Abstract
Juvenile and adult Cossura pygodactylata Jones 1956 from the White Sea were studied using confocal laser scanning microscopy, light microscopy, scanning and transmission electron microscopy. Transformations of the anterior musculature and digestive tract during ontogenesis were investigated. The early juveniles were shown to be lecithotrophic; their pharyngeal cavities were not connected to the intestines, which contained yolk granules. The juveniles bore prototrochs, which are used for movement, although juveniles had parapodial musculature similar to that of the adults. The juveniles presumably inhabit the upper semi-liquid layer of the silt. The muscles of the prostomium and circumbuccal complex change dramatically during ontogenesis. The ultrastructure of the buccal tentacles is redescribed. The tentacles consist of outer ciliated epithelial cells and an inner cylinder formed by epithelio-muscle cells. The blood sinus is situated between the central cylinder and the epithelium. Both juveniles and adults have developed circulatory systems. The whole dorsal vessel forms the heart with walls that consist of cells with circular cross-striated muscular fibres. The inner lumen is occluded by the heart body which is formed by a single row of cells that are tightly pressed together and connected by adherens junctions along their anterior and posterior surfaces. They contain granules and vesicles and bear numerous processes on the outer surface. The heart body most likely has a secretory haemopoetic function. A hypothetical mechanism of protraction and retraction of the buccal tentacles is suggested, and the participation of muscle contraction and relaxation in these movements is described. It is proposed that the protraction of the tentacles is provided by cell rigidity and increases in the blood volume in the tentacles blood sinuses. The development of the circulatory system is likely related to the need to keep the tentacles exposed during feeding while the anterior part of the body cavity is filled with muscle cell processes and there is no coelomic liquid flow. The proposed mechanism of feeding inside the sediment contrasts with that of surface feeding suggested by Tzetlin (Mém Mus Natl Hist Nat 162:137–143, 1994).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cossurids are small, burrowing worms that grow to approximately 15 mm and are usually present in mixed sandy and muddy sediments. Cossurids have a very peculiar morphology with long unpaired branchial filaments and an unusual buccal apparatus; their eversible buccal tentacles are attached to the dorsal side of the mouth cavity (Tzetlin 1994). The data regarding cossurid internal structure are very sparse and come from a single species, i.e., Cossura pygodactylata Jones 1956 from the White Sea. The feeding apparatus was first studied by Tzetlin (1994), and the body wall and gametogenesis were investigated by Rouse and Tzetlin (1997). Zhadan et al. (2014) studied the musculature of adult specimens of C. pygodactylata with F-actin labelling and confocal laser scanning microscopy (CLSM). These authors showed that the buccal tentacles represent a transformed dorsal part of the axial non-muscular pharynx. It has been shown that cossurid tentacles are paired structures and that their numbers are always even and their musculature is rooted symmetrically to the right and left dorsal longitudinal bands of the body wall musculature by thick retractor muscles. It remains unclear how the protraction of the buccal tentacles occurs. There is no coelomic cavity in the tentacles, and the anterior region of the coelom is filled with the cytoplasmic processes of myocytes (Tzetlin 1994; Rouse and Tzetlin 1997); therefore, the creation of extra hydrostatic pressure via contraction of the anterior muscles is unlikely to be the underlying protraction mechanism.
Little is known about the reproduction and larval development of cossurids with the exception of some data about the early juvenile stages of C. pygodactylata (Bachelet and Laubier 1994). In the present study, we describe changes in the external characteristics and anatomy of the anterior end during ontogenesis and attempt to explain the functioning of the buccal apparatus of C. pygodactylata.
Materials and methods
The specimens were collected with an epibenthic sledge from the muddy sediment at a depth of 30–60 m in the Kandalaksha Bay near the White Sea biological station of Moscow State University (66°52 N 33°19 W) in September 2011 and October 2012. The samples were washed using seawater and a 100-µm mesh and sorted with a stereomicroscope. The specimens were relaxed in an isotonic MgCl2 solution and fixed with a 4 % paraformaldehyde solution in 0.1 M phosphate buffer for 4 h in a refrigerator (4 °C) with constant mixing. After fixation, the worms were washed three times for 20 min in PBS with constant stirring. Next, the specimens were placed in Phallacidin 60 or Phalloidin 488 (Sigma‐Aldrich, St. Louis, MO, USA) diluted to 1:70 in 0.1 M PBS for 1 h in a refrigerator (4 °C) with constant stirring. After staining, the animals were washed three times for 20 min with 0.1 M PBS with constant stirring and subsequently placed into a drop of 0.1 M PBS on a glass cover slip coated with poly-l-lysine (Sigma) to enable adhesion to the glass. The samples were then dehydrated in isopropyl alcohol solutions with ascending concentrations (70, 85, 95, 100, and 100 %) and cleared using Murray Clear [MC, benzyl benzoate with benzyl alcohol (2:1)], MC1, MC2, and MC3. The worms were placed in each solution for 30 s. The specimens were then mounted in MC between two cover slips and placed in a refrigerator (4 °C) overnight. After staining with phallacidin or phalloidin, the slides were kept in the dark. The examinations of the muscular systems were conducted using a Nikon A1 (Tokyo, Japan) confocal laser scanning microscope (CLSM) with laser emissions of 514 and 488 nm for the phallacidin and phalloidin, respectively. Three-dimensional (3D) reconstructions were built using Imaris 7.0 (Bitplane AG, Zurich, Switzerland) software.
The specimens for the electron microscopy studies were relaxed in an isotonic MgCl2 solution, fixed in 2.5 % glutaraldehyde in 0.1 M phosphate buffer (pH 7.2–7.4). After rinsing in the same buffer, the specimens were postfixed with buffered 1 % osmium tetroxide, dehydrated in a graded ethanol series, and then transferred to acetone. For TEM, the specimens were embedded in Spur resin. Semithin and ultrathin sections were cut on Dupont MT 5000 (Sorvall, Newtown, USA) and Leica EM UC 6 (Solms, Germany) ultramicrotomes. The semithin sections were stained with 1 % toluidine blue and 1 % methylene blue on 1 % sodium tetraborate and studied with a Leica light microscope DM2500 (Leica Microsystems, Wetzlar, Germany). The ultrathin sections were stained with lead citrate (15 min) and uranylacetate (1 %, 40 min, 35°) and examined with a Jeol JEM 1011 transmission electron microscope. Light microscopic images were processed using the software Leica AS V3.6.0. Final panels were designed using Adobe (San Jose, CA, USA) Photoshop CS5 and Illustrator CS3.
Totally eight specimens were investigated using SEM (three juveniles and five adults) and 17 specimens using CLSM (five juveniles and 12 adults); two series of semithin transversal sections and two series of longitudinal sections obtained from four juvenile specimens were made and investigated with light microscopy; two longitudinal and two transversal series of ultra-thin sections obtained from four adult specimens were studied with TEM.
The nomenclature used here for the muscles was introduced by Filippova et al. (2005). The following abbreviations are used for the body sections: prostomium (p), buccal tentacles (bt), pharynx (ph), intestine (i), and body wall (bw). The abbreviations are followed by the orientation and the location of the muscle, e.g., “bw/dl” indicates “body wall, dorsal longitudinal muscles”. To keep the abbreviations short, the “muscle” is generally omitted.
Results
External morphology of the juveniles (Fig. 1a, b)
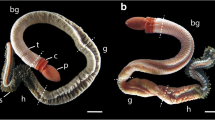
Juveniles with as few as six chaetigers were studied. The juvenile specimens had short cylindrical bodies without clear divisions between the regions and resembled the adult specimens in that they had long branchial filaments and the same type of chaetae (Fig. 1a). The main differences between the adults and juveniles were the absences of the buccal tentacles and the anal cirri and the intercirral processes, the presence of the prototroch in the juveniles, and the shape of the prostomium. The juvenile prostomium is shorter and rounder than that of the adults (Fig. 1b, c) and has a thick basal swelling bearing a prototroch. This swelling forms a collar with a thin anterior fold that is turned-up and backward. Cilia are located on the middle and posterior parts of the swelling (Figs. 1b, 2a, b). Distal to the basal swelling, a constriction that is covered with cilia is present. No nuchal organs were observed (Fig. 1b). The peristomium is shorter than the first chaetiger and approximately the same length as prostomial basal swelling.
External morphology of C. pygodactylata. a Drawing of 17-chaetiger juvenile. b Anterior region of 21-chaetiger juvenile, SEM, arrowheads show anterior fold of prostomium. c Anterior region of adult specimen with buccal tentacles retracted, latero-ventral view, SEM. d Anterior region of adult specimen with four buccal tentacles protracted, ventral view, SEM. I and II first and second chaetigers, bf branchial filament, bt buccal tentacles, ll lower lip, m mouth, ne neurochaetae, no notochaetae, p prostomium, pe peristomium, pr prototroch, pyg pygidium, ul upper lip. Scale bar b–d 30 µm
Histological parasagittal sections of 6-chaetiger juvenile of C. pygodactylata. a Whole specimen. b Anterior end. Arrowheads show anterior fold of prostomium (p). b brain, bv blood vessel, i intestine, m mouth, np neuropile, p prostomium, pe peristomium, ph pharynx, pr prototroch, vnc ventral nerve cord. Scale bar a 40 µm, b 20 µm
The only one juvenile stage can be distinguished. Transformation to adult stage occurs gradually. Specimens with fewer than 25–30 chaetigers maintain their juvenile appearance. Bigger specimens loose the prototroch, their prostomium becomes more elongated without basal swelling, the buccal tentacles, anal cirri and the intercirral processes appear, and the body regionalization becomes more conspicuous.
Anatomy of the juveniles (Figs. 2a, b, 3a–d, 4a–e)
Digestive system
The mouth opening is situated on the ventral part of the peristomium, behind the prototroch (Fig. 2b). The mouth leads to the pharyngeal cavity, which is blindly closed at the level of posterior border of the first chaetiger and does not connect with the intestine (Fig. 2a). The epithelium of the pharyngeal cavity is thick and ciliated. The buccal tentacles are not developed in early juveniles. In the stage with 21–25 chaetigers, four ciliated outgrowths (buccal tentacles) appear near the dorsal wall of the pharynx at the level of branchial filament. They contain neither inner cavities nor muscle fibres; thin blood sinuses are present inside (Figs. 3c, 4e). The intestine is straight, stretches along the whole body, and is blindly closed at the posterior end. The intestinal epithelium is thick and ciliated, and the cells have basally located nuclei and contain numerous yolk granules (Figs. 2a, 3d).
Histological transverse sections of 21-chaetiger juvenile of C. pygodactylata. a On level of peristomium-first chaetiger border, note four buccal tentacles (bt) in pharyngeal cavity. b On level of first chaetiger, tentacle retractors (bt/r) closely abut pharynx (ph). c On level of second chaetiger, tentacle retractors are rooted in dorsal longitudinal bands (bw/dl). d On level of fourth chaetiger, note heart (h) with heart body occluding entire heart lumen and intestine (i) with yolk granules. bw/dl dorsal longitudinal bands, bw/vl ventral longitudinal bands, bt buccal tentacles, i intestine, pbs periintestinal blood sinus, ph pharynx, vnc ventral nerve cord. Scale bar a–d 20 µm
CLSM micrographs showing phalloidin staining of musculature of C. pygodactylata, 2D projections. a–e 21-chaetiger juvenile. a Dorsal view of anterior end showing dorsal longitudinal bands (bw/dl) with processes to prostomium (arrows). b Dorsal view of anterior end with dorsal longitudinal bands omitted to show ventro-rostral fibres (p/vr) of the prostomium. c Dorso-lateral view, dorsal longitudinal bands mostly omitted, note ventro-rostral fibres joining each other and forming unpaired fibre that bends down near tip of the prostomium. d Dorsal view of anterior end showing ventral longitudinal bands (bw/vl) with processes to prostomium (arrows). e Dorsal view of anterior end showing dorsal (p/dr) and ventro-rostral fibres (p/vr). f Adult specimen, dorsal view, dorsal longitudinal bands omitted to show heart (h) and intestinal circular fibres (i/c). nu retractors of nuchal organs, Scale bar a 30 µm, b–d 40 µm, e 20 µm, f 30 µm
Body cavity is divided by the full dissepiments (Fig. 7). The dorsal and ventral mesenteries connect the intestine with the body wall. The whole space between the organs in the anterior part of the body is filled with large transparent vacuolated cells with randomly distributed nuclei (Figs. 2a, b, 3a–d, 7).
The circulatory systems are developed in the juveniles and adults (Fig. 2a). The sinuses of the blood periintestinal plexus closely abut the intestine (Fig. 3d). The dorsal blood vessel (heart) contains the heart body, which occupies its entire lumen (Fig. 3d).
Nervous system
The entire inner space of the prostomium is occupied by the brain. The neuropile is centrally positioned and connected with the circumesophageal connectives, whereas the pericaria are located in more periferic positions. No clear border with the prostomial epithelium is present (Fig. 2b). The ventral nerve cord has large ganglia in each segment (Fig. 2a, b). In transverse sections, the neuropile occupies an upper position and has two lateral lobes. The pericaria are located ventrally and rise in the middle and from both side of the neuropile (Figs. 2a, b, 3a, b, d).
Muscular system
Body wall muscles
The juveniles have developed dorsal (bw/dl) and ventral (bw/vl) longitudinal bands (Figs. 3a–d, 4a, c). The bands start from the peristomium, and dorsal and ventral bands give rise only to thin and short processes to the prostomium, and the ventral outgrowths are shorter than dorsal (Figs. 4b, d, 5a–d). The transverse and circular elements of the body wall are absent in the anterior and middle regions of the body and weakly developed in the posterior segments in the juveniles (Figs. 4a–d, 5a–c).
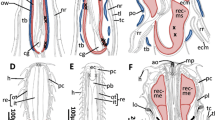
Anterior end musculature of juvenile C. pygodactylata, a, c schematic reconstructions, b, d 3D reconstructions. a Dorso-lateral view showing dorsal longitudinal bands (bw/dl) with anterior processes to prostomium (arrows). b Ventral view. c Dorso-lateral view, dorsal longitudinal bands omitted to show prostomial muscles and rudiments of circumbuccal complex. d Lateral view, ventral longitudinal bands omitted. bw/dl dorsal longitudinal bands, bw/vl ventral longitudinal bands, ll lower lip; nu retractors of nuchal organs, p prostomium, pe peristomium, p/dr dorso-rostral muscles of prostomium, p/dv orso-ventral prostomium bundles, p/t transverse muscle of prostomium, p/vr ventro-rostral muscles of prostomium, ul upper lip, ul/r upper lip retractors. Scale bar b, d 30 µm
Prostomium muscles
The most developed muscles of the prostomium are the paired ventro-rostral muscle fibres (p/vr), which start from the ventral longitudinal bands and proceed forward and upward where they join each other and form unpaired fibre that bends down and then proceed forward nearly to the tip of the prostomium (Figs. 4b–d, 5a, c). The only pair of dorso-ventral prostomium bundles (p/dv) is located close to the end of the unpaired fibre. The dorso-rostral muscles (p/dr) are thinner; they start from the dorsal longitudinal bands and run forward and downward to connect with the ventro-rostral muscles before they join. At the point at which the ventro-rostral muscle fibres join, the retractor muscles of the nuchal organ (nu) start (Figs. 4b–d, 5a, c).
Two bundles located more posteriorly than the nuchal fibres (nu) stretch dorsally. Between their bases runs a short transverse muscle fibre (p/t) connecting the ventro-rostral muscle fibres. The transverse bundle comprises an upper lip (ul) that connects the basal parts of the ventral longitudinal muscles (bw/vl; Figs. 4b–d, 5a, c).
The parapodial muscle complexes (pp) consisting of the chaetal retractors, chaetal protractors and dorsal and ventral external parapodial muscles are developed in juveniles (Fig. 4f).
The circumbuccal complex is not completely developed in the juveniles. The upper lip (ul) contains only transverse muscle fibres and has only one group of retractors (ul/r). Two bundles of retractors go from the upper lip muscle fibre to the anterior parts of the ventro-rostral muscle fibres but do not reach them. The lower lip (ll) is represented by two lateral rudiments of bundles that begin from the dorsal longitudinal bands (bw/dl) but do not connect to each other (Figs. 4b–e, 5a, c).
The circumbuccal transversal and oblique muscles, which are situated posteriorly to the main lower lip muscle in the adults, are absent in the juveniles.
Muscles of buccal tentacles
When the first four tentacles appear, they do not contain any muscle fibres (Fig. 3c). At this stage, only the retractors of the buccal tentacles have begun to form, and they stretch from the dorsal longitudinal bands to the pharynx wall but do not penetrate it (Fig. 3a–b).
Fine structure of the adult specimens
Ultrastructure of the buccal tentacles (Fig. 6a–d)
The buccal tentacles are flattened dorso-ventrally, and the ratios of the long and short axes on transverse section range from 3:2 to 3:1. The ciliated epithelium occupies the majorities of the areas of both the transversal and longitudinal sections of the tentacles (Figs. 1d, 6a, c, d). The epithelial cells of the dorsal side are higher and bear more cilia than those of the ventral side. The cuticle is thin and penetrated by long microvilli, some of which are dichotomously branching. The epithelial cells are connected by adherens and septate junctions in their distal parts (Fig. 6b). The cilia have normal 9 + 2 axonemes and long striated rootlets originating from the basal bodies (Fig. 6b). The nuclei are large and situated medially or basally. The cytoplasm contains many mitochondria, vesicles, and granules, and the Golgi apparatus and the endoplasmic reticulum are well developed. The nerve fibres stretch along the tentacle axis between the epithelial cells (Fig. 6a, c). The inner space of the tentacle is occupied by a central cylinder consisting of epithelio-muscle cells (Fig. 6a, c, d). The blood sinus is situated between the basement membrane and the epithelio-muscle cells and occasionally extends between the epithelial cells (Fig. 6a, c, d).
Buccal tentacles of C. pygodactylata, TEM. a Transverse section of tentacle. b Outer part of epithelial cells showing cilia, rootlets, and contacts between cells. c Distal part of tentacle, sagittal section. d Central cylinder of tentacle, transverse section. aj adherens junctions, bb basal bodies, bm basement membrane, bs blood sinus, c cilia, ec epithelial cells, emc epithelio-muscle cells, mf muscle fibres, mv microvilli, n nuclei, nf nerve fibres, r rootlets, sj septate junctions. Scale bar a 2 µm, b 1 µm, c 2 µm, d 5 µm
Ultrastructure of the circulatory system (Figs. 4f, 7, 8a–d)
The circulatory system consists of a periintestinal blood sinus, dorsal and ventral vessels, and segmentary vessels. The dorsal blood vessel forms the heart. It originates from the perintestinal blood sinus at the level of the 13–15 chaetiger and proceeds to the posterior border of chaetiger 4 where it splits into a few vessels that proceed forward (Fig. 4f). The entire lumen of the heart is occluded by the heart body, and blood runs in the narrow space between the heart wall and the heart body (Figs. 7, 8a, d). The heart wall is formed by epithelio-muscular cells lined with basement membrane from the lumenal side (Fig. 8d). These cells contain cross-striated circular muscular fibres in their basal parts (Figs. 4f, 8b, c). In the CLSM, they appear as short longitudinal fibres arranged in regular rings (Fig. 4f). The distal cytoplasmic parts of these cells contain large nuclei and many mitochondria (Fig. 8d). The cells of the heart are interconnected by gap junctions along their lateral sides (Fig. 8d).
Anterior part of the body of C. pygodactylata from 3rd to 10th chaetiger, sagittal section, TEM. bw/o oblique muscles of the body wall, cem cytoplasmic elements of myocytes, d dissepiments, h heart, hb heart body, i intestine, pbs periintestinal blood sinus, ph pharynx, sbv segmentary blood vessel, vnc ventral nerve cord. Scale bar 50 µm
Heart and heart body of C. pygodactylata, TEM. a Sagittal section through heart and heart body. b, c Section parallel to heart surface showing cross-striation of heart muscle fibres. d Heart and heart body, sagittal section, close-up. aj adherens junctions, bm basement membrane, cp cell processes, g granules, hbc heart body cells, hc heart cells, m mitochondria, mf muscle fibres, n nuclei, gj gap junctions, v vesicles. Scale bar a 2 µm, b 3 µm, c 2 µm, d 1 µm
The heart body is a plug consisting of one row of cells that are tightly pressed to each other (Fig. 8a). Basement membrane is not observed. The cytoplasm of these cells contains many dark granules forming aggregations of different shapes and sizes and in some cases large electron-light vesicles (Fig. 8a, d). The outer surfaces of the cells form numerous round processes that are exposed to the lumen of the heart and contact the blood (Fig. 8d). The cells of the heart body are interconnected by numerous adherens junctions along their anterior and posterior surfaces (Fig. 8a, d).
Discussion
Development of C. pygodactylata
Little is known about reproduction of Cossuridae, and no data about larval development are available. Zhadan et al. (2012) provided a review of the information related to the reproduction and development of cossurids, described the outer morphology of C. pygodactylata juveniles, and provided suggestions about the lifespan and continuous reproduction over the year of this species. In the present study, some details about the internal structure of juveniles, particularly the digestive system and musculature, are described for the first time.
The important finding of this study is that the intestine is not functional in juveniles because their pharyngeal cavity is not connected to the intestine. This fact, together with presence of yolk granules in the intestinal cells, is indicative of the lecithotrophic development of early C. pygodactylata juveniles in the White Sea.
Development of the musculature during ontogeny
The main changes occur in the prostomial muscles and circumbuccal complex. The main muscle of the juvenile prostomium is the ventro-rostral muscle, and this condition is similar to that of the prostomium of other polychaetes, such as dorvilleids (Filippova et al. 2010), in which the rostral muscles reach the distal end of the prostomium. In adult cossurids, the prostomial rostral muscles persist, but they become much shorter relative to the length of the prostomium and are completely covered by the dorsal and ventral longitudinal bands, which start at about the middle of the prostomium. The distal part of the adult cossurid prostomium is occupied by the dorso-ventral bundles; these muscles have not been described in other polychaetes (Zhadan et al. 2014). The dorsal and ventral longitudinal bands give rise to only thin and short outgrowths to the prostomium in juveniles. These muscles resemble longitudinal prostomial muscles, which also originate from the longitudinal bands in other polychaetes, such as Spaerodoridae and Magelonidae (Filippova et al. 2005). The difference is that in the latter families, the prostomial longitudinal muscles originate from the ventral bands, whereas in Cossura juveniles, mostly the dorsal bands give rise to this elongation. In adult cossurids, both the dorsal and ventral longitudinal bands reach the middle of the prostomium.
The muscles of nuchal organs are developed in juveniles although they do not yet have visible nuchal organs. It is likely that one of the functions of these bundles is to support the brain because these bundles encircle the brain from the ventral and lateral sides.
The muscles of the tentacles appear later than the tentacles themselves. There is a stage in which four tentacles are already present but do not have muscle fibres (Fig. 3c). The retractors of the tentacles develop first; they arise from the dorsal longitudinal bands and stretch to the pharynx. Subsequently, they penetrate the pharynx wall and grow into tentacles.
Mechanisms of movement of juveniles and adults
The differences in the architectures of the anterior ends of adult and juvenile C. pygodactylata lead us to suggest that their movements also differ. Because the juveniles do not feed, their prototroch most likely serves a locomotor function. The prostomial rostral muscles provide support for the prostomium. In adults, the anterior prostomium contains numerous dorso-ventral bundles that flatten in the prostomium dorso-ventrally. This motion is most likely used to create a wedge shape of the prostomium that is presumably used to propagating crack during burrowing (Dorgan et al. 2006; Zhadan et al. 2014). We suppose that the juveniles inhabit different sediment layers with less resistance in the uppermost few millimetres of the semi-liquid silt and use their prototroch for slow sliding. Both juveniles and adults have anterior coelom that are filled with cytoplasmic processes of muscle cells as described by Rouse and Tzetlin (1997) and confirmed in the present study. These processes most likely provide rigidity to the anterior end and help to maintain a constant body shape during movement.
The fine structure of the buccal tentacles and mechanisms of their protraction and feeding (Fig. 9)
In living conditions, cossurids keep their mouth closed and their tentacles retracted (our observations). After relaxation in magnesium chloride solution, the majority of the specimens protract their tentacles and cannot pull them back inside the mouth cavity. Tzetlin (1994) suggested that the central cylinder of the buccal tentacles forms a type of chord that supports the constant shape and volume of the tentacle and compared cossurid tentacles to the palps of Protodriloides chaetifer studied by Purschke (1993) in terms of the presence of longitudinal muscles and supporting (coelenchyme-like) elements. He hypothesized that the buccal tentacles are exposed to the substratum when the mouth is opened very wide. In such situations, the tentacles may be pressed down onto the sediment. Based on our knowledge of cossurid musculature and fine structure, we propose the following mechanism of tentacle protraction and retraction. The retraction of the tentacles is elicited by the contractions of the longitudinal muscle fibres inside buccal tentacles and the tentacle retractors. Simultaneously, the muscles of the upper and lower lips contract to close the mouth, and the retractors of the upper lip and the oblique circumbuccal muscles are relaxed (Fig. 9a). During tentacle protraction, the longitudinal fibres and retractors are relaxed as are the upper and lower lips. The retractors of the upper lip pull the lip forward and upward, and the oblique circumbuccal muscles contract and pull the lower lip backward (Fig. 9b). Although we found no specialized supporting structures in the central cylinder of the buccal tentacles, their protraction might be enabled by the rigidity of the epithelial and epithelio-muscular cells and increases in blood volume in the tentacular blood sinuses. We assume that the dorsal longitudinal bands also participate in the opening of the mouth and the protraction of the tentacles because the specimens with open mouths typically have elevated prostomium (Fig. 9b). This position of the prostomium during feeding contrasts with the position suggested by Tzetlin (1994, Fig. 4). It seems more probable that cossurids feed inside the sediment and expose their tentacles to cracks while burrowing.
Schema of muscles contraction and relaxation during retraction (a) and protraction (b) of buccal tentacles. a Muscle of buccal tentacles (bt), tentacle retractors (bt/r) muscles of upper (ul) and lower (ll) lips are contracted; dorsal longitudinal bands (bw/dl), retractors of the upper lip (ul/r) and oblique circumbuccal muscles (cbm) are relaxed, mouth (m) is closed. b Muscle of buccal tentacles (bt), tentacle retractors (bt/r), muscles of the upper (ul) and lower (ll) lips are relaxed; dorsal longitudinal bands (bw/dl), retractors of the upper lip (ul/r) and the oblique circumbuccal muscles (cbm) are contracted to open mouth (m). i intestine, p prostomium, pe peristomium, p/vr ventro-rostral muscles of prostomium
Structure and functioning of circulatory system
Although cossurids in general and C. pygodactylata in particular are small worms (C. pygodactylata are up to 350 µm in width), they have well-developed circulatory systems even in the early juvenile stage. This system is typically built for polychaetes and includes a periintestinal blood sinus, dorsal and ventral longitudinal vessels, and segmentary vessels. The very long branchial filament is also equipped with a blood vessel (Zhadan et al. 2012). The dorsal vessel is transformed to the heart with a muscular wall and a heart body, which occludes the entire lumen of the heart. The buccal tentacles also have blood sinuses in their central parts, and increases in the blood volumes of these sinuses are thought to participate in the protraction of the tentacles.
The heart body is present in many polychaete families, including Alvinellidae, Acrocirridae, Ampharetidae, Arenicolidae, Cirratulidae, Flabelligeridae, Parergodrilidae, Pectinariidae, Sabellariidae, Spionidae, and Terebellidae (Fransen 1988; Rouse and Pleijel 2001; Radashevsky 2012). The functions of the heart body in polychaetes are the secretion of blood pigment, the sequestration of foreign material, and possibly mechanically ensuring the flow of blood or performing a valvular function (Kennedy and Dales 1958; Braunbeck and Dales 1984; Fransen 1988). In terebellids, the heart body is formed by an invaginated wall of the dorsal vessel and contains cells similar to the chloragogen cells outside of the vessel (Kennedy and Dales 1958). The peculiarity of the cossurid heart body is that it consists of one row of cells; the cells are connected to each other with numerous adherens junctions, but we did not observe basement membrane around these cells, which indicates that the mechanism of the formation of the heart body in Cossura is likely different from that in terebellids. The presence of vesicles, aggregations of dark granules, and cell processes on their surface support the suggestions of the secretory haemopoetic function of the heart body in cossurids. The mechanical role of the heart body preventing blood floating backwards also could not be rejected. The lumen between the heart wall and the heart body is very thin although all specimens were relaxed before fixation. We do not know exactly whether systole or diastole was observed. On CLSM (f-actin staining), the heart body looks like a number of regular transverse partitions (Zhadan et al. 2014, Fig. 9b). We hypothesize that the adherens junctions between the cells of the heart body are stained with phalloidin due to the content of actin in this type of cell contact (Green et al. 2010).
It can be assumed that development of the circulatory system (i.e., the presence of all common components and the differentiation of a heart with a muscular wall) in cossurids is because the anterior part of the coelom is filled with cell processes, and there is no free coelomic liquid flow. The circulatory system transports oxygen and provides nutrients. Small-sized annelids, for example Polygordius, Microphthalmus, Paraonis, and Dinophilus are shown to have functionally acoelomate organization without free space between the body wall and the gut (Fransen 1988). In Cossura, we observed similar condition. Another reason for the development of the circulatory system could be the need to keep the buccal tentacles exposed during feeding by increasing the blood volumes in the tentacle sinuses.
References
Bachelet G, Laubier L (1994) Morphology, ecology and juvenile development of Cossura pygodactylata Jones (Polychaeta, Cossuridae) in Arcachon Bay, SW France, with a reassessment of the geographical distribution of C. pygodactylata and C. soyeri Laubier. Mém Mus Natl Hist Nat 162:355–369
Braunbeck T, Dales RP (1984) The role of the heart-body and of the extravasal tissue in disposal of foreign cells in two polychaete annelids. Tissue Cell 16(4):557–563
Dorgan KM, Jumars PA, Johnson BD, Bordeaux BP (2006) Macrofaunal burrowing: the medium is the message. Oceanogr Mar Biol Annu Rev 44:85–121
Filippova A, Purschke G, Tzetlin AB, Müller MC (2005) Reconstruction of the musculature of Magelona cf. mirabilis (Magelonidae) and Prionospio cirrifera (Spionidae) (Polychaeta, Annelida) by phalloidin labeling and cLSM. Zoomorphology 124:1–8
Filippova A, Purschke G, Tzetlin AB, Müller MC (2010) Musculature in polychaetes: comparison of Myrianida prolifera (Syllidae) and Sphaerodoropsis sp. (Sphaerodoridae). Invertebr Biol 129:184–198
Fransen M (1988) Coelomic and vascular systems. In: Westheide W, Hermans CO (eds) The ultrastructure of Polychaeta. Microfauna Mar. vol 4. Gustav Fischer Verlag, Stuttgart, New York, pp 199–213
Green KJ, Getsios S, Troyanovsky S, Godsel LM (2010) Intercellular junction assembly, dynamics, and homeostasis. Cold Spring Harb Perspect Biol 2(2):a000125
Jones ML (1956) Cossura pygodactylata, a new annelid from San Francisco Bay (Polychaeta: Cirratulidae). J Wash Acad Sci 46:127–130
Kennedy GY, Dales PR (1958) The function of the heart-body in polychaetes. J Mar Biol Assoc UK 37(1):15–31
Purschke G (1993) Structure of the prostomial appendages and the central nervous system in the Protodrilida (Polychaeta). Zoomorphology 113(1):1–20
Radashevsky VI (2012) Spionidae (Annelida) from shallow waters around the British Islands: an identification guide for the NMBAQC Scheme with an overview of spionid morphology and biology. Zootaxa 3152:1–35
Rouse GW, Pleijel F (2001) Polychaetes. Oxford University Press, Oxford
Rouse GW, Tzetlin AB (1997) Ultrastructure of the body wall and gametogenesis in Cossura cf. longocirrata (Cossuridae Polychaeta). Invertebr Reprod Dev 32(1):41–54
Tzetlin AB (1994) Fine morphology of the feeding apparatus of Cossura sp. (Polychaeta, Cossuridae) from the White Sea. Mém Mus Natl Hist Nat 162:137–143
Zhadan AE, Vortsepneva EV, Tzetlin AB (2012) Redescription and biology of Cossura pygodactylata Jones, 1956 (Polychaeta: Cossuridae) in the White Sea. Invertebr Zool 9:115–125
Zhadan A, Vortsepneva E, Tzetlin A (2014) Three-dimensional reconstruction of the musculature of Cossura pygodactylata Jones, 1956 (Annelida: Cossuridae). Zool Anz 253(3):181–191
Acknowledgments
This study was supported by grant from the Russian Foundation of Basic Research, Grant No. 15-04-05875 (the collection of the material) and No. 13-04-00078 (transmission and scanning electron microscopy investigations). The confocal laser scanning microscopy investigations were supported by Russian Scientific Foundation, Grant 14-50-00029. The electron microscopical investigations were performed at User Facilities Center of M.V.Lomonosov Moscow State University and at the Institute for Biology of Inland Waters of the Russian Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by A. Schmidt-Rhaesa.
Rights and permissions
About this article
Cite this article
Zhadan, A., Vortsepneva, E. & Tzetlin, A. Ontogenetic development and functioning of the anterior end of Cossura pygodactylata Jones, 1956 (Annelida: Cossuridae). Zoomorphology 134, 509–521 (2015). https://doi.org/10.1007/s00435-015-0282-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00435-015-0282-7