect of season have focused on variation in size that generally decreases late in the year in insect species with complex life-history shifts (Sweeney and Vannote 1978; Rowe and Ludwig 1991; Nylin and Gotthard 1998). Usually, this type of phenotypic variation has been ascribed to an adaptation of growth rate to variations in temperature or photoperiod, rather than interpreted as a non-adaptive reaction (Rowe and Ludwig 1991). Other kinds of seasonal polyphenisms concern wing colour changes, especially in butterflies (Brakefield and Larsen 1984; Kingsolver and Wiernasz 1991; Kingsolver 1995), which mainly links with thermoregulation and mimicry.
In some species, seasonal adaptation includes the adoption of alternative conditions or phenotype-dependent mating tactics which differ between early and late emergers and which increase fitness under different environmental conditions (Brockmann 2001). Commonly, in males, these mating tactics can be divided in a territorial strategy, in which individuals hold territories with female-relevant resources and court females, and non-territorial strategies, in which males do not defend territories and try to mate with females without defending a resource (i.e. Gross 1996; Plaistow et al. 2004). The adoption of such strategies may be genetically determined, a product of phenotypic plasticity or depend on male condition (Oliveira et al. 2008). In flying insects, mating tactics are often associated with different physiological (i.e. fat content, age or immunocompetence: Marden and Waage 1990; Plaistow and Siva-Jothy 1996; Kemp and Alcock 2003; Kemp and Wiklund 2004; Koskimäki et al. 2004) or morphological characters. Specifically, flight morphologies, which are selected for specific flight needs, are closely correlated with flight performance (Dudley 2002; Berwaerts et al. 2002). This flight performance is the key to the outcome of territorial competition, to energy consumption necessary for locating or chasing females, and to other fitness relevant behaviours (e.g. predator avoidance). So, different behavioural strategies have different flight morphology optima, and if strategies change during the season, also optimal flight morphology is expected to change accordingly in an adaptive way. Evidence for this assumption has been reported for the butterfly Parage aegeria, in which males can be territorial perchers or non-territorial patrollers that adopt a permanent searching flight. This species shows a seasonal reduction in territoriality, which correlates with morphological variation in males. They change from a territorial to a non-territorial flight morphology, an adaptive seasonal plasticity, probably triggered by environmental cues (Van Dyck and Wiklund 2002).
In this study we tested whether a pattern of seasonal adaptation in morphology, similar to that described above, exists in the damselfly Calopteryx splendens (Harris 1780) in relation to a variation in viability of the territorial behaviour along the season that also occurs in this species. This member of the family Calopterygidae (Odonata) ranges from the European Atlantic coast to north-west of China (Dijkstra and Lewington 2006) and shows sexually dimorphic colouration, with a greenish blue body in males and greenish brown body colour in females. Male wing colouration in this species, and across the genus, is condition-dependent since it correlates with several aspects of individual condition such as immunocompetence and competitive ability (Siva-Jothy 2000; Córdoba-Aguilar and Cordero-Rivera 2005; Rantala et al. 2010). Hardersen (2010) showed that the area of wing spots in C. splendens males decreases during the reproductive season.
A male of C. splendens can adopt either territorial or non-territorial behaviour. When territorial, a male defends a territory and actively courts females, displaying its wing spots, the size of which acts as secondary sexual characters signalling male’s quality to male competitors and to females (reviewed in Córdoba-Aguilar and Cordero-Rivera 2005). Territorial males experience high competitive costs but have higher fitness than non-territorial ones (Plaistow and Siva-Jothy 1996; Oliveira et al. 2008). When individuals are unable to defend a territory, they adopt a non-territorial strategy, and try to obtain opportunistic matings by pursuing females. Since these strategies are usually considered to be dependent on the physiological conditions (e.g. fat content), males can adopt these sequentially, being initially territorial and later switching to non-territorial behaviour, when fat reserves are depleted (Forsyth and Montgomerie 1987; Plaistow and Siva-Jothy 1996; Outomuro et al. 2014). Because of this, hereafter, when referring to non-territorial males, we intend males that are currently adopting a non-territorial strategy, which can be adopted after a period of territoriality (Outomuro et al. 2014). This switching to an alternative reproductive tactic is interpreted as a behaviour which maximizes fitness. The fraction of males adopting a non-territorial behaviour during their lives increases with population density because of the excessive competition for the fixed number of available territories (Forsyth and Montgomerie 1987; Cordero-Rivera 1999). Consequently, the percentage of males showing non-territorial behaviour increases with the advancing breeding season because population density increases from early spring onwards. At high-population densities, mating attempts largely depend on pursuing flights and forced copulations (Hilfert and Rüppell 1997; Cordero-Rivera 1999; Córdoba-Aguilar and Cordero-Rivera 2005; Hilfert-Rüppell and Rüppell 2009) and females perform convenience polyandry (Cordero-Rivera and Andrés 2002).
Wing morphology in C. splendens is strongly sexually dimorphic with shorter and more “rounded” wings in males, probably subject to sexual selection (Outomuro and Johansson 2011; Outomuro et al. 2012), and more tapered wings in females, closer to the aerodynamic optimum for less energetically demanding flight selected by natural selection (Betts and Wootton 1988; Bots et al. 2009; Outomuro et al. 2012; Sacchi and Hardersen 2013). Wing shape differs between alternative reproductive tactics in this species (Outomuro et al. 2014): territorial males have shorter and broader wings than non-territorial ones, which have wings slightly more similar to those of females. This phenotypic difference might be related to improved manoeuvrability in territorial males and/or to the co-evolution between shape and wing spot size (Outomuro et al. 2014). Indeed, wing shape constrains the area available for the deposition of pigments and consequently affects the attractiveness of individual males to females (Outomuro and Johansson 2011; Outomuro et al. 2013a). Furthermore, a more tapered and less sexually dimorphic wing may improve success rate when pursuing females thanks to a flight morphology more similar to that of females, increasing lifetime reproductive success as more opportunistic matings are obtained (Forsyth and Montgomerie 1987; Outomuro et al. 2014).
The reasons for the differences in wing shape between territorial and non-territorial males are not known, and phenotypic plasticity as well as genetic differentiation between “morphs” is the possible cause (Outomuro et al. 2014).
Here, we test the hypothesis that average wing shape of males shifts from a “territorial” morphology to “non-territorial” wing shape, which should be less sexually dimorphic, as population density increases during the season and as ever less males are able to adopt a territorial behaviour for most of their life time. This predicted shift should allow for higher manoeuvrability and larger wing spots when male density is low early in the season, and for faster flight, more similar to females, when pursuing females later in the season, when male density is high. This pattern may result in wing shape phenotypic adjustment, in which wing development adaptively changes, depending on the more likely strategy males are going to adopt at the specific time of the year when sexually active. Wing shape in females is not expected to change to the same extent because females do not show alternative mating strategies and always benefit from having slender wing adapted to reduce flight energy demand. Therefore, if males shift from a territorial to a non-territorial strategy, we predict that the extent of sexual dimorphism in wing shape will decrease as the season advances.
Materials and methods
Sampling
Females and territorial males of C. splendens were sampled from May to September in 2009 and 2010 from a stream near Pavia (Northern Italy: 45°10′04″N, 9°04′40″E). We sampled territorial males because these males won at least some territorial contests and are likely to have wings which are the most sexually dimorphic. Therefore, any correlation between wings shape, sexual dimorphism and season should be most obvious in this group. Males were considered territorial only if they were resident in a territory and defended it against intruders, and if they were courting females. A total of 769 individuals were caught, 412 males and 357 females, during six sampling sessions in 2009 and four in 2010 (Sample details are given in Online Resource 1). Individuals were classified as early, middle, and late depending on sampling date as follows: early season, before the 3rd July (2009: two sampling sessions for 110 males and 81 females; 2010: two sampling sessions for 71 males and 70 females), middle season from 4th July to 11th of August (2009: two sampling sessions including 93 males and 78 females; 2010: one sampling session including 47 males and 41 females), and late season encompassed all dates after 12th August (2009: two sampling sessions including 51 males and 45 females; 2010: one sampling session including 40 males and 42 females). Individuals were weighed to the nearest 0.01 mg using a precision balance (Sartorius research, R200D), and digital images of their wings were obtained with a scanner (HP Scanjet G4010, resolution of 600 DPI). One individual was not weighed because it had lost its head. Damselflies were scanned dorsally, while the wings were blocked by two pieces of polyurethane foam held down with a weight of about 50 g (Cigognini et al. 2014). This system prevented animal movement. A strip of graph paper was included in the scan to provide a scale. Three individuals had damaged wings (one had damaged front wings and two damaged hind wings); consequently, these were not used in further analysis. After image acquisition, each individual was marked prior to release at the site of origin in order to avoid recapture. Several marked individuals were found the days after they had been released, and this suggests that handling did not interfere with short-term survival and population viability.
Image processing and analysis
Wing shape was quantified using geometric morphometrics methods (Rohlf and Marcus 1993; Bookstein 1997; Adams et al. 2004). These methods are a statistical framework for shape analysis that allow for the quantification of shape using Generalized Procrustes Analysis (GPA, Rohlf and Slice 1990; Adams et al. 2004), which scales, translates and rotates landmark configurations removing the effect of non-shape variation (Rohlf and Marcus 1993; Bookstein 1997). Information about size is retained as centroid size (CS) and is defined as the square root of the sum of the squared distances between each landmark and the specimen centroid. Ten homologous landmarks located where veins meet the edge of the wing (Fig. 1) were recorded on the right front wing and on the right hind wing using TpsDig2 software (Rohlf 2010). To test whether landmark positioning was repeatable, front and hind wing landmarks of a sub-sample of ten animals were digitalized three times, with a day interval between measurements. Repeatability resulted very high (ICC > 0.99, F 9,20 > 1.6 × 106 and p < 0.001 for all x and y coordinates of both front and hind wing landmarks).
Locations of the 10 landmarks, defined by the intersection between wing margin and: (1) nodus, (2) first radius, (3) third radius, (4) third radius intercalary, (5) fourth + fifth radius, (6) medius, (7) first cubitus, (8) second cubitus, (9) proximal apex of anal triangle, (10) Connection Costa—Subcosta. Nomenclature follows Dumont (1991). The grey area represents the spot of males
After landmark digitalization, the abdomen length of all individuals was measured using tools available in TpsDig2 software (Rohlf 2010), except for 27 individuals whose abdomens were not completely visible. Repeatability of abdomen measurements was checked using three replicates with a day interval between measurements on a sub-sample of ten animals was highly significant (F 17,36 = 4.9 × 103 and p < 0.001).
Afterwards, a GPA was performed on digitalized landmark configurations, for front and hind wings, obtaining aligned configurations. The natural logarithm of the centroid size (LnCS) for front and hind wing was chosen as an index of wing size instead of using wing lengths (for a distribution of raw data of LnCS see Online Resources 2). A principal component analysis (PCA) was carried on the variance–covariance matrix of the landmark coordinates of the aligned configurations (Claude 2008). Since the dimensionality of the data is 2 × p − 4 (i.e. 2 × 10 − 4 = 16), where p is the number of landmarks (Dryden and Mardia 1998), we used the scores of the first 16 PCs as a set of shape variables.
Statistical analysis
Body weight, abdomen length, the size of front and hind wing, estimated as LnCS, were only weakly correlated (Pearson’s correlation coefficient r p ranking from 0.33 to 0.63), whereas the size of front and hind wing were strongly correlated (r p = 0.97). The variation of traits along the breeding season was analysed using linear models including the factors SEX, SEASON (with three levels corresponding to early, middle and late season), YEAR and all interactions among them as predictors. An independent model was performed for each trait. In these analyses, the main effect of SEX stands for sexual dimorphism, the main effect of SEASON is for the plastic response to seasonal variation of environmental constraints and the main effect of YEAR accounts for differences in the environmental constraints among breeding seasons, the SEX × SEASON interaction measures the context-dependent effect on sexual dimorphism due to constraints within the season, SEX × YEAR account for yearly variability of sexual dimorphism due to yearly variability of ecological factors. Also the SEASON × YEAR interaction was included to account for possible not synchronous variations of the traits in the 2 years. The SEX × SEASON × YEAR interaction accounts for yearly differences in the variation of sexual dimorphism between the three periods.
In order to assess whether sexual dimorphism in wing shape varies with the season the set of shape variables was used as dependent variable in a MANCOVA including the three factors SEX, SEASON, and YEAR, and the covariate SIZE as predictors. Additionally, all 2- and 3-way interactions between predictors were tested. Two independent models were performed for front and hind wings, respectively. In order to verify whether the models showed a pattern of variation in sexual dimorphism of wing shape consistent with our hypothesis, we computed the Procrustes distances between mean shapes of males and females of our sample for each period of the season.
All models in the analysis were simplified by removing non-significant terms (significance threshold: α = 0.01) starting from interactions (Zuur et al. 2009).
All statistical analyses were performed using the software R, version 3.2.1 (R Development Core Team 2015). Geometric morphometrics analyses were performed using the R package “geomorph” (version 2.1.6, Adams and Otárola-Castillo 2013).
Results
Flight related traits
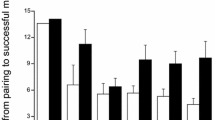
Statistically significant sexual dimorphism was found in weight and in the size of front and hind wings, males being in general lighter and with smaller wings than females (Fig. 2) . A less clear pattern emerged in the abdomen length, which did not differ between sexes (Table 1). The extent of sexual dimorphism in wing size was independent from year (Table 1), but it was less pronounced in 2009 with respect to 2010 in weight (Table 1; Fig. 2). The advancing season resulted in a general reduction in size for all traits, individuals at the end of the breeding season being lighter, having shorter abdomens and smaller wings than those which had emerged earlier. The extent of variation of these characters was similar in males and females (interactions SEASON × SEX were never significant in any model), resulting in a stable sexual dimorphism along the breeding season for all traits.
Wing shape
The MANCOVAs performed on shape variables showed that the deformation of front and hind wings were subject to multiple factors (the minimum significant models are reported in Table 2). All main effects were significant in both analyses: SEX suggested that wing shapes were sexually dimorphic, SEASON suggested that shapes varied along the breeding season and YEAR accounted for yearly differences in environmental constraints among breeding seasons. The main effects of SIZE revealed the allometric variation of wing shape (Outomuro and Johansson 2011), thus accounting for the amount of shape variability explained by the body size variation along the season, because even small variations in size may cause modifications in wing morphology (Johansson et al. 2009; Outomuro et al. 2013b). The SEASON × SEX interaction was significant for front wings as well as for hind wings (Table 2), which suggests that wing shape varied during the season differently in the two sexes, thus affecting sexual dimorphism. This is confirmed by the plot of Procrustes distances between predicted mean shapes of males and females, which showed that sexual dimorphism decreased sharply between the first and the second period, remaining relatively stable later in the season (Fig. 3). This reduction in sexual dimorphism was mainly due to changes in the shape of male wings, which became more similar to the wings of females later in the season (Fig. 3). Indeed, the shape of the wings of males changed more during the season, measured as Procrustes distances between mean shape of each period (Table 3), when compared to changes in the shape of the wings of females (Table 3). This variation in wing shape mainly involved the landmarks in the hind margin and in the wing tip, corresponding to the area of the greatest curvature of the wing (Fig. 3).
Variation of sexual dimorphism in wings along the season: arrows indicate differences in wing shape from females (grey line) to males (black line) in the three periods of the season (front wing: a1, a2, a3; hind wing: b1, b2, b3), the effect is magnified 4 times to better appreciate wing changes. a4 and b4 show variation of sexual dimorphism measured as Procrustes distance between males and females mean wing shape of each season period
Front wing also showed a significant SIZE × YEAR interaction, suggesting that the allometric relationship between shape and size varied between the sampling years, without affecting sexual dimorphism (the SEX × YEAR × SIZE interaction was not significant). Finally, the YEAR × SEASON interaction was significant in both wings, accounting for unknown environmental factors whose effects on wing shape are not consistent over years, leading to a lack of synchrony in seasonal patterns between years.
Discussion
Weight and size in both wings of C. splendens resulted highly sexually dimorphic, but not the abdomen length, and these traits decreased along the breeding season to the same extent in males and females.
During their larval stage, animals are often confronted with time stress imposed by seasonality and several other stressors simultaneously (Stoks et al. 2008) which are the likely driving forces for the observed patterns. Such environmental factors may include water temperature but especially photoperiod, which is a more stable cue for the advancing season (Nylin and Gotthard 1998 and references therein). Size and weight of both sexes resulted affected to the same extent by this phenomenon, probably because males and females need to emerge and reproduce before winter, so they need to speed up development more and more as winter approaches, shifting the trade-off between size and age at metamorphosis (Rowe and Ludwig 1991) that leads to reduced size at metamorphosis as the season advances. This phenomenon has been observed in many insect species (e.g. Chown and Gaston 2010), including Odonata (e.g. Hardersen et al. 1999; Corbet 1999).
Also the shapes of front and hind wings were sexually dimorphic and changed progressively along the season. The observed size reduction in the other traits studied probably affected the seasonal variation of wing shape, because wing shape and size co-varied, but this allometric relationship was independent from sex and advancing season. Consequently, same-sized males collected in different months shared the same wing shape, and so did females. This pattern is probably caused by the aerodynamic constraints on wing morphology modifications imposed by body size variation during the season in both sexes. These changes probably improve flight performance when size, weight and wing dimensions change.
The main result of this study is that territorial males and females underwent non-synchronous modifications in wing shape along the season, which reduced the extent of sexual dimorphism. This finding is in accordance with the predictions and resulted in a seasonal variation in wing shape. At the beginning of the breeding season, males had more rounded wings when compared with females. In this period the mating system is primarily territorial, so males probably benefit from rounded “territorial” wings which can bear wider spots (Outomuro et al. 2013a) and this form also improves manoeuvrability and probably is more attractive to females (Outomuro et al. 2014). This strategy is highly profitable early in the season since territoriality is generally associated with high fitness (Oliveira et al. 2008; Plaistow and Siva-Jothy 1996) and the relative low number of males in relation to the abundance of available territories favours territorial behaviour. As the season advances and the population density increases, the competition for territories becomes very high and only a small fraction of males succeed in maintaining territories for long. Consequently, the number of males adopting the non-territorial strategy during their lives increases to maximize fitness. This selective pressure leads to males that emerge in different times of the season with different wing shapes and that become ever more similar to that of females as the season advances. Towards the end of the season, males with rounded wings would incur high energetic costs if they switched to non-territorial behaviour as this wing shape is optimized for the territorial mating system (Berwaerts et al. 2006; Outomuro et al. 2014). When male density is highest, it becomes increasingly difficult to defend territories for long, and territorial males with short rounded wings, which are suited for territorial behaviour might be unable to pursue females as efficiently as males with more tapered wings. So, for late emerging males, which are unable to defend a territory for any length of time, is more profitable to have less dimorphic wings that permit to switch to the non-territorial strategy and maximize fitness by pursuing and mating females opportunistically (Outomuro et al. 2014).
Interestingly, Hilfert-Rüppell and Rüppell (2009) found evidence that males that pursue females also try to signal to females, using their wing spots. Thus, also in situations where the territorial system is disrupted, secondary sexual characters might still be positively selected for.
It seems that the selective pressures imposed by the seasonal modification of the mating system promoted and maintains phenotypic plasticity which adjusts wing shape during the season and results in the observed variation in average wing shape of the population. The reduction in sexual dimorphism is additive to, but independent from, the allometric effect formerly recognized; indeed, the effects of sex and size in our models were always independent. This reduction in sexual dimorphism was mostly caused by changes in the shape of male wings. The handicap principle states that secondary sexual traits evolve as honest signals of the bearer’s quality because only individuals of higher quality can meet the costs needed to produce and maintain them (Zahavi 1975; Andersson 1982; Grafen 1990) and thus the degree of condition dependence should be greater in sexually selected traits than in non-sexual traits (e.g. Cotton et al. 2004). If time constraints induced by the advancing season are interpreted as stress, it follows that wing shape, which showed a higher condition dependence when compared to size and weight, is likely to be a sexually selected character. This is a further indication that wing shape acts as a sexual character in Calopteryx (Outomuro et al. 2012, 2014). So far it had been shown only for spot size of the wings of Calopteryx that they are sexually selected traits (e.g. Hardersen 2010; Outomuro et al. 2013a).
In contrast, females showed smaller changes in wing shape with respect to males, and the observed pattern of variation for wing shape in females during the season can be attributed to allometric variation in response to the decrease in size and weight. These two distinct patterns resulted in a clear decrease of sexual dimorphism of wing shape during the season.
We interpreted our data in the light of phenotypic plasticity of individuals to season, but we cannot exclude that the observed pattern of shape variation in males might result also from genetic differentiation between early and late emergers, and both constitutive expression and plasticity may co-operate to drive wing shape development (Outomuro et al. 2014).
Even if wing shape of males is mainly selected through male–male competition, females also may apply a selective pressure on territorial male wing shape through courtship, since the exhibition of wing ornamentation needs manoeuvrability and a correlation between wing pigmentation and shape (Outomuro et al. 2012, 2013a). As population density becomes higher later in the season mating attempts largely depend on pursuing flight and forced copulations (Hilfert and Rüppell 1997; Cordero-Rivera 1999; Córdoba-Aguilar and Cordero-Rivera 2005; Hilfert-Rüppell and Rüppell 2009). In this context, females suffer male harassment and become less choosy, performing convenience polyandry (Cordero-Rivera and Andrés 2002), consequently relaxing the selective pressure on male ornaments and on sexually selected rounded wing shape. Thus, males with wings more similar to the aerodynamic optimum for fast and long flight (Betts and Wootton 1988; Outomuro et al. 2012; Sacchi and Hardersen 2013), promoted by natural selection for predator avoidance and predation, are advantaged. The mechanisms proposed, which result from a reduced level of sexual selection by females, and the one determined from the direct effect of the advancing season on larval development, are not mutually exclusive but may work together in shaping the wings of male C. splendens.
In conclusion, wing shape of male C. splendens shows a clear pattern of adaptive variation along the season, in response to a predictable variation in environmental factors as well as in predictable changes in mating strategies adopted by both sexes. Obviously, our results are only correlative, and further studies, under environmentally controlled conditions of genetically homogeneous individuals, are needed to determine whether the phenotypic change and the reduction in sexual dimorphism observed is caused by phenotypic plasticity induced by environmental factors or primarily a product of heritable wing shape expression.
References
Adams DC, Otárola-Castillo E (2013) geomorph: an r package for the collection and analysis of geometric morphometric shape data. Methods Ecol Evol 4:393–399. doi:10.1111/2041-210X.12035
Adams DC, Rohlf FJ, Slice DE (2004) Geometric morphometrics: ten years of progress following the “revolution”. Ital J Zool 71:5–16. doi:10.1080/11250000409356545
Andersson M (1982) Sexual selection, natural selection and quality advertisement. Biol J Linn Soc 17:375–393. doi:10.1111/j.1095-8312.1982.tb02028.x
Berwaerts K, Van Dyck H, Aerts P (2002) Does flight morphology relate to flight performance? An experimental test with the butterfly Pararge aegeria. Funct Ecol 16:484–491. doi:10.1046/j.1365-2435.2002.00650.x
Berwaerts K, Aerts P, Van Dyck H (2006) On the sex-specific mechanisms of butterfly flight: flight performance relative to flight morphology, wing kinematics, and sex in Pararge aegeria. Biol J Linn Soc 89:675–687. doi:10.1111/j.1095-8312.2006.00699.x
Betts CR, Wootton RJ (1988) Wing shape and flight behaviour in butterflies (Lepidoptera: Papilionoidea and Hesperioidea): a preliminary analysis. J Exp Biol 138:271–288
Bookstein FL (1997) Morphometric tools for landmark data: geometry and biology. Cambridge University Press, Cambridge
Bots J, Breuker CJ, Van Kerkhove A et al (2009) Variation in flight morphology in a female polymorphic damselfly: intraspecific, intrasexual, and seasonal differences. Can J Zool 87:86–94. doi:10.1139/Z08-141
Brakefield PM, Larsen TB (1984) The evolutionary significance of dry and wet season forms in some tropical butterflies. Biol J Linn Soc 22:1–12. doi:10.1111/j.1095-8312.1984.tb00795.x
Brockmann HJ (2001) The evolution of alternative strategies and tactics. Adv Stud Behav 30:1–51. doi:10.1016/S0065-3454(01)80004-8
Chown SL, Gaston KJ (2010) Body size variation in insects: a macroecological perspective. Biol Rev Camb Philos Soc 85:139–169. doi:10.1111/j.1469-185X.2009.00097.x
Cigognini R, Gallesi MM, Mobili S et al (2014) Does character displacement demonstrate density-dependent expression in females? A test on the wing shape of two species of European damselflies. Evol Ecol 28:941–956. doi:10.1007/s10682-014-9711-1
Claude J (2008) Morphometrics with R. Springer, New York
Corbet PS (1999) Dragonflies: behavior and ecology of Odonata. Cornell University Press, New York
Cordero-Rivera A (1999) Forced copulations and female contact guarding at a high male density in a calopterygid damselfly. J Insect Behav 12:27–37. doi:10.1023/A:1020972913683
Cordero-Rivera AR, Andrés JA (2002) Male coercion and convenience polyandry in a calopterygid damselfly. J Insect Sci 2:14. doi:10.1093/jis/2.1.14
Córdoba-Aguilar A, Cordero-Rivera A (2005) Evolution and ecology of Calopterygidae (Zygoptera: Odonata): status of knowledge and research perspectives. Neotrop Entomol 34:861–879. doi:10.1590/S1519-566X2005000600001
Cotton S, Fowler K, Pomiankowski AP (2004) Do sexual ornaments demonstrate heightened condition-dependent expression as predicted by the handicap hypothesis? Proc R Soc Lond B 271:771–783. doi:10.1098/rspb.2004.2688
Dijkstra K-DB, Lewington R (2006) Field guide to the dragonflies of Britain and Europe: including Western Turkey and North-western Africa. British Wildlife Publishing, Dorset
Dryden IL, Mardia KV (1998) Statistical shape analysis. Wiley, Chichester
Dudley R (2002) The biomechanics of insect flight: form, function, evolution. Princeton University Press, Princeton
Dumont HJ (1991) Odonata of the Levant. Israel academy of sciences and humanities, Jerusalem, IL
Forsyth A, Montgomerie RD (1987) Alternative reproductive tactics in the territorial damselfly Calopteryx maculata: sneaking by older males. Behav Ecol Sociobiol 21:73–81. doi:10.1007/PL00020230
Gotthard K, Nylin S (1995) Adaptive plasticity and plasticity as an adaptation: a selective review of plasticity in animal morphology and life history. Oikos 74:3–17. doi:10.2307/3545669
Gotthard K, Nylin S, Wiklund C (1994) Adaptive variation in growth rate: life history costs and consequences in the speckled wood butterfly, Pararge aegeria. Oecologia 99:281–289. doi:10.1007/BF00627740
Grafen A (1990) Biological signals as handicaps. J Theor Biol 144:517–546. doi:10.1016/S0022-5193(05)80088-8
Gross MR (1996) Alternative reproductive strategies and tactics: diversity within sexes. Trends Ecol Evol 11(2):92–98. doi:10.1016/0169-5347(96)81050-0
Hardersen S (2010) Seasonal variation of wing spot allometry in Calopteryx splendens (Odonata Calopterygidae). Ethol Ecol Evol 22:365–373. doi:10.1080/03949370.2010.510042
Hardersen S, Wratten SD, Frampton CM (1999) Does carbaryl increase fluctuating asymmetry in damselflies under field conditions? A mesocosm experiment with Xanthocnemis zealandica (Odonata: Zygoptera). J Appl Ecol 36:534–543. doi:10.1046/j.1365-2664.1999.00417.x
Hilfert D, Rüppell G (1997) Alternative mating tactics in Calopteryx splendens (Odonata: Calopterygidae). Mitt Dtsch Ges Allg Angew Ent 11:411–414
Hilfert-Rüppell D, Rüppell G (2009) Males do not catch up with females in pursuing flight in Calopteryx splendens (Odonata: Calopterygidae). Int J Odonatol 12:195–203. doi:10.1080/13887890.2009.9748339
Johansson F, Söderquist M, Bokma F (2009) Insect wing shape evolution: independent effects of migratory and mate guarding flight on dragonfly wings. Biol J Linn Soc 97:362–372. doi:10.1111/j.1095-8312.2009.01211.x
Kemp DJ, Alcock J (2003) Lifetime resource utilization, flight physiology, and the evolution of contest competition in territorial insects. Am Nat 162:290–301. doi:10.1086/376890
Kemp DJ, Wiklund C (2004) Residency effects in animal contests. Proc R Soc Lond B 271:1707–1712. doi:10.1098/rspb.2004.2775
Kingsolver JG (1995) Viability selection on seasonally polyphenic traits: wing melanin pattern in western white butterflies. Evolution 49:932–941. doi:10.2307/2410415
Kingsolver JG, Wiernasz DC (1991) Seasonal polyphenism in wing-melanin pattern and thermoregulatory adaptation in Pieris butterflies. Am Nat 137:816–830
Koskimäki J, Rantala MJ, Taskinen J et al (2004) Immunocompetence and resource holding potential in the damselfly, Calopteryx virgo L. Behav Ecol 15:169–173. doi:10.1093/beheco/arg088
Marden JH, Waage JK (1990) Escalated damselfly territorial contests are energetic wars of attrition. Anim Behav 39:954–959. doi:10.1016/S0003-3472(05)80960-1
Nijhout HF (2003) Development and evolution of adaptive polyphenisms. Evol Dev 5:9–18. doi:10.1046/j.1525-142X.2003.03003.x
Nylin S (1994) Seasonal plasticity and life-cycle adaptations in butterflies. In: Danks HV (ed) Insect life-cycle polymorphism. Springer Netherlands, Dordrecht, pp 41–67
Nylin S, Gotthard K (1998) Plasticity in life-history traits. Annu Rev Entomol 43:63–83. doi:10.1146/annurev.ento.43.1.63
Oliveira RF, Taborsky M, Brockmann HJ (2008) Alternative reproductive tactics: an integrative approach. Cambridge University Press, New York
Outomuro D, Johansson F (2011) The effects of latitude, body size, and sexual selection on wing shape in a damselfly. Biol J Linn Soc 102:263–274. doi:10.1111/j.1095-8312.2010.01591.x
Outomuro D, Bokma F, Johansson F (2012) Hind wing shape evolves faster than front wing shape in Calopteryx damselflies. Evol Biol 39:116–125. doi:10.1007/s11692-011-9145-4
Outomuro D, Adams DC, Johansson F (2013a) The evolution of wing shape in ornamented-winged damselflies (Calopterygidae, Odonata). Evol Biol 40:1–10. doi:10.1007/s11692-012-9214-3
Outomuro D, Adams DC, Johansson F (2013b) Wing shape allometry and aerodynamics in calopterygid damselflies: a comparative approach. BMC Evol Biol 13:118. doi:10.1186/1471-2148-13-118
Outomuro D, Rodríguez-Martínez S, Karlsson A, Johansson F (2014) Male wing shape differs between condition-dependent alternative reproductive tactics in territorial damselflies. Anim Behav 91:1–7. doi:10.1016/j.anbehav.2014.02.018
Plaistow S, Siva-Jothy MT (1996) Energetic constraints and male mate-securing tactics in the damselfly Calopteryx splendens xanthostoma (Charpentier). Proc R Soc Lond B Biol 263:1233–1239. doi:10.1098/rspb.1996.0181
Plaistow SJ, Johnstone RA, Colegrave N, Spencer M (2004) Evolution of alternative mating tactics: conditional versus mixed strategies. Behav Ecol 15(4):534–542. doi:10.1093/beheco/arh029
Rantala MJ, Honkavaara J, Suhonen J (2010) Immune system activation interacts with territory-holding potential and increases predation of the damselfly Calopteryx splendens by birds. Oecologia 163:825–832. doi:10.1007/s00442-010-1582-8
R Development Core Team (2015) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Rohlf JF (2010) tpsDig2. Department of Ecology and Evolution, State University of New York, Stony Brook
Rohlf JF, Marcus LF (1993) A revolution morphometrics. Trends Ecol Evol 8:129–132. doi:10.1016/0169-5347(93)90024-J
Rohlf FJ, Slice D (1990) Extensions of the procrustes method for the optimal superimposition of landmarks. Syst Biol 39:40–59. doi:10.2307/2992207
Rowe L, Ludwig D (1991) Size and timing of metamorphosis in complex life cycles: time constraints and variation. Ecology 72:413–427. doi:10.2307/2937184
Sacchi R, Hardersen S (2013) Wing length allometry in Odonata: differences between families in relation to migratory behaviour. Zoomorphology 132:23–32. doi:10.1007/s00435-012-0172-1
Shapiro AM (1976) Seasonal polyphenism. In: Hecht MK, Steere WC, Wallace B (eds) Evolutionary biology. Springer, US, pp 259–333
Siva-Jothy MT (2000) A mechanistic link between parasite resistance and expression of a sexually selected trait in a damselfly. Proc R Soc Lond B Biol 267:2523–2527. doi:10.1098/rspb.2000.1315
Stoks R, Johansson F, De Block M (2008) Life-history plasticity under time stress in damselfly larvae. In: Córdoba-Aguilar A (ed) Dragonflies and damselflies: model organisms for ecological and evolutionary research. Oxford University Press, Oxford, pp 39–50
Sweeney BW, Vannote RL (1978) Size variation and the distribution of hemimetabolous aquatic insects: two thermal equilibrium hypotheses. Science 200:444–446. doi:10.1126/science.200.4340.444
Van Dyck H, Wiklund C (2002) Seasonal butterfly design: morphological plasticity among three developmental pathways relative to sex, flight and thermoregulation. J Evol Biol 15:216–225. doi:10.1046/j.1420-9101.2002.00384.x
Zahavi A (1975) Mate selection—a selection for a handicap. J Theor Biol 53:205–214. doi:10.1016/0022-5193(75)90111-3
Zuur A, Ieno EN, Walker N et al (2009) Mixed effects models and extensions in ecology with R. Springer, New York
Acknowledgments
We would like to thank Elisa Riservato for her support and for the suggestions about the sampling site, and for her help and advice during the sampling. Also, thanks go to Fabio Pupin for the help during the field work. We want to thank also two anonymous reviewers that helped us to improve the manuscript.
Funding
This study was partially supported by a PhD grant of the University of Pavia to M.M.G.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national and/or institutional guidelines for the care and use of animals were followed.
Additional information
Communicated by A. Schmidt-Rhaesa.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gallesi, M.M., Mobili, S., Cigognini, R. et al. Season matters: differential variation of wing shape between sexes of Calopteryx splendens (Odonata: Calopterygidae). Zoomorphology 135, 313–322 (2016). https://doi.org/10.1007/s00435-016-0309-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00435-016-0309-8