Abstract
Purpose
Tumor-infiltrating lymphocytes (TILs) have shown remarkable clinical responses in some patients with advanced solid tumors. As a rare subset of TILs, CD4−/CD8− double-negative T cells (DNTs) were poorly known. This study aims to investigate the characteristics and function of CD3+CD4−CD8− TILs (double-negative TIL, DN-TILs) derived from solid tumor.
Methods
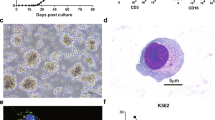
DN-TILs were derived and expanded ex vivo from resected gastric carcinoma tissue and phenotyped by flow cytometry. The cytotoxicity of DN-TILs was determined against established tumor cell lines in vitro or through in vivo adoptive transfer into xenograft models. K562 cells were transferred with the HLA gene to verify whether the cytotoxicity of DN-TILs was MHC-independent.
Results
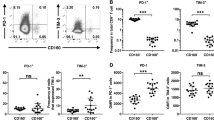
Flow cytometric analysis revealed a high-purity population of DN-TILs (> 97%) within CD3+ TILs, which expanded more than 800-folds in 2 weeks, consisting of a mixture of alpha–beta (αβ) and gamma–delta (γδ) T-cell receptor (TCR)—expressing cells (with the majority being αβ-TCR, > 95%). Using single-cell RNA sequencing, the expanded DN-TILs were categorized into four main subsets, Natural Killer T cells (approximately 80%, 5563 in 7028), Progenitor cells, Germ cells and T helper2 cells. DN-TILs exhibited a broad anticancer cytotoxicity in a donor-unrestricted manner against various cancer cell lines derived from pancreatic cancer (Panc-1), gastric cancer (HGC-27), ovarian cancer (SKOV-3), malignant melanoma (A375). The cytotoxicity was MHC-independent, which was not altered in K562 transferring with HLA gene or not. DN-TILs significantly reduced tumor volume in xenograft models with superior tumor-homing ability and low off-target toxicity.
Conclusion
Gastric carcinoma derived DN-TIL can target tumor cells in vitro and in vivo. DN-TILs have the potential to be used as a adoptive cell therapy for solid cancers with both the advantages of DNT and TIL.




Similar content being viewed by others
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
(2008) Umbilical cord blood T cells express multiple natural cytotoxicity receptors after IL-15 stimulation but only NKp30 is functional. J Immunol 181(7):4507–4515. https://doi.org/10.4049/jimmunol.181.7.4507
Alnaggar M et al (2019) Allogenic Vgamma9Vdelta2 T cell as new potential immunotherapy drug for solid tumor: a case study for cholangiocarcinoma. J Immunother Cancer 7(1):36
van den Berg JH et al (2020) Tumor infiltrating lymphocytes (TIL) therapy in metastatic melanoma: boosting of neoantigen-specific T cell reactivity and long-term follow-up. J Immunother Cancer 8(2):1
Di Blasi D et al (2020) Unique T-cell populations define immune-inflamed hepatocellular carcinoma. Cell Mol Gastroenterol Hepatol 9(2):195–218
Brenner MK (2012) Will T-cell therapy for cancer ever be a standard of care? Cancer Gene Ther 19(12):818–821
Chen B et al (2018) Targeting chemotherapy-resistant leukemia by combining DNT cellular therapy with conventional chemotherapy. J Exp Clin Cancer Res 37(1):88
Chitadze G et al (2017) The ambiguous role of gammadelta T lymphocytes in antitumor immunity. Trends Immunol 38(9):668–678
Creelan BC et al (2021) Tumor-infiltrating lymphocyte treatment for anti-PD-1-resistant metastatic lung cancer: a phase 1 trial. Nat Med 27(8):1410–1418
Fang L et al (2019) Targeting late-stage non-small cell lung cancer with a combination of DNT cellular therapy and PD-1 checkpoint blockade. J Exp Clin Cancer Res 38(1):123
Feng X, Yan J, Wang Y, Zierath JR, Nordenskjöld M, Henter J-I, et al (2010) The proteasome inhibitor bortezomib disrupts tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) expression and natural killer (NK) cell killing of TRAIL receptor-positive multiple myeloma cells. Mol Immunol 47(14):2388–2396
Fischer K et al (2005) Isolation and characterization of human antigen-specific TCR alpha beta+ CD4(−)CD8-double-negative regulatory T cells. Blood 105(7):2828–2835
Fisher JP et al (2014) Gammadelta T cells for cancer immunotherapy: a systematic review of clinical trials. Oncoimmunology 3(1):e27572
Gomes AQ, Martins DS, Silva-Santos B (2010) Targeting gammadelta T lymphocytes for cancer immunotherapy: from novel mechanistic insight to clinical application. Cancer Res 70(24):10024–10027
Hall M et al (2016) Expansion of tumor-infiltrating lymphocytes (TIL) from human pancreatic tumors. J Immunother Cancer 4:61
Koch J, Steinle A, Watzl C, Mandelboim O (2013) Activating natural cytotoxicity receptors of natural killer cells in cancer and infection. Trends Immunol 34(4):182–191. https://doi.org/10.1016/j.it.2013.01.003
Kabelitz D, Wesch D, He W (2007) Perspectives of gammadelta T cells in tumor immunology. Cancer Res 67(1):5–8
Kazemi MH et al (2022) Tumor-infiltrating lymphocytes for treatment of solid tumors: It takes two to tango? Front Immunol 13:1018962
Koch J, Steinle A, Watzl C, Mandelboim O. Activating natural cytotoxicity receptors of natural killer cells in cancer and infection. Trends Immunol. 2013;34:182–191.
Lee J et al (2018) Allogeneic human double negative T cells as a novel immunotherapy for acute myeloid leukemia and its underlying mechanisms. Clin Cancer Res 24(2):370–382
Liu Z et al (2017) Tumor-infiltrating lymphocytes (TILs) from patients with glioma. Oncoimmunology 6(2):e1252894
Low-dose bortezomib increases the expression of NKG2D and DNAM-1 ligands and enhances induced NK and γδ T cell-mediated lysis in multiple myeloma.
Lu Y et al (2019) Double-negative T cells inhibit proliferation and invasion of human pancreatic cancer cells in co-culture. Anticancer Res 39(11):5911–5918
Merims S et al (2011) Anti-leukemia effect of ex vivo expanded DNT cells from AML patients: a potential novel autologous T-cell adoptive immunotherapy. Leukemia 25(9):1415–1422
Niu C, Jin H, Li M, Zhu S, Zhou L, Jin F, et al. Low-dose bortezomib increases the expression of NKG2D and DNAM-1 ligands and enhances induced NK and γδ T cell-mediated lysis in multiple myeloma. Oncotarget. 2017;8:5954–5964.
Paijens ST et al (2021) Tumor-infiltrating lymphocytes in the immunotherapy era. Cell Mol Immunol 18(4):842–859
Poschke IC et al (2020) The outcome of ex vivo TIL expansion is highly influenced by spatial heterogeneity of the tumor T-cell repertoire and differences in intrinsic in vitro growth capacity between T-cell clones. Clin Cancer Res 26(16):4289–4301
Qin M et al (2022) Tumor-infiltrating lymphocyte: features and prognosis of lymphocytes infiltration on colorectal cancer. Bioengineered 13(6):14872–14888
Ramachandran V, Kolli SS, Strowd LC (2019) Review of graft-versus-host disease. Dermatol Clin 37(4):569–582
Rosenberg SA, Spiess P, Lafreniere R (1986) A new approach to the adoptive immunotherapy of cancer with tumor-infiltrating lymphocytes. Science 233(4770):1318–1321
Sarnaik AA et al (2021) Lifileucel, a tumor-infiltrating lymphocyte therapy in metastatic melanoma. J Clin Oncol 39(24):2656–2666
Schumacher TN, Schreiber RD (2015) Neoantigens in cancer immunotherapy. Science 348(6230):69–74
Stankovic B et al (2018) Immune cell composition in human non-small cell lung cancer. Front Immunol 9:3101
Stevanović S et al (2019) A Phase II study of tumor-infiltrating lymphocyte therapy for human papillomavirus-associated epithelial cancers. Clin Cancer Res 25(5):1486–1493
Stuart T et al (2019) Comprehensive integration of single-cell data. Cell 177(7):1888-1902 e21
Tang Q, Grzywacz B, Wang H, Kataria N, Cao Q, Wagner JE, et al. Umbilical cord blood T cells express multiple natural cytotoxicity receptors after IL-15 stimulation, but only NKp30 is functional. J Immunol. 2008;181:4507–4515.
Titov A et al (2021) Adoptive immunotherapy beyond CAR T-cells. Cancers (basel) 13(4):1
Vantourout P, Hayday A (2013) Six-of-the-best: unique contributions of γδ T cells to immunology. Nat Rev Immunol 13(2):88–100
Wu Z et al (2022) CD3(+)CD4(−)CD8(−) (double-negative) T cells in inflammation, immune disorders and cancer. Front Immunol 13:816005
Yao J et al (2019) Human double negative T cells target lung cancer via ligand-dependent mechanisms that can be enhanced by IL-15. J Immunother Cancer 7(1):17
Funding
Clinical research project of Shanghai Municipal Health Commission (No. 201940492).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception or design. Clinical samples were provided by XG and QZ. Material preparation was performed by RX. Cell assays were performed by JL, CH and RH. Data collection and analysis were performed by JL, CH, YL and QX. JL, CH and QX contributed to the study conception and design. The first draft of the manuscript was written by JL and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript. JL and CH are equal first authors of this publication.
Corresponding author
Ethics declarations
Conflict of interest
Chen Huang is an employee of Shanghai Juncell Biotechnology Co., LTD. The other authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lu, J., Huang, C., He, R. et al. CD4−/CD8− double-negative tumor-infiltrating lymphocytes expanded from solid tumor tissue suppress the proliferation of tumor cells in an MHC-independent way. J Cancer Res Clin Oncol 149, 9007–9016 (2023). https://doi.org/10.1007/s00432-023-04823-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-023-04823-x