Abstract
Purpose
The determination of the programmed death ligand-1 (PD-L1) expression is part of the diagnostic algorithm for advanced non-small cell lung cancer (NSCLC) patients. We aimed to analyze the diagnostic performance of EBUS-TBNA performed as first-choice nodal staging procedure for the determination of PD-L1 expression in NSCLC patients.
Methods
Longitudinal-prospective study including NSCLC patients diagnosed between January 2018 and October 2019, for whom a primary tumor biopsy sample and an EBUS-TBNA cytological malignant sample were available. Samples with fewer than 100 malignant cells were considered inadequate. PDL-1 IHC 22C3 pharmDx antibody was used. The percentage of tumor cells expressing PD-L1, setting 1% and 50% as cutoff points, was collected. The weighted kappa coefficient was used to assess the concordance of PD-L1 expression. The PD-L1 expression was compared in precision terms.
Results
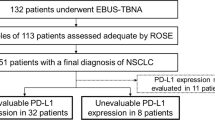
From a total of 43 patients, 53 pairs of samples were obtained, of which 23 (43.4%) were adequate and included for analysis. The weighted kappa coefficient for PD-L1 expression was 0.41 (95% CI 0.15–0.68) and 0.56 (95% CI 0.23–0.9) for cutoff values ≥ 1% and ≥ 50%, respectively. In advanced stages, the weighted kappa coefficient was 0.6 (95% CI 0.3–0.9) and 1 (95% CI 1–1) for PD-L1 expression cutoff values ≥ 1% and ≥ 50%, respectively. EBUS-TBNA showed a sensitivity, specificity, positive predictive value, and negative predictive value of 1 to detect PDL-1 expression ≥ 50% in advanced stages.
Conclusion
EBUS-TBNA performed as first nodal staging procedure in advanced NSCLC patients provides reliable specimens for the detection of PD-L1 expression ≥ 50% and could guide immunotherapy.


Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ALK:
-
Anaplastic lymphoma kinase
- CI:
-
Confidence interval
- CNS:
-
Coagulation necrosis sign
- CT:
-
Computed tomography
- EBUS-TBNA:
-
Endobronchial ultrasound transbronchial needle aspiration
- EBUS:
-
Endobronchial ultrasound
- EGFR:
-
Epidermal growth factor receptor
- FDT:
-
Fast diagnostic track
- FNA:
-
Fine needle aspiration
- H&E:
-
Hematoxylin and eosin
- IASLC:
-
International association for the study of lung cancer
- IHC:
-
Immunohistochemistry
- NPV:
-
Negative predictive value
- NSCLC:
-
Non-small cell lung cancer
- PD-1:
-
Programmed death protein-1
- PD-L1:
-
Programmed death protein-1 ligand
- PPV:
-
Positive predictive value
- TBNA:
-
Transbronchial needle aspiration
- TNM:
-
Tumor node metastases system
- TPS:
-
Tumor proportion score
References
Birchard KR (2011) Transthoracic needle biopsy. Semin Interv Radiol 28(1):87–97. https://doi.org/10.1055/s-0031-1273943
Biswas A, Leon ME, Drew P, Fernandez-Bussy S, Furtado LV, Jantz MA, Mehta HJ (2018) Clinical performance of endobronchial ultrasound-guided transbronchial needle aspiration for assessing programmed death ligand-1 expression in nonsmall cell lung cancer. Diagn Cytopathol 46(5):378–383. https://doi.org/10.1002/dc.23900
Buttner R, Gosney JR, Skov BG, Adam J, Motoi N, Bloom KJ, Dietel M, Longshore JW, Lopez-Ríos F, Penault-Llorca F, Viale G, Wotherspoon AC, Kerr KM, Tsao MS (2017) Programmed death-ligand 1 immunohistochemistry testing: a review of analytical assays and clinical implementation in non-small-cell lung cancer. J Clin Oncol 35(34):3867–3876. https://doi.org/10.1200/JCO.2017.74.7642
De Leyn P, Dooms C, Kuzdzal J, Lardinois D, Passlick B, Rami-Porta R, Turna A, Schil PVan, Venuta F, Waller D, Weder W, Zielinski M (2014) Revised ests guidelines for preoperative mediastinal lymph node staging for non-small-cell lung cancer. Eur J Cardio-Thoracic Surg 45(5):787–798. https://doi.org/10.1093/ejcts/ezu028
Du Rand IA, Blaikley J, Booton R, Chaudhuri N, Gupta V, Khalid S, Mandal S, Martin J, Mills J, Navani N, Rahman NM, Wrightson JM, Munavvar M (2013) British Thoracic Society guideline for diagnostic flexible bronchoscopy in adults. Thorax. https://doi.org/10.1136/thoraxjnl-2013-203618
Fernandez-Bussy S, Pires Y, Labarca G, Vial MR (2018) PD-L1 expression in a non-small cell lung cancer specimen obtained by EBUS-TBNA. Arch Bronconeumol 54(5):290–292. https://doi.org/10.1016/j.arbres.2017.10.008
Goldstraw P, Chansky K, Crowley J, Rami-Porta R, Asamura H, Eberhardt WEE, Nicholson AG, Groome P, Mitchell A, Bolejack V, Ball D, Beer DG, Beyruti R, Detterbeck F, Edwards J, Galateau-Sallé F, Giroux D, Gleeson F, Huang J, Yokoi K (2016) The IASLC lung cancer staging project: proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM Classification for lung cancer. J Thoracic Oncol 11(1):39–51. https://doi.org/10.1016/j.jtho.2015.09.009
Guinde J, Roy P, Dutau H, Musani A, Quadrelli S, Stratakos G, Vergnon JM, Tremblay A, Fortin M (2020) An international survey of mediastinal staging practices amongst interventional bronchoscopists. Respiration 99(6):508–515. https://doi.org/10.1159/000507096
Haragan A, Field JK, Davies MPA, Escriu C, Gruver A, Gosney JR (2019) Heterogeneity of PD-L1 expression in non-small cell lung cancer: implications for specimen sampling in predicting treatment response. Lung Cancer 134:79–84. https://doi.org/10.1016/j.lungcan.2019.06.005
Heymann JJ, Bulman WA, Swinarski D, Pagan CA, Crapanzano JP, Haghighi M, Fazlollahi L, Stoopler MB, Sonett JR, Sacher AG, Shu CA, Rizvi NA, Saqi A (2017) PD-L1 expression in non-small cell lung carcinoma: comparison among cytology, small biopsy, and surgical resection specimens. Cancer Cytopathol 125(12):896–907. https://doi.org/10.1002/cncy.21937
Jamal-Hanjani M, Wilson GA, McGranahan N, Birkbak NJ, Watkins TBK, Veeriah S, Shafi S, Johnson DH, Mitter R, Rosenthal R, Salm M, Horswell S, Escudero M, Matthews N, Rowan A, Chambers T, Moore DA, Turajlic S, Xu H, Swanton C (2017) Tracking the evolution of non–small-cell lung cancer. New Engl J Med 376(22):2109–2121. https://doi.org/10.1056/nejmoa1616288
Kerr KM, Nicolson MC (2016) Non-small cell lung cancer, PD-L1, and the pathologist. Arch Pathol Lab Med 140(3):249–254. https://doi.org/10.5858/arpa.2015-0303-SA
Landis JR, Koch GG (1977) The Measurement of Observer Agreement for Categorical Data. Biometrics 33(1):159. https://doi.org/10.2307/2529310
Perrotta F, Nankivell M, Adizie JB, Maqsood U, Elshafi M, Jafri S, Lerner AD, Woolhouse I, Munavvar M, Evison M, Booton R, Baldwin DR, Janes SM, Kerr KM, Bianco A, Yarmus L, Navani N (2020) Endobronchial ultrasound-guided transbronchial needle aspiration for PD-L1 testing in non-small cell lung cancer. Chest 158(3):1230–1239. https://doi.org/10.1016/j.chest.2020.04.059
Planchard D, Popat S, Kerr K, Novello S, Smit EF, Faivre-Finn C, Mok TS, Reck M, Van Schil PE, Hellmann MD, Peters S (2018) Metastatic non-small cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 29:192–237. https://doi.org/10.1093/annonc/mdy275
Polanco D, Pinilla L, Gracia-Lavedan E, Mas A, Bertran S, Fierro G, Seminario A, Gómez S, Barbé F (2021) Prognostic value of symptoms at lung cancer diagnosis: a three-year observational study. J Thoracic Dis 13(3):1485–1494. https://doi.org/10.2103/jtd-20-3075
Reck M, Rabe KF (2017) Precision diagnosis and treatment for advanced non–small-cell lung cancer. New Engl J Med 377(9):849–861. https://doi.org/10.1056/NEJMra1703413. (Massachussetts Medical Society)
Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe S, O’Brien M, Rao S, Hotta K, Leiby MA, Lubiniecki GM, Shentu Y, Rangwala R, Brahmer JR (2016) Pembrolizumab versus chemotherapy for PD-L1–positive non–small-cell lung cancer. N Engl J Med 375(19):1823–1833. https://doi.org/10.1056/nejmoa1606774
Rusch VW, Asamura H, Watanabe H, Giroux DJ, Rami-Porta R, Goldstraw P (2009) The IASLC lung cancer staging project: a proposal for a new international lymph node map in the forthcoming seventh edition of the TNM classification for lung cancer. J Thoracic Oncol 4(5):568–577. https://doi.org/10.1097/JTO.0b013e3181a0d82e
Sacher AG, Gandhi L (2016) Biomarkers for the clinical use of PD-1/PD-L1 inhibitors in non-small-cell lung cancer: a review. JAMA Oncol 2(9):1217–1222. https://doi.org/10.1001/jamaoncol.2016.0639
Sakakibara R, Inamura K, Tambo Y, Ninomiya H, Kitazono S, Yanagitani N, Horiike A, Ohyanagi F, Matsuura Y, Nakao M, Mun M, Okumura S, Inase N, Nishio M, Motoi N, Ishikawa Y (2017) EBUS-TBNA as a promising method for the evaluation of tumor PD-L1 expression in lung cancer. Clin Lung Cancer 18(5):527-534.e1. https://doi.org/10.1016/j.cllc.2016.12.002
Sakata KK, Midthun DE, Mullon JJ, Kern RM, Nelson DR, Edell ES, Schiavo DN, Jett JR, Aubry MC (2018) Comparison of programmed death ligand-1 immunohistochemical staining between endobronchial ultrasound transbronchial needle aspiration and resected lung cancer specimens. Chest 154(4):827–837. https://doi.org/10.1016/j.chest.2018.07.017
Shafiek H, Fiorentino F, Peralta AD, Serra E, Esteban B, Martinez R, Noguera MA, Moyano P, Sala E, Sauleda J, Cosío BG (2014) Real-time prediction of mediastinal lymph node malignancy by endobronchial ultrasound. Arch Bronconeumol 50(6):228–234. https://doi.org/10.1016/j.arbr.2014.05.003
Smith A, Wang H, Zerbo A, Beaudoin S, Ofiara L, Fiset PO, Benedetti A, Gonzalez AV (2020) Programmed death ligand 1 testing of endobronchial ultrasound-guided transbronchial needle aspiration samples acquired for the diagnosis and staging of non-small cell lung cancer. J Bronchol Interventional Pulmonol 27(1):50–57. https://doi.org/10.1097/LBR.0000000000000623
Stoy SP, Rosen L, Mueller J, Murgu S (2018) Programmed death-ligand 1 testing of lung cancer cytology specimens obtained with bronchoscopy. Cancer Cytopathol 126(2):122–128. https://doi.org/10.1002/cncy.21941
Sukari A, Nagasaka M, Al-Hadidi A, Lum LG (2016) Cancer immunology and immunotherapy. Anticancer Res 36(11):5593–5606. https://doi.org/10.21873/anticanres.11144
Wahidi MM, Herth F, Yasufuku K, Shepherd RW, Yarmus L, Chawla M, Lamb C, Casey KR, Patel S, Silvestri GA, Feller-Kopman DJ (2016) Technical aspects of endobronchial ultrasound-guided transbronchial needle aspiration CHEST guideline and expert panel report. Chest 149(3):816–835. https://doi.org/10.1378/chest.15-1216
Wang Memoli JS, El-Bayoumi E, Pastis NJ, Tanner NT, Gomez M, Huggins JT, Onicescu G, Garrett-Mayer E, Armeson K, Taylor KK, Silvestri GA (2011) Using endobronchial ultrasound features to predict lymph node metastasis in patients with lung cancer. Chest 140(6):1550–1556. https://doi.org/10.1378/chest.11-0252
Wang L, Wu W, Hu Y, Teng J, Zhong R, Han B, Sun J (2015) Sonographic features of endobronchial ultrasonography predict intrathoracic lymph node metastasis in lung cancer patients. Ann Thorac Surg 100(4):1203–1209. https://doi.org/10.1016/j.athoracsur.2015.04.143
Wang H, Agulnik J, Kasymjanova G, Wang A, Jiménez P, Cohen V, Small D, Pepe C, Sakr L, Fiset PO, Auger M, Camilleri-Broet S, Alam El Din M, Chong G, van Kempen L, Spatz A (2018) Cytology cell blocks are suitable for immunohistochemical testing for PD-L1 in lung cancer. Ann Oncol 29(6):1417–1422. https://doi.org/10.1093/annonc/mdy126
Yoshimura K, Inoue Y, Karayama M, Tsuchiya K, Mori K, Suzuki Y, Iwashita Y, Kahyo T, Kawase A, Tanahashi M, Ogawa H, Yokomura K, Inui N, Funai K, Shinmura K, Niwa H, Suda T, Sugimura H (2019) Heterogeneity analysis of PD-L1 expression and copy number status in EBUS-TBNA biopsy specimens of non-small cell lung cancer: comparative assessment of primary and metastatic sites. Lung Cancer 134:202–209. https://doi.org/10.1016/j.lungcan.2019.06.002
Zhou J, Gong Z, Jia Q, Wu Y, Yang ZZ, Zhu B (2018) Programmed death ligand 1 expression and CD8+ tumor-infiltrating lymphocyte density differences between paired primary and brain metastatic lesions in non-small cell lung cancer. Biochem Biophys Res Commun 498(4):751–757. https://doi.org/10.1016/j.bbrc.2018.03.053
Acknowledgements
LP is the recipient of a predoctoral fellowship from the Ministry of Universities of Spain (FPU19/01555).
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All the authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by DP, LP, EG-L, SG, MZ, MP, SG and FB. The first draft of the manuscript was written by DP, and all the authors commented on previous versions of the manuscript. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of the University Hospital Arnau de Vilanova and Santa María (Date September 17th,2018/No.1959).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Polanco, D., Pinilla, L., Gracia-Lavedan, E. et al. Performance of endobronchial ultrasound transbronchial needle aspiration as the first nodal staging procedure for the determination of programmed death ligand-1 expression in non-small cell lung cancer patients. J Cancer Res Clin Oncol 149, 12459–12468 (2023). https://doi.org/10.1007/s00432-023-05039-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-023-05039-9