Abstract
The ocular morphology, morphological characteristics and topography of ganglion cell distribution were studied in four eyes of Globicephala melas to estimate the retinal resolution. The ganglion cell layer was composed of a single row of ganglion cells with a primarily round shape and a cell size which varied from 10 to 75 µm (mean 33.5 µm) in diameter. The typical feature was that 65 % of ganglion cells had a diameter larger than 25 µm, with a similar average size in all regions of the retina. The total number of ganglion cells (183,000–218,000; mean 203,000) was distributed in several isodensity lines with two definite areas of high cell density: one area was located in the temporal retinal area, and the other one in the nasal retina, at 65° ± 5° from the optic disk. A surprising result was the presence of a third cell density peak in the dorsal region of one retina. The mean peak cell densities of three retinas were 268 and 267 cells/mm2 in the nasal and temporal areas, respectively, and 287 cells/mm2 in the third peak of the dorsal region. Finally, the underwater retinal resolution, calculated from posterior nodal distance and the peak cell density, was 10.9′ (2.8 cycles/degree) in both nasal and temporal retinal areas, whereas the aerial resolution was 14.5′ (2.1 cycles/degree). These data suggest that G. melas has a visual acuity similar to other cetaceans investigated so far.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cetaceans are aquatic mammals unable to move, reproduce or feed on land. As they are considered acoustic specialists with a well-developed echolocation system, studies of their sensory capacities have concentrated on this aspect. But abundant evidence indicates that cetacean vision shows adaptation to their visual environment and that vision plays an important role both in water and air and participates in prey detection and capture, social interactions, reproductive behavior, as well as in spatial orientation (Spong and White 1971; White et al. 1971; Dral 1977; Nachtigall 1986; Madsen and Herman 1980; Dawson 1988; Herman 1990; Mass and Supin 1990; Mobley and Helweg 1990; Griebel 2002). Since Cetacea evolved from terrestrial ancestor (Fordyce and Barnes 1994; Thewissen 1994; Thewissen et al. 2001, 2007; Thewissen and Williams 2002), they offer a unique opportunity to study the evolution of the visual system in its adaptation from the terrestrial to the aquatic environment.
From the terrestrial mammalian eye which evolved in an environment with a wide range of light intensities, specialization in an aquatic life required morphological adaptations to seeing in a world characterized by low light levels. Thus, as many other vertebrates, cetaceans show a tapetum lucidum highly developed as an adaptation to a dim light environment in underwater vision (Ollivier et al. 2004; Mass and Supin 2007; Mass et al. 2012; Buono et al. 2012). Moreover, as temperature and pressure conditions fluctuate widely between the surface and deeper waters, the morphology of the cetacean eye also differs from the mammalian eye in a number of features. For example, the cetacean eye has well-developed extraocular muscles, thick cornea and sclera for protection against increased pressure and to prevent excessive heat loss in cold water by the ophthalmic rete and finally insulation in the eyelids. Moreover, a retractor bulbi muscle can retract the eye into the orbit for protection (Dral 1972; Dawson 1988; Buono et al. 2012). Strangely enough, the cetacean eye has some adaptations that converge with those of other vertebrates, such as fishes that develop their life in the same habitat; for example, the shape of the eyeball is elliptic, and the lens is almost spherical (Birndorf and Pérez 1972; Dawson 1988).
However, cetaceans use their eyes also in air for prey detection and capture (e.g., dolphins use the vision for circling sea birds) and orientation. That is the reason they are not completely detached from the characteristics that allow the vision in the air (Perez et al. 1972; Pepper and Simmons 1973; Dawson 1988). In this sense, it has been suggested that the pupil, acting as a single or double pinhole, is an important mechanism accommodative to allow an amphibious vision. However, to date mechanisms for well-focused images in both water and air have not been fully studied.
In the study of the visual system, the determination of the spatial resolution of a given species from the retinal ganglion cell distribution determined in Nissl-stained retinal wholemounts has received particular attention (Stone 1981; Stone and Halasz 1989; Collin and Pettigrew 1989). In this sense, retinal ganglion cell topography is an unrivalled source of information about the visual capability of a species when it is combined with visual optics (Hughes 1975, 1977, 1985; Stone and Fukuda 1974). Of particular interest are the retinal areas with high ganglion cell density, so that they are characterized by a higher visual acuity than other retina sites, and the shape, size and density of ganglion cells in them are associated with the ecology of the species (Hughes 1977). High-resolution retinal areas in mammals are shaped either as the area centralis or as the visual streak, or a combination of both area centralis and the visual streak. Area centralis is a retinal specialization in a specific retinal location, as it occurs in humans, and provides acute vision mostly in the frontal direction. Horizontal visual streak is an elongated arrangement of high ganglion cell density, characteristic of animals that inhabit open environments, and provides satisfactory vision in the horizontal plane (Hughes 1977; Peichl 1992; Williams et al. 1993; Collin 1999; Coimbra et al. 2013). Analyses on the topographic distribution of the retinal ganglion cells on the estimation of visual acuity have been conducted on a variety of species of cetaceans to compare visual specialization (Dral 1974, 1977, 1983; Murayama et al. 1992, 1995; Mass and Supin 1989, 1995, 1997, 2002; Mass et al. 1986, 2012). These studies have revealed two areas of greatest ganglion cell density in the temporal and nasal quadrants of the visual field, which correspond the best-vision areas (Dral 1977, 1983; Gao and Zhou 1987; Mass and Supin 1995, 1997, 2002; Mass et al. 2012; Murayama et al. 1992, 1995; Supin et al. 2001). The higher ganglion cell density in the temporal quadrant of the retina suggests its use for binocular vision, whereas high ganglion cell density in the nasal zone may be responsible for panoramic vision (Mass and Supin 1990). Thus, cetaceans can scan a large visual field with high resolution with a little rotation of the eye (Kröger and Kirschfeld 1993).
Areas of increased density have been thought to be retinal regions having greater resolving power than the remainder of the retina. These areas determine the acuity of the animal’s vision, that is, the upper limit of spatial resolving power of the eye can be obtained from the interganglion cell distance (Rodieck 1973; Frisen and Frisen 1976; Collin and Pettigrew 1989; Mass and Supin 1995, 1997, 2002; Mass et al. 2012). Thus, visual acuity in cetacean is ranged between 7′ in minke whale, Balaenoptera acutorostrata (Murayama et al. 1992), and 12′ in gray whale, Eschrichtius robustus (Mass and Supin 1997). These low spatial resolution values suggest that although vision is important in cetaceans, probably it is not the most important sense.
But the most typical feature in the cetacean retina is the presence of ganglion cells named giant cells (mainly 50–80 µm) separated by wide intercellular spaces (Dawson and Perez 1973; Dawson et al. 1982; Dral 1983; Mass 1996; Mass and Supin 1995, 1997, 2002; Mass et al. 1986, 2012; Murayama et al. 1992, 1995). These ganglion cells are characterized by large soma size, large dendritic fields and thick axons as named alpha ganglion cells (Peichl et al. 1987; Peichl 1991). Although the functional significance of these giant ganglion cells is not known, electrophysiological studies in cat, Felis catus, have demonstrated that alpha ganglion cells exhibit a fast-conducting properties and respond to moving stimuli (Wässle et al. 1981). These ganglion cells may play an important role in detecting contrast and movement at the ocean depths.
At present, the functional significance of retinal topography, and the presence, size, form and composition of retinal specializations in vertebrates, remains a matter for speculation. For many years, different distribution patterns of ganglion cells have been related to the lifestyle and ecological niche of the animal (Hughes 1977; Collin 1999, 2008). But regional specialization might be also closely related to phylogenetic ancestor. In this sense, to obtain further evidence about the presence of retinal specializations and its relationship to the visual acuity and lifestyle of cetaceans, we have studied the morphological characteristics and ocular parameters, and the retina of the long-finned pilot whale (Globicephala melas, Traill 1809). Pilot whales are highly gregarious, and they live in stable family groups of 10–50 individuals in both pelagic and coastal aquatic biomes. Similar to other members of the Delphinidae family, the long-finned pilot whales echolocate when foraging for prey (fish, cephalopods and crustaceans), and most feeding occurs at night in deep water between depths of 200–500 m for 10–16 min (Shirihai and Jarrett 2006). Although in these low light levels, the echolocation system is very important, vision plays an essential role in other aspects of their life, including orientation and navigation in space, identification of conspecifics and coordination of group movements. As other members of cetaceans, in order to extract information at low light levels of the aquatic medium, probably G. melas presents different adaptations in the retina.
This paper reports a quantitative analysis of the topographic distribution of ganglion cells in wholemounted retinae of the long-finned pilot whale, G. melas, paying particular attention to the retinal specializations which function in visual acuity.
Materials and methods
Animals
The eyes of four dead long-finned pilot whales were obtained at the Faroes coast. All applicable international, national and/or institutional guidelines for the care and use of animals were followed. Three adult whales (5.1, 4.8 and 4.7 m in length) and a juvenile (2.8 m) were used in this study.
Histological procedure
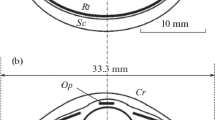
The eyes were collected 1–2 h after death. After fixation in 10 % formalin, the orbit of one adult subject was examined with computed tomography (CT) to obtain cross-sectional images of the orbital eyes. These images were used to estimate the main optic dimensions in order to calculate the visual acuity.
Following eye extraction and before fixation, three eyes were dissected and the anterior portion including cornea, lens and iris was cut away. After elimination of vitreous humor, the retina was excised from the eyecup and used to prepare retinal wholemounts. Before the eyeball was excised, its orientation in the orbit (dorsal, ventral, nasal and temporal sectors) was noted. Three retinas were prepared as wholemounts in order to measure the size and density of ganglion cells according to Stone’s method (1981). Each retina was sectioned in sectors, flattened on several slides with the ganglion cell layer upward, weighted in 10 % buffered formalin solution for several hours and air-dried. The orientation of each retina and each sector was made by making an incision at the dorsal and nasal retinal margins. Then, the retina was stained by the Nissl method with cold 0.1 % cresyl violet for 12–15 min, dehydrated in graded ethanol solutions, cleared with xylene and mounted. With these methods, shrinkage is usually negligible or occurs primarily at the edge of the retina (Stone and Halasz 1989).
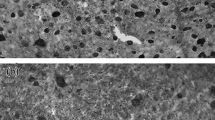
We identified the ganglion cells based on the criteria which are accepted generally (Stone 1965; Hughes 1975; Mass et al. 1986), that is, the presence of a clear cytoplasm ring surrounding the nucleus with well-stained Nissl substance and the presence of a distinct nucleolus. Amacrine and glial cells were easily identified by their morphological characteristics and excluded from the cell analysis.
Data processing
To map the ganglion cell density distribution, photomicrographs were taken every 2 mm on the complete retina surface of the Nissl-stained wholemount. In each frame, the number of cells was counted within 0.25 mm2. In regions of higher ganglion cell densities, sampling points were taken every 1 mm. These counts were converted into the number of cells per 1 mm2. Isodensity contours were drawn by interpolation between the values of retinal ganglion cell density. These maps were made by overlapping and bonding each image over the next using the vascular ramifications as a reference (Fig. 1). Thus, we reconstructed each row, then each sector (Fig. 1) and finally the entire retina.
Morphological parameter of ganglion cells (cellular diameter, soma area, perimeter) was analyzed from 62 photomicrographs and 2901 ganglion cells, at a total magnification of ×100, taken in different regions of the three retinas. Measurements of morphological parameters were performed using the Visilog 5.0.1 (Noesis, France) software for image analysis. To obtain cell size, the following parameters were measured: perimeter, area, width and length diameter (minimum and maximum diameters). From minimum and maximum diameter, we calculated the mean value.
In order to identify possible different groups of ganglion cells, we used the cluster analysis by Ward method to get the squared Euclidean distance between all pairs. Variables included in the analysis were mean diameter, area and perimeter.
The spatial resolving power was calculated by converting ganglion cell density to ganglion cells per degree of visual arc according to Mass et al. (2012). The average distance between adjacent cells (L) was calculated as
where L is the intercellular distance and D is the density of cells (per 1 mm2).
To convert the intercellular distance to angular measure of visual resolution, the posterior nodal distance (PND) of the eye must be known. As the cetacean eye is emmetropic in water, the PND of the long-finned pilot whale may be considered to be the distance from the lens center to the central part of the retina. This distance, calculated from CT images, was 19.3 mm.
The retinal resolution was calculated according to the formula:
where V is the retinal resolution (angle degree), L is the intercellular distance (in mm) and N is the PND (in mm).
The retinal resolution in air was calculated based on the fact that retinal image size in water is 1.33 times bigger than that in air because of the difference in refractive index between these media.
Results
Eye dimensions
Like other odontocetes, the eyes of G. melas are placed laterally in the head. The eyeball is hemispherical, flattened along the visual axis (axial length = 24.5 mm; dorsoventral diameter = 36.70 mm). The lens was almost spherical: The axial and transverse diameters were 9.5 and 9.6 mm, respectively. Finally, the distance from the center of the lens to the retina (PND) was 19.3 mm (Fig. 2).
The thick sclera surrounded the eyeball from the cornea to the exit of the optic nerve. The thickness was variable: About 2.9 mm thick near to the corneoscleral junction, it increased markedly toward the caudal pole (6.2 mm), and it decreased to 3.1 mm where it joins with the optic nerve.
The cornea is oval shaped (rostro-caudal diameter = 24.1 mm, dorsoventral diameter = 20.3 mm), and the outer surface is flattened. Its thickness is variable, from 0.9 mm in the center to 2 mm at the edges. The pupil was horizontally elongated.
Finally, the well-developed tapetum lucidum had a green–blue coloring and covered the major part of the eyecup. It was made up of large choroidal vessels and closely and regularly arranged collagen fibers and was parallel to the retinal surface.
Identification of ganglion cells
The ganglion cell layer of the long-finned pilot whale retina consisted of a single row of cells separated by wide intercellular spaces. Based on morphological features, two types of cells could be distinguished. One type, the ganglion cells, had a substantial cytoplasm with Nissl granules and a clear nucleus with prominent nucleolus. The other type, the amacrine cells, was small (typically <10 µm), with a round or oval outline, a paler cytoplasm and a smaller nucleolus. Darkly stained cytoplasm corresponds to glial cells. These cells were not included in the analysis of ganglion cell distribution (Fig. 3).
Retinal ganglion cell size
A total of 2091 cells were measured in different regions of the three retinas. Although the diameter was variable (ranged from 10 to 75 µm, but 33.5 ± 3.4 µm in average), the majority of ganglion cells (over 65 %) were 25–70 µm in diameter (Table 1). Moreover, 8–10 % of cells were bigger than 60 μm in diameter. These values were similar in all areas of the three retinas, including the peaks of maximum density.
Using cluster analysis to identify the existence of cell groups of ganglion cells, we noted two groups in the three retinas. Figure 4 shows the dendrogram to represent the distance at which clusters are combined. Vertical axis indicates the linkage distance so that the position of vertical lines in this axis denotes the distance at which clusters are joined. The distances observed are rescaled to fall into the range of 1–100. In the three retinas, we can observe two different groups of ganglion cells, thus combining two fairly dissimilar clusters (the distance between two groups is 100). The predominance of a population of giant ganglion cells is observed.
Density distribution of ganglion cells
Figure 5 shows the topographic map of ganglion cell density for the total ganglion cell population in each retina. Distribution of the ganglion cells was nonuniform across the retina, and the cells were distributed in isodensity contours starting from the optic disk outwards almost in circles, with peaks and decrease in cell density. Two definite areas of high cell density were identified: One area was located in the temporal retinal area, and the other in the nasal retinal area, at 65° ± 5° from the optic disk (this value was calculated as the distance in degrees from geometric center). Moreover, in the wholemount 3, a dorsal area was found. The ganglion cell density decreases toward the central and peripheral retina.
The total number of ganglion cells for each retina was 183,000, 209,000 and 218,000, with a mean of 203,000. The averaged peak cell densities for the three wholemounts were 268.7 and 267.3 cells/mm2 in the nasal and temporal areas, respectively (Table 2). However, in wholemount 3, the distribution of ganglion cells was different due to the presence of a third peak cell density of 287 cells/mm2 in the dorsal area.
Retinal resolution and visual acuity of Globicephala melas
As described in Methods, we used the PND value and ganglion cell densities to calculate the angle subtending 1 mm on the retina and subsequently calculated the number of cells subtended by one degree of visual arc (average 5, 5 in both areas). Data are given in Table 2.
Discussion
Eye characteristics
The general anatomy and size of the eye in this whale are similar to those reported in other cetaceans (Buono et al. 2012; Rodrigues et al. 2014). The thick sclera of G. melas is a typical feature of the cetacean eye (Dawson 1988; Mass and Supin 2007). Although the functional role of this trait has not been revealed, it has been pointed that the thickened sclera stabilizes the eye because of its high mass. Together with the periorbital fat, it may also have an insulating effect that would enable the animals to regulate the temperature of the retina (Kröger and Katzir 2008). Moreover, as in other cetaceans, the thick sclera probably helps to avoid deformations of the eyeball during protrusion and retraction eyes into the orbit (Dawson 1988; Kastelein et al. 1990; Zhu et al. 2001; Bjerager et al. 2003; Buono et al. 2012; Mass and Supin 1997, 2007). Finally, a thick sclera may be necessary as withstanding pressure to avoid deformation of the cornea when animal is diving (Kastelein et al. 1990; Buono et al. 2012).
In G. melas, the cornea is thick but as in other cetaceans is thinner in the center and thicker at the periphery (Dawson et al. 1987; Supin et al. 2001; Mass and Supin 2007; Mass et al. 2012). In contrast to terrestrial mammals, where the cornea acts as the major refractive element of the eye, in cetaceans it is optically absent and does not contribute significantly to the focusing of images on the retina. It is due to identical refractive indices both in front and behind the cornea (Dawson et al. 1987; Kröger and Kirschfeld 1994). Although the cornea cannot be neglected completely in cetaceans (Kröger and Kirschfeld 1990; Mass and Supin 2007), the most powerful refractive structure is the lens nearly spherical in shape, as in fishes (Sivak 1980; Fernald 1990).
Morphological characteristics of ganglion cells
A significant feature of the retina of G. melas, as in other cetaceans, is the presence of giant ganglion cells. We have found that more of 65 % of ganglion cells have a diameter from 25 to 70 µm (mean 33.7 µm), and about 10 % of ganglion cells were bigger than 60 μm in diameter. These large neurons, named giant cells, have been observed in different cetacean species as the fin whale, Balaenoptera physalus (Pilleri and Wandeler 1964), the bottlenose dolphin, Tursiops truncatus (Perez et al. 1972; Dawson et al. 1982; Mass and Supin 1995), common dolphin, Delphinus delphis (Dral 1983), harbor porpoise, Phocoena phocoena (Mass et al. 1986), the minke whale, B. acutorostrata (Murayama et al. 1992), the gray whale, E. robustus (Mass and Supin 1997), beluga whale, Delphinapterus leucas (Mass and Supin 2002), and killer whale, Orcinus orca (Mass et al. 2012).
Large ganglion cells about 25–30 μm have been found in the retina of several orders of placental and marsupial mammals, and they were named alpha cell (Fukuda and Stone 1974; Hughes 1975, 1981; Peichl 1991). These cells account for less than 10 % of the ganglion cells, their somata are regularly spaced, and their dendritic fields cover the retina in a mosaic-like fashion (Wässle et al. 1981; Peichl et al. 1987; Peichl 1991). In a physiological view, alpha ganglion cells exhibit brisk-transient and fast-conducting properties (Peichl et al. 1987). Compared to terrestrial mammals, included large terrestrial mammals as the elephant (Stone and Halasz 1989) and big artiodactyls, e.g., bovines (Hebel 1976) and giraffe (Coimbra et al. 2013), where ganglion cell diameter does not exceed 25 μm, the giant cells (presumed alpha-like ganglion cells) of cetaceans are much larger; in fact, most ganglion cells are larger in cetaceans than in terrestrial mammals. There are ecological implications for this feature in the low light intensity of cetacean environment. Huge receptive field with a complete coverage of the retina might allow an efficient spatial summation and collecting and integrating photon signals from large retinal area. Thus, these cells might contribute to the detection of contrast and movement at the ocean depths. In this sense, Mass and Supin (2003) suggest that the big size of the ganglion cells would associate with the way of aquatic life.
Related to the big size of ganglion cells in cetaceans, Mazzatenta et al. (2001) reported that a population of giant optic axons (up to 15 μm in diameter) was present in both of the fin whale (B. physalus) and the striped dolphin (Stenella coeruleoalba). This implies that the fiber density of optic nerve is two times lower than in terrestrial mammals. These authors argued that these features probably are the result of adaptations to the aquatic lifestyle, so that giant optic fibers might guarantee rapid detection of movement prey. Previously, it had been reported axons of this size in the optic nerve of several odontocetes (Dawson and Perez 1973; Dawson et al. 1982; Gao and Zhou 1992). All these observations suggest the existence of a system of alpha cells with big receptive fields and big sensitivity for low spatial frequencies and the movement perception; however, these cells are not effective in the analysis of static objects (Wässle and Boycott 1991). In this way, the system of giant fibers in cetaceans would have evolved to satisfy the need of fast motion detection (Mazzatenta et al. 2001) and alerting the animal to threatening during navigation.
Number and topographic distribution of ganglion cells
In the long-finned pilot whale, the mean number of ganglion cells was 203,233, which is higher to those reported in E. robustus (mean is 174,000, Mass and Supin 1997) and in Delphinapteurs leucas (mean is 122,360, Mass and Supin 2002).
In regard to ganglion cell topography, our results demonstrate the presence of two areas of high ganglion cell density in the retinas of long-finned pilot whale. These areas, as it has been described in other cetaceans, are located in the nasal and temporal quadrants of the retina. In the mammal retina, ganglion cells are distributed in a nonuniform manner, with regions of different density of ganglion cells. Areas of greatest ganglion cell density determine the best-vision areas and location of these regions correspond to the eye position, eye mobility, illumination level and lifestyle (Hughes 1985; Collin and Pettigrew 1989; Mass and Supin 2007). As cetaceans move three-dimensionally in deeper open waters, they have not prominent visual streaks. It is likely that ancestral cetaceans retained a visual streak, because it occurs in all extant artiodactyls (Hebel 1976; Hughes 1977). The streak may have been modified by the obliteration of the center resulting in two areas of high ganglion cell density, as found in cetaceans (Kröger and Katzir 2008).
In cetaceans, the existence of two high-density zones, in temporal and nasal areas, was described firstly by Peers (1971) in the dolphin T. truncatus and corroborated many researchers in different species: bottlenose dolphin, T. truncatus (Dral 1974, 1977, 1983; Mass and Supin 1995), harbor porpoise, P. phocoena (Mass et al. 1986), minke whale, B. acutorostrata (Murayama et al. 1992), Dall’s porpoise, Phocoenoides dalli (Murayama et al. 1995), gray whale, E. robustus (Mass and Supin 1997), pacific white-sided dolphin, Lagenorhynchus obliquidens (Murayama and Somiya 1998), tucuxi dolphin, Sotalia fluviatilis (Mass and Supin 1999), and beluga whale, D. leucas (Mass and Supin 2002). Supin et al. (2001) and Mass and Supin (2007) suggest that the presence of these two high-density areas may be related to the cetacean’s capability for good vision in both aquatic and aerial environments. These high-density areas are placed just opposite two pupil slits in aerial vision when light intensity is high and the pupil is constricted (Herman et al. 1975). Thus, light falls onto the high-resolution areas of the retina to prevent aerial myopia (Mass and Supin 2007). Moreover, Mass and Supin (1995) suggest that the presence of the two best-vision areas compensates the restricted mobility of the head of cetaceans.
In G. melas, the peak cell density in high-density areas (267–268 cells/mm2) is higher than mysticetes as B. acutorostrata (118–161 cells/mm2, Murayama et al. 1992) and E. robustus (142–200 cells/mm2, Mass and Supin 1997), but is lower than other odontocetes as T. truncatus (510–826 cells/mm2, Dral 1977; Mass and Supin 1995), D. delphis (700 cells/mm2, Dral 1983), P. phocoena (700 cells/mm2, Mass et al. 1986), P. dalli (538–541 cells/mm2, Murayama et al. 1995), D. leucas (300–400 cells/mm2, Mass and Supin 2002) and O. orca (250–300 cells/mm2, Mass et al. 2012). Both areas in G. melas show the same peak cell density. However, in other cetacean species, the cell density in the temporal area (which serves the frontal visual field and binocular vision) is higher than in the nasal region (related to side vision), as it had been reported in the harbor porpoise, P. phocoena (Mass et al. 1986), Dall’s porpoise, P. dalli (Murayama et al. 1992, 1995), beluga whale, D. leucas (Mass and Supin 2002), and killer whale, O. orca (Mass et al. 2012). On the other hand, the peak cell density of cetaceans is markedly lower than those of terrestrial vertebrates: 7000–10,000 cells/mm2 in the cat (Stone 1978; Wong and Hughes 1987; Williams et al. 1993), 6000–14,000 in the dog, 12,000–14,000 in the wolf (Peichl 1992) and 13,000–15,000 in giraffe (Coimbra et al., 2013). As we observed, compared to terrestrial mammals, cetaceans have a relatively low ganglion cell density even the areas with maximum acuity. However, they have huge ganglion cells with large dendritic trees, which are consistent with the low light intensity of their environment. Then, visual sensitivity and spatial resolution are different in cetaceans and terrestrial mammals.
But a surprising result of this work is that one retina presented a different topographic organization of ganglion cells. In addition to the presence of two areas located at the horizontal diameter of the retina, the retina of one specimen showed a third high-density dorsal area with 287 cells/mm2. Moreover, this value did not differ from those of temporal and nasal areas. Although the present data are unusual and contradict earlier reports about the presence of two high-density areas in cetaceans, and the available material for this study was very limited (only three retinas), the high density of ganglion cells in dorsal area conducts us to consider the possibility that it is not due to an individual difference. The reason could be that this retina belonged to a juvenile specimen of 2.8 m long and retinal topography might be different. Firstly, visual ecology of juvenile specimens might be different to adult ones. In this sense, as juveniles are usually positioned over their mothers head when swimming to protect them from a predator attack from below, a high-density dorsal area may be very important to cover the lower portion of the visual field. In the same manner, fishes that predominantly feeding from the bottom possess high-density areas located in dorsal region (Shand et al. 2000). Secondly, from birth to adulthood, a probable transformation of the number and topography of the retinal ganglion cells take place. In different mammal species (Henderson et al. 1988), nonuniform cell generation, cell death or cell migration have each been shown to contribute to the development of topographic specializations in the retina. But there is not any report about the distribution and changes in the number or retinal ganglion cells with age in cetacean retinas. Additional research is necessary to provide new insights into this area.
Estimation of visual acuity in pilot whale
The best visual acuity of the long-finned pilot whale can be estimated as about 10.9′ in both temporal and nasal areas, comparable to the visual acuity to both mysticetes and odontocetes. In killer whale, O. orca (Mass et al. 2012), the retinal resolution is 9.6′ and 10.5, and in dolphins the data are 8′ in bottlenose dolphin, T. truncatus (Mass and Supin 1995), 8′ and 9.5′ in common dolphin, D. delphis, and 11′ and 13′ in harbor porpoise, P. phocoena (Mass et al. 1986). In mysticetes, the retinal resolution is 7′ and 7.6′ in minke whale, B. acutorostrata (Murayama et al. 1992), and 10′ and 12′ in gray whale, E. robustus (Mass and Supin 1997).
Compared with terrestrial mammals, even nocturnal terrestrial mammals, ocular anatomy and optics in G. melas are different and their visual acuity is inferior. However, this study suggests a visual acuity similar to other cetaceans in both aquatic and aerial environments. It could be that cetaceans do not depend on vision as primary sense, considering that their acoustic sense is more important in their ecological niche. As Collin and Pettigrew (1989) have been suggested, variations in visual acuity reflect the various foraging strategies of the animals. In this sense, the long-finned pilot whale, G. melas, probably does not require a high resolving power in the retina for their lifestyle (e.g., most feeding occurs at night in deep water between depths of 200–500 m).
References
Birndorf L, Pérez J (1972) Gross anatomy and optics of the dolphin eye. Cetology 10:1–12
Bjerager P, Heegaard S, Tougaard J (2003) Anatomy of the eye of the sperm whale (Physeter macrocephalus L.). Aquat Mammal 29:31–36
Buono MR, Fernández MS, Herrera Y (2012) Morphology of the eye of the southern right whales (Eubalaena australis). Anat Rec 295:355–368
Coimbra JP, Hart NS, Collin SP, Manger PR (2013) Scene from above: retinal ganglion cell topography and spatial resolving power in the giraffe (Giraffa camelopardalis). J Comp Neurol 521(9):2042–2057
Collin SP (1999) Behavioural ecology and retinal cell topography. In: Archer SN, Djamgoz MBS, Loew ER, Partridge JC, Vellarga S (eds) Adaptive mechanism in the ecology of vision. Chapman & Hall, London, pp 509–535
Collin SP (2008) A web-based archive for topographic maps of retinal cell distribution in vertebrates. Clin Exp Optom 91(1):85–95
Collin SP, Pettigrew JD (1989) Quantitative comparison of the limits on visual spatial resolution set by the ganglion cell layer in twelve species of reef teleosts. Brain Behav Evol 34:184–192
Dawson WW (1988) The Cetacean eye. In: Herman LM (ed) Cetacean behaviour: mechanisms and functions. Willey Interscience, New York, pp 53–100
Dawson WW, Perez JM (1973) Unusual retinal cells in the dolphin eye. Science 181:747–749
Dawson WW, Hawthorne MN, Jenkins RL, Goldston RT (1982) Giant neural system in the inner retina and optic nerve of small whales. J Comp Neurol 205:1–7
Dawson WW, Shroeder JP, Sharpe SN (1987) Corneal surface properties of two marine mammal species. Mar Mamm Sci 3:186–197
Dral ADG (1972) Aquatic and aerial vision in the bottle-nosed dolphin. Neth J Sea Res 5:510–513
Dral ADG (1974) Some quantitative aspects of the retina of Tursiops truncatus. Aquat Mamm 2:28–31
Dral ADG (1977) On the retinal anatomy of Cetacea (mainly Tursiops truncatus). In: Harrison RJ (ed) Functional anatomy of marine mammals. Academic Press, London, pp 81–134
Dral ADG (1983) The retinal ganglion cells of Delphinus delphis and their distribution. Aquat Mamm 10:57–68
Fernald RD (1990) The optical system of fishes. In: Douglas R, Djamgoz M (eds) The visual system of fish. Chapman and Hall, New York, pp 45–61
Fordyce RE, Barnes LG (1994) The evolutionary history of whales and dolphins. Annu Rev Earth Planet Sci 22(1):419–455
Frisen L, Frisen M (1976) A simple relationship between the probability distribution of visual acuity and density of retinal output channels. Acta Opthalmol 54:437–443
Fukuda Y, Stone J (1974) Retinal distribution and central projections of Y-, X-, and W-cells of the cat’s retina. J Neurophysiol 37:749–772
Gao A, Zhou K (1987) On the retinal ganglion cells of Neophocaena and Lipotes. Acta Zool Sinica 33:316–323
Gao A, Zhou K (1992) Fiber analysis of the optic and cochlear nerves of small cetaceans. In: Thomas JA, Kastelein RA, Supin AY (eds) Marine mammal sensory systems. Plenum, New York, pp 39–52
Griebel U (2002) Color vision in marine mammals: a review. In: Bright M, Dworschak PC, Stachowitsch M (eds) The Vienna School of Marine Biology: a tribute to Jörg Ott. Facultas Universitätsverlag, Wien, pp 73–87
Hebel R (1976) Distribution of retinal ganglion cells in five mammalian species (pig, sheep, ox, horse, dog). Anat Embriol 150:45–51
Henderson A, Finlay BL, Wikler KC (1988) Development of ganglion cell topography in ferret retina. J Neursoci 8(4):1194–1205
Herman LM (1990) Cognitive performance of dolphins in visually guided tasks. In: Thomas JA, Kastelein RA (eds) Sensory abilities of cetaceans. Plenum Press, New York, pp 455–462
Herman LM, Peacock MF, Yunker MP, Madsen CJ (1975) Bottlenose dolphin: doubles lit pupils yields equivalent aerial and underwater diurnal acuity. Science 189:650–652
Hughes A (1975) A quantitative analysis of the cat retinal ganglion cell topography. J Comp Neurol 163:107–128
Hughes A (1977) The topography of vision in mammals of contrasting life style: comparative optics and retinal organization. In: Crescitelli F (ed) Handbook of sensory physiology. Springer, New York, pp 613–756
Hughes A (1981) Population magnitudes and distribution of the major modal classes of cat retinal ganglion cell as estimated from HRP filling and systematic survey of the soma diameter spectra for classical neurons. J Comp Neurol 197:303–339
Hughes A (1985) New perspectives in retinal organization. In: Osbdorne N, Chader G (eds) Progress in retinal research. Pergamon Press, Oxford, pp 243–313
Kastelein RA, Zweypfennig RCVJ, Spekreijse H (1990) Anatomical and histological characteristics of the eyes of a month-old and an adult harbor porpoise (Phocoena phocoena). In: Thomas JA, Kastelein RA (eds) Sensory abilities of cetaceans. Plenum, New York, pp 463–480
Kröger R, Katzir G (2008) Comparative anatomy and physiology of vision in aquatic tetrapods. In: Thewissen JGM and Numela S (eds) Sensory evolution on the threshold: adaptations in secondarily aquatic vertebrates. University of California Press, Berkeley, Los Angeles, California, pp 121–148
Kröger R, Kirschfeld K (1990) The cornea as an optical element in the cetacean eye. In: Thomas J, Kastelein R (eds) Sensory abilities of cetaceans. Plenum Press, New York, pp 97–106
Kröger R, Kirschfeld K (1993) Optics of the harbor porpoise eye in water. J Opt Soc Am A: 10(7):1481–1489
Kröger R, Kirschfeld K (1994) Refractive index in the cornea of a harbor porpoise (Phocoena phocoena) measured by two-wavelengths laser-interferometry. Aquat Mamm 20:99–107
Madsen CJ, Herman LM (1980) Social and ecological correlates of cetacean vision and visual appearance. In: Herman LM (ed) Cetacean behaviour: mechanisms and functions. Willey Interscience, New York, pp 101–147
Mass AM (1996) Regions of increased density of the ganglion cells and resolution of the retina of the grey whale Eschrichtius robustus. Dokl Akad Nauk 350:139–142
Mass AM, Supin AY (1989) Distribution of ganglion cells in the retina of an Amazon river dolphin Inia geoffrensis. Aquat Mamm 15:49–56
Mass AM, Supin AY (1990) Best vision zones in the retinae of some cetaceans. In: Thomas J, Kastelein R (eds) Sensory abilities of cetaceans. Plenum Press, New York, pp 505–517
Mass AM, Supin AY (1995) Ganglion cells topography of the retina in the bottlenosed dolphin, Tursiops truncatus. Brain Behav Evol 45:257–265
Mass AM, Supin AY (1997) Ocular anatomy, retinal ganglion cell distribution, and visual resolution in the gray whale, Eschichtius robustus. Aquat Mamm 23:17–28
Mass AM, Supin AY (1999) Retinal topography and visual acuity in the riverine tucuxi (Sotalia fluviatilis). Mar Mammal Sci 15:351–365
Mass AM, Supin AY (2002) Visual field organization and retinal resolution of the beluga, Delphinapterus leucas (Pallas). Aquat Mamm 28:241–250
Mass AM, Supin AY (2003) Retinal topography of the harp seal Pagophilus groenlandicus. Brain Behav Evol 62:212–222
Mass AM, Supin AY (2007) Adaptive features of aquatic mammals’ eye. Anat Rec 290:701–715
Mass AM, Supin AY, Severtsov AN (1986) Topographic distribution of sizes and density of ganglion cells in the retina of a porpoise Phocoena phocoena. Aquat Mamm 12:95–102
Mass AM, Supin AY, Mukhametov LM, Rozanova EI, Abramov AV (2012) Morphological estimation of retinal resolution of a killer whale (Orcinus orca). Dokl Biol Sci 442:34–37
Mazzatenta A, Caleo M, Baldaccini N, Maffei L (2001) A comparative morphometric analysis of the optic nerve in two cetacean species, the striped dolphin (Stenella coeruleoalba) and fin whale (Balaenoptera physalus). Visual Neurosci 18:319–325
Mobley JR, Helweg DA (1990) Visual ecology and cognition in cetaceans. In: Thomas JA, Kastelein RA (eds) Sensory abilities of cetaceans. Plenum Press, New York, pp 519–536
Murayama T, Somiya H (1998) Distribution of ganglion cells and object localizing ability in the retina of three cetaceans. Fish Sci 64:27–30
Murayama T, Fujise Y, Aoki I, Ishii T (1992) Histological characteristics and distribution of ganglion cells in the retina of the Dall’s porpoise and Minke whale. In: Thomas JA, Kastelein RA (eds) Sensory abilities of cetaceans. Plenum, New York, pp 137–145
Murayama T, Somiya H, Aoki I, Ishii T (1995) Retinal ganglion cell size and distribution predict visual capabilities of Dall’s porpoise. Mar Mammal Sci 11:136–149
Nachtigall PE (1986) Vision, audition and chemoreception in dolphins and other marine mammals. In: Schusterman JA, Thomas JA, Wood FG (eds) Dolphin cognition and behavior: a comparative approach. Lawrence Erlbaum Associates, Hillsdale, pp 79–113
Ollivier FJ, Samuelson DA, Brooks DE, Lewis PA, Kallberg A, Komáromy M (2004) Comparative morphology of the tapetum lucidum (among selected species). Vet Ophthalmol 7(1):11–22
Peers B (1971) The retinal histology of the atlantic bottlenose dolphin Tursiops truncatus (Montagu, 1821). Thesis, Univ. of Guelph
Peichl L (1991) Alpha ganglion cells in mammalian retinae: common properties, species differences, and some comments on other ganglion cells. Vis Neurosci 7:155–169
Peichl L (1992) Topography of ganglion cells in the dog and wolf retina. J Comp Neurol. 324:603–620
Peichl L, Ott H, Boycott BB (1987) Alpha ganglion cells in mammalian retinae. Proc R Soc Lond B Biol Sci 231:169–197
Pepper RL, Simmons JV (1973) Air visual acuity of the bottlenose dolphin. Exp Neurol 41:271–276
Perez JM, Dawson WW, Landau D (1972) In air visual acuity of the bottlenose dolphin (Tursiops truncatus). Cetology 11:1–11
Pilleri G, Wandeler A (1964) Developmental and functional anatomy of the eyes of the fin whale Balenoptera physalus. Acta Anat Suppl 57:1–74
Rodieck RW (1973) The vertebrate retina: principles of structure and function. Freeman WH, San Francisco
Rodrigues FM, Silva FM, Trompieri-Silveira AC, Vergara-Parente JE, Miglino MA, Guimarães JP (2014) Morphology of the eyeball from the Humpback whale (Megaptera novaeangliae). Microsc Res Tech 77(5):348–355
Shand J, Chin SM, Harman AM, Moore S, Collin SP (2000) Variability in the location of the retinal ganglion cell area centralis is correlated with ontogenetic changes in feeding behavior in the black bream, Acanthopagrus butcheri (Sparidae, teleostei). Brain Behav Evol 55(4):176–190
Shirihai H, Jarrett B (2006) Whales dolphins and other marine mammals of the world. Princeton. Princeton University Press, Princeton, pp 82–84
Sivak JG (1980) Accommodation in vertebrates: a contemporary survey. Curr Top Eye Res 3:281–330
Spong P, White D (1971) Visual acuity and discrimination learning in the dolphin (Lagenorhynchus obliquidens). Exp Neurol 31:431–436
Stone J (1965) A quantitative analysis of the distribution of ganglion cells in the cat’s retina. J Comp Neurol 124:337–352
Stone J (1978) The number and distribution of ganglion cells in cat´s retina. J Comp Neurol 180:753–771
Stone J (1981) The wholemount handbook. A guide to the preparation and analysis of retinal wholemounts. Maitland, Sydney
Stone J, Fukuda Y (1974) Properties of cat retinal ganglion cells: a comparison of W-Cells with X- and Y-cells. J Neurophysiol 37:722–748
Stone J, Halasz P (1989) Topography of the retina in the elephant Loxodonta africana. Brain Behav Evol 34:84–95
Supin AY, Popov VV, Mass AM (2001) The sensory physiology of aquatic mammals. Kluwer Akademic Publishers, Boston
Thewissen JGM (1994) Phylogenetic aspects of cetacean origins: a morphological perspective. J Mammal Evol 2(3):157–184
Thewissen JGM, Williams EM (2002) The early radiations of cetacea (mammalia). Evolutionary pattern and developmental correlations. Annu Rev Ecol Syst 33(1):73–90
Thewissen JGM, Williams EM, Roe LJ, Hussain ST (2001) Skeletons of terrestrial cetaceans and the relationship of whales to artiodactyls. Nature 413(6853):277–281
Thewissen JGM, Cooper LN, Clementz MT, Bajpai S, Tiwari BN (2007) Whales originated from aquatic artiodactyls in the Eocene epoch of India. Nature 450(7173):1190–1194
Traill TS (1809) Description of a new species of whale, Delphinus melas. In a letter from Thomas Stewart Traill, M. D. to Mr. Nicholson. J Nat Phil Chem Arts 1809:81–83
Wässle H, Boycott BB (1991) Functional architecture of the mammalian retina. Physiol Rev 71:447–480
Wässle H, Peichl L, Boycott BB (1981) Morphology and topography of on- and off-alpha cells in the cat retina. Proc Soc Lond B Biol Sci 212:157–175
White D, Cameron N, Spong P, Bradford J (1971) Visual acuity in the killer whale (Orcinus orca). Exp Neurol 32:230–236
Williams R, Cavada C, Reinoso-Suárez F (1993) Rapid evolution of the visual system: a cellular assay of the retina and dorsal lateral geniculate nucleus of the spanish wildcat and the domestic cat. J Neurosci 13:208–228
Wong ROL, Hughes A (1987) The morphology, number and distribution of a large population of confirmed displaced amacrine cells in the adult cat retina. J Comp Neurol 255:159–177
Zhu Q, Hillmann DJ, Henk WG (2001) Morphology of the eye and surrounding structures of the bowhead whale, Balaena Mysticetus. Mar Mammal Sci 17:729–750
Acknowledgment
We thank Dra. Dorete Bloch, Department of Zoology, Natural History Museum Faroe Islands, for getting permission to obtain the samples for this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by A. Schmidt-Rhaesa.
Rights and permissions
About this article
Cite this article
Mengual, R., García, M., Segovia, Y. et al. Ocular morphology, topography of ganglion cell distribution and visual resolution of the pilot whale (Globicephala melas). Zoomorphology 134, 339–349 (2015). https://doi.org/10.1007/s00435-015-0258-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00435-015-0258-7