Abstract
Purpose
Prostate cancer can undergo curative effects by radical prostatectomy or radical radiotherapy. However, the best treatment for more aggressive high-risk prostate cancer remains controversial. Insufficient infiltration capacity and dysfunction are commonly occurrences in engineered T lymphocytes expressing chimeric antigen receptor (CAR-T), characterizing cancer immunotherapy failure. We conducted this study to investigate whether the combinative application of docetaxel and PSMA-CAR-T cells could be a more effective treatment to prostate cancer.
Methods
Expressions of prostate specific membrane antigen (PSMA) on prostate cancer cells were examined by Flow cytometry. The efficaciousness of PSMA-CAR-T was evaluated in vitro using ELISA and RTCA. The effect of intermixed therapy was assessed in vivo utilizing a human prostate cancer liver metastasis mouse model and a human prostate cancer cell xenograft mouse model.
Results
The outcome of cytokine discharge and cell killing assays demonstrated that PSMA-CAR-T cells have characteristic effector capacity against PSMA+ prostate cancer cells in vitro. Additionally, collaborative treatment of PSMA-CAR-T cells and docetaxel have cooperative efficacy in a mouse model of human prostate cancer. The merged strategy could be seen as an undeveloped avenue to augmenting adoptive CAR-T cell immunotherapy and mitigating the adverse side effects of chemotherapy.
Conclusions
Cooperation of PSMA-specific CAR-T cells and the chemotherapy drug docetaxel can impressively ameliorate antitumor effectiveness against an installed metastatic human prostate cancer model in NPG mice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prostate cancer is the second most prevailing cancer among men in the whole world. The new emergency cases of prostate cancer provoked in last few years and caused considerable deaths in the USA (Siegel et al. 2021). Surgical resection and local radiotherapy can effectively treat early stage of prostate cancer, while advanced prostate cancer is mostly treated with palliative treatment which leading to the high mortality rate (Siegel et al. 2020). The medical world craving for new strategy to increase the curative effect and prolonged patient prognosis.
Adaptive immunotherapy with chimeric antigen receptor-modified T (CAR-T) cells, as the most propitious tumor remedy, involves inserting the CAR gene into T lymphocytes through genetic engineering to stimulate autoimmune cells to kill cancerous cells. This approach has brought new hope to countless patients with malignant tumors, especially hematological malignancies (Porter et al. 2011). The success of CAR-T cells in the remedy of hematological tumours is also bringing optimism to the treatment of solid tumors. The hinder of the efficaciousness of CAR-T cells in the therapy of solid tumors was owing to the convoluted dynamic repressive tumor microenvironment partly. It contains various immunosuppressive factors, including myeloid-derived suppressor cells (MDSCs), tumor-associated macrophages (TAMs), regulatory T cells (Tregs) (Verma et al. 2011), and ligands which restrain the capability of T cells to abbreviate tumor cells (such as PD-L1) (Zhao et al. 2018). Therefore, it is necessary to adopt approaches to take full advantage of the effect of CAR-T cell in solid tumor immunotherapy.
Docetaxel (DTX) is an antineoplastic agent that has a unique mechanism of action as an inhibitor of cellular mitosis and that currently acts a pivotal part in castration-resistant prostate cancer (CRPC) therapy. Although the use of these drugs has improved clinical outcomes, prolonging lifespans, many patients develop resistance. In a recent study, it was proved that DTX pretreatment in non-small cell lung cancer could induce increased expression of high mobility group protein B1 (HMGB1) in tumor cells, followed by increased emission of chemokine CXCL11, and tumor infiltration of CD8+ T cells was also enhanced (Gao et al. 2019). These results demonstrated the regulatory role of DTX in the tumor immune microenvironment, suggesting a possible therapeutic method: the DXT therapy might enhance CAR-Ts’ functions and achieve better therapeutic effects.
In this study, we design a second-generation CAR, which is aiming to PSMA with a costimulatory domain of CD137. The PSMA-CAR-T cells’ capability of killing and discharge lymphokines were tested in vitro. The collaborative curative efficaciousness and mechanism of synthesized therapy with PSMA-CAR-T and docetaxel are verified, both in a mouse liver metastasis model and in a mouse xenograft model of human prostate cancer. We found DTX and PSMA-CAR-T cells together may down-regulated the immune checkpoint molecules, including programmed cell death protein-1 (PD-1), cytotoxic T-lymphocyte antigen 4 (CTLA-4), lymphocyte activation gene-3 (LAG-3), T-cell immunoglobulin and mucin-domain containing-3 (TIM-3). In mechanism, we found DTX enhances CAR-T therapy by advancing infiltration of CAR-T cells and diminishing MDSCs.
Materials and methods
Cell culture
Human primary T cells were expanded in X-VIVO 15 with 10% FBS, 1% penicillin/streptomycin and IL-2 (500 U/mL) within a moisten 5% (v/v) CO2 atmosphere at 37 ℃. To set up a durable overexpressing PSMA cell line, the PC3 cell line was transduced with a lentivirus bearing the human PSMA gene. All FBS was heat inactivated prior to use.
Reagents
Docetaxel was purchased from MedChemExpress company (Shanghai, China). Antibodies used to perform flow cytometry were purchased from BD Biosciences or BioLegend. Mouse anti-c-myc monoclonal antibody was obtained from Sigma-Aldrich. Phycoerythrin-conjugated mouse antihuman PSMA antibody was obtained from R&D Systems.
T cells transduction and expansion
Human peripheral blood mononuclear cells (PBMCs) obtained from healthy donors were separated with Lymphoprep density separation (Stemcell, Canada) and then stimulated by Human T-Activator CD3/CD28 Dynabeads. On day 2, T lymphocytes were then transduced with lentiviral particles at a multiplicity of infection of 5 in the presence of RetroNectin. The beads were removed at day 6 after stimulation. The transduction efficiency was detected by the percentage of c-myc+ cells using flow cytometry. The CAR-T cells were expanded and maintained at a cell density of 1 × 106 cells/mL.
Flow cytometry
Each sample requires at least 10,000 events, and data achieved from BD FACS Canto II were analyzed using the software Flowjo 10. All of the staining steps were accomplished in staining buffer and fixed in 1% paraformaldehyde.
Cytokine production assay
The release of cytokine is detected by cytometric bead array assay, Ctrl-CAR-Ts or PSMA-CAR-Ts (1 × 104) were co-incubated with target cells (1 × 104) in 96-well plates without adding extra exogenous cytokines. One day later, supernatant is gathered and cytokine levels are measured in duplicate utilizing the specific cytometric bead array assay (BD Biosciences). Statistics are attained through FACS Diva software and analyzed by FCAP software.
Real-time cell assay (RTCA)
The cytotoxic capability of PSMA-CAR-T cells in vitro is measured by xCELLigence RTCA TP instrument (ACEA Biosciences). The RTCA system continuously records impedance data of cell attachment and growth, and the statistics are acquired to keep track of the CAR-T cytotoxic activity which is based on the viability of attached target cells, as reflected by cell index values.
Immunohistochemistry
Paraffin-embedded prostate cancer tumor sections are heat fixed, blocked with 3% H2O2, and incubated with specific antibodies overnight at 4 ℃. Images are acquired using fluorescence microscopy (200, Olympus).
Human prostate cancer liver metastasis mouse model
6-week old male NPG (NOD. Cg-Prkdcscid Il2rgtm1Vst/Vst) mice (Vital Star Laboratory Animal Technology, Beijing, China) were injected i.v. with PC3PSMA+/luci+ cells (1 × 106 cells/mouse). On day 7, Docetaxel (15 mg/kg, MCE) is administered continuously by intraperitoneal injection on day 7 after tumor inoculation. Simultaneously, 2.5 × 106 PSMA-CAR-T cells are injected i.v. into the mice on day 8. Mice are matched based on the tumor bioluminescence before assignment to control or treatment groups. All of the animal procedures are approved and manipulated in strict accordance with the Institutional Ethical Care and Use Committee.
Human prostate cancer cell xenograft mouse model
For the prostate cancer cell xenograft mouse model, PC3PSMA+ cells (1 × 106 cells/mouse) are surgically injected into NPG mice in the right flank. On day 7 after tumor inoculation, docetaxel is administered by intraperitoneal injection, respectively. On day 8, PSMA-CAR-T cells (2.5 × 106 cells/mouse) are injected intravenously via tail injection (i.v.). The tumor volume is calculated by the formula: (major axis of tumor) × (minor axis of tumor)2/2. Mice are matched based on the tumor volume before assignment to control or treatment groups. At the endpoint, mice are euthanized when the tumor volumes reached ~ 1000 mm3 in the control group or signs of discomfort were noticed.
Statistical analysis
Data are analyzed and plotted with GraphPad Prism software (GraphPad, San Diego, CA, USA). Comparisons between 2 groups are performed by Student’s unpaired 2-tailed t-test. The two-sided log-rank test is applied for the mouse survival test. P < 0.05 was considered significant.
Results
Cytotoxic capability of PSMA-CAR-T cells against PSMA + prostate cancer cells
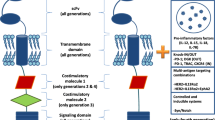
We design a PSMA CAR involving a mouse anti-human PSMA-scFv, a CD137 costimulatory molecule and a CD3 zeta intracellular activation domain. The control-CAR is the same construct without the anti-PSMA-scFv (Fig. 1A). When expansion is over (day 10), both Ctrl-CAR-T cells and PSMA-CAR-T cells are stained with c-myc antibody which is followed by Alexa Fluor647-conjugated secondary antibody and measured their transduction effectiveness by flow cytometry. As shown in Fig. 1B, the transduction effectiveness of activated PSMA-CAR-T cells and Ctrl-CAR-T cells are 59.8% and 55.9%, separately.
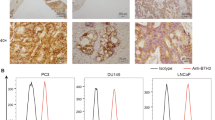
Cytotoxic Capability of PSMA-CAR-T Cells Against PSMA+ Prostate Cancer Cells A abridged general view of lentiviral vectors expressing Ctrl-CAR and PSMA-CAR. B Surface expression of PSMA-CAR on T cells transduced with lentivirus was verified by FACS with anti-c-myc antibody (red line, isotype control). C Surface expression of PSMA in human cancer cell lines. D Cytokine release of PSMA-CAR-T cells. A total of 1 × 104 Ctrl-CAR-T or PSMA-CAR-T cells were cocultured with 1 × 104 target cells in 200 μL of medium per well in round-bottom 96-well plates in triplicate. E Cytotoxicity examination of PSMA-CAR-T cells against PSMA+ or PSMA− target cells by RTCA (E:T = 1:1). The expansion of the target cells is incessantly recorded. *P < 0.05; **P < 0.01; ***P < 0.001; ns, not significant
To possess PC3PSMA+, PC3 cell line is transduced by lentivirus. The density of PSMA expression in various prostate cancer cell lines is assessed by flow cytometry analysis using a mouse anti-human PSMA monoclonal antibody. As shown in Fig. 1C, flow cytometry analysis efficiently detects high expression of PSMA in PC3PSMA+ and LNCap cells and nonexpression in DU145 and PC3 cells, which are used as negative controls.
To assess the level of the cytokine releasing by PSMA-CAR-T cells against PSMA+ cells, we coculture CAR-T cells together with target cells, then pick up the supernatant including the cytokines assayed by ELISA. As shown in Fig. 1D, compared with Ctrl-T cells, much higher levels of IFN-γ, perforin, granzyme B and IL-2 released by PSMA-CAR-T cells are detected while co-incubated with PC3PSMA+ and LNCap cells. The difference in cytokine release between PSMA-CAR-T cells and Ctrl-T cells co-incubated with 293 T, DU145 and PC3 was insignificant. These results reveal PSMA-CAR-T cells might be specifically stimulated by PSMA+ target cells and secrete specifically more cytokines than encountering with PSMA− target cells. Tumor cells which are expressing low levels of PSMA may trigger considerably less cytotoxic capability of PSMA-CAR-T cells.
Then, we use RTCA to further check the cytotoxic capacity of PSMA-CAR-T cells. As shown in Fig. 1E, PSMA-CAR-T cells against LNCap cells result in an instant and sharp reduction related with time in cell index values, similar to PC3PSMA+ cells. Approximately 50 h after the addition of CAR-T cells, the LNCap cell index closely drop to baseline (cell index = 0.0), indicating almost completely cytolysis of the target cells. When co-incubated with Ctrl-CAR-T cells, however, the LNCap cells undergo insubstantial effects. The cytotoxicity of PSMA-CAR-T cells is significantly weakened in DU145 and wild type PC3 cells, indicating the targeting capacity of PSMA-CAR-T cells. In summary, upper data establish that PSMA-CAR-T cells possess particular cytotoxic capability against PSMA+ target cells.
Docetaxel and PSMA-CAR-T cells show collaborative curative efficaciousness against the Mouse liver metastasis model of human prostate cancer
Liver metastasis is one of the most significant metastasis routes for advanced prostate cancer; therefore, we establish the mouse liver metastasis model of human prostate cancer to investigate the collaborative curative efficaciousness of the synthesized therapy. We first test the effect of docetaxel on the proliferation and function of CAR-T cells in vitro. In summary, upper data establish that docetaxel therapy causes no notable side effects on the proliferation, cytokine release, and cytotoxicity of PSMA-CAR-T cells in a clinical setting (S1A, B, C).
We establish a liver metastasis model of human prostate cancer. When each bioluminescence intensity (BLI) of the tumors is average, mice were divided into 4 groups (n = 8). On day 7, mice are infused with docetaxel intraperitoneal (i.p.). On day 8, mice are infused with PSMA-CAR-T cells via tail vein injection. The treating schedule of the mice in the CAR-T + DTX group is shown in Fig. 2A. To eliminate the influence of docetaxel's strong curative effect on the experiment, we select the proper concentration of docetaxel so that it would not show an overwhelming effect on tumor growth but only inhibit tumor growth slightly. PSMA-CAR-T cells or docetaxel therapy alone demonstrate slightly antitumor curative effects. However, synthesized treatment with docetaxel and PSMA-CAR-T cells confer a considerable reduction in tumor burden (Fig. 2B, C), and viability is prolonged at the same time (Fig. 2D). Compared with monotherapy, the tumor burden decrease by nearly 50%, and the average life span almost double, extending to 66 days instead of 35 days. A significant increase in PSMA-CAR-T cells in mouse peripheral blood caused by docetaxel treatment is observed (Fig. 2E, F). Upper data establish that docetaxel and PSMA-CAR-T cells showed collaborative efficaciousness in the mouse liver metastasis model of human prostate cancer.
Docetaxel and PSMA-CAR-T Cells Show Collaborative Curative Efficaciousness Against the Mouse Liver Metastasis Model of Human Prostate Cancer A Abridged general view indicating the remedy planning of the mice. B Representative bioluminescence images of PC3PSMA+/luci+ tumor growth in the human prostate cancer model shown in A. C Bioluminescence kinetics of PC3PSMA+/luci+ tumor growth (5 mice/group) in the human prostate cancer model in (A). D General viability of NPG immunodeficient mice carrying an settled liver metastatic model of human prostate cancer after collaborative treatment of PSMA-CAR-T cells and docetaxel. E and F, Summarized statistics and representative dot plots demonstrating human T cells in the peripheral blood of NPG mice. Error bars denote SEM; **P < 0.01; ***P < 0.001
Docetaxel and PSMA-CAR-T cells show collaborative curative efficaciousness against the xenograft model of human prostate cancer
The triumph of CAR-T therapy in treating solid tumours has been limited (Rafiq et al. 2020). To further examine the curative efficaciousness of collaborative therapy with docetaxel and PSMA-CAR-T cells, we apply the PC3PSMA+ cell line to set up a xenograft model of human prostate cancer in NPG mice (Fig. 3A). We observe obvious differences between the single curative efficaciousness of CAR-T cells and collaborative therapy. When the experiment is ended, all of the tumor tissues are separated and lined up to be imaged. Similar to the liver metastasis model, single PSMA-CAR-T cells treatment or single docetaxel treatment provide poor antitumor curative benefits. Collaborative therapy of docetaxel and PSMA-CAR-T cells lead to significant tumor growth suppression (Fig. 3B–D). Compared with monotherapy, the tumor volume is decreased nearly 75%, decreasing from an average of 400 mm3 to 50 mm3, and the tumor weight decrease from an average of 0.5 g to 0.1 g. Upper statistics establish that docetaxel and PSMA-CAR-T cells show collaborative curative efficaciousness against a mouse xenograft model of human prostate cancer. Docetaxel therapy also provoke a notable growth of PSMA-CAR-T cells in mouse peripheral blood (Fig. 3E, F). After the treatment, we notice that collaborative therapy multiply raises the frequentness of T cells in tumor tissue compared with single treatment, as demonstrated by immunohistochemical staining, from approximately 0.2% to 0.6% CD3-positive cells (Fig. 3G, H). Next, we investigated expressions of several T cells functional markers, including PD-1, TIM-3 and CTLA-4. Collaborative therapy with docetaxel decreased expressions of PD1, TIM-3 and CTLA-4 in CAR-T cells (Fig. 3I–K), suggesting docetaxel ameliorated CAR-T exhaustion in tumor tissues. We also detected the Ki67 levels of CAR-T cells, and the results revealed that docetaxel increased the expression of Ki67 in CAR-T cells (Fig. 3L). These results indicated that collaborative therapy enhance CAR-T efficacy by down-regulating the immune checkpoint molecules and promoting CAR-T cells proliferation.
Docetaxel and PSMA-CAR-T Cells Show Collaborative Curative Efficaciousness Against the Xenograft Model of Human Prostate Cancer A Abridged general view indicating the remedy planning of the mice. B Line chart of tumor growth during the experiment. C, D Representative tumor images (C) and weights (D) from different treatment groups. E, F Summarized statistics and representative dot plots demonstrating human T cells in the peripheral blood of NPG mice. G and H, Representative IHC staining for CD3 (H) and quantitative results (G) of tumor infiltrating T cells in the CAR-T or CAR-T + docetaxel groups. CD3-positive rates (G) were analyzed using Image-Pro Plus software based on 3 representative visual areas in each section from each tumor (n = 6 per group). I, J. and K, T exhaustion markers include PD-1 (I), TIM-3 (J), and CTLA4 (K) in tumor-infiltrated CAR-T cells were detected by flow cytometry. L, Ki67 expressions in tumor-infiltrated CAR-T cells were detected by flow cytometry (n = 5). Error bars denote SEM; *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001; ns, not significant
Docetaxel treatment decreased the frequency and immunosuppression of MDSCs to PSMA-CAR-T cells
According to previous reports, docetaxel could ameliorate tumor microenvironment by inhibiting MDSCs (Kodumudi et al. 2010; Djeu and Wei 2012), therefore, we assess the frequency of MDSCs in peripheral blood. We exert a drastic reduction of MDSCs in peripheral blood in combination therapy group, compared with single PSMA-CAR-T treatment (Fig. 4A, B). Then, the MDSCs are isolated from the PC3 tumor tissue and the repressing effects of mouse MDSCs against human PSMA-CAR-T cells are detected by RTCA and ELISA. The separated MDSCs are co-incubated with PSMA-CAR-T cells at a ratio of 2.5:1 for a day. Next, the CAR-T cells are extracted from the mixed cells and co incubated with PC3PSMA+ cells at an E: T ratio of 1:1 in an E-plate for approximately 50 h, and the cytotoxicity of the CAR-T cells is detected by RTCA. When RTCA is ended, the supernatants are gathered and ELISA is using to evaluate the level of IFN-γ released by CAR-T cells. As shown in Fig. 4C, D, compared with untreated MDSC CAR-T cells group, target cells in the MDSC-treated CAR-T cells (MDSC: CAR-T = 2.5:1) expand remarkably quicker, indicating the lower cytotoxicity of MDSC-treated CAR-T cells. The ELISA results show that the IFN-γ levels of MDSC-treated CAR-T cells (MDSCs: CAR-T = 2.5:1) were remarkably lower than those secreted by MDSC-untreated CAR-T cells (Fig. 4E). In summary, upper data establish that downregulation of MDSCs triggered by docetaxel therapy could be a considerable factor in the collaborative effects of docetaxel and PSMA-CAR-T cells.
Docetaxel Treatment Decreased the Frequency of MDSCs and Inhibited the Cytotoxicity of Human PSMA-CAR-T Cells by Mouse MDSCs. A and B Summarized statistics and representative dot plots demonstrating MDSCs in peripheral blood. The cells were stained with antibodies anti-mouse CD45, mouse CD11b, mouse GR-1 for MDSCs. C MDSCs were isolated from PC3PSMA+ tumors of NPG mice. The separated MDSCs are co-incubated with PSMA-CAR-T cells at a ratio of 2.5:1 for a day. Next, the CAR-T cells are extracted from the mixed cells and co incubated with PC3PSMA+ cells at an E:T ratio of 1:1 in an E-plate for approximately 50 h, and the cytotoxicity of the CAR-T cells was detected by RTCA. D Representative results and summarized RTCA data showing the cytotoxicity of PSMA CAR-T cells treated with or without mouse MDSCs. E, IFN-γ release of PSMA-CAR-T cells that received mouse MDSC treatment or not were detected by ELISA (n = 2). E: T, effector/target; IFN, interferon-gamma; MDSC, myeloid-derived suppressor cell; RTCA, real-time cell assay. **P < 0.01; ***P < 0.001
Discussion
Docetaxel, a clinically available antimicrotubular agent, has been used as an efficacious remedy to cure various solid tumors, including breast cancer and prostate cancer (Beltran and Morris 2020; Nabholtz and Gligorov 2005). However, the capacity of high-dose docetaxel in clinical practice would be jeopardized by inappropriate control of side effects, such as cytopenia, peripheral edema, myalgia, arthralgia, alopecia, and ototoxicity neurotoxicity (Xuan et al. 2020; da Costa et al. 2020; Rochigneux et al. 2018). In our study, combination therapy with low-dose docetaxel and PSMA-CAR-T cells decreased the side effects caused by docetaxel treatment that the treated mice had no obvious symptoms and still experienced a magnified curative effect.
CAR-T cells containing immunotherapy has indicated highly unique and practical results in treating hematological malignancies, such as acute/chronic lymphoblastic leukemia, lymphoma, and multiple myeloma (Achkova and Pule 2018; Xu et al. 2017). However, no progress has been made on CAR-T therapy applied to remedy patients suffering solid tumors, and genesis of the obstacle have been broadly investigated (D’Aloia et al. 2018). As the trickiest dilemma, the unfriendly immunosuppressive microenvironment of solid tumors would extraordinarily hinder the capacity of CAR-T cells (D’Aloia et al. 2018; Gorchakov et al. 2019). Aiming to solve this handicap, we look into the possibility of employing various clinical frontline chemotherapy drugs to strengthen the capability of CAR-T cells. The unprecedented function of docetaxel in the overturning of immunosuppression and regulation of the tumor microenvironment has been established in up-to-date investigations (Hu et al. 2020; Wen et al. 2018). Collectively, we theorize that collaborative treatment containing docetaxel could ameliorate the capability of PSMA-CAR-T cells against prostate cancer to a greater extent. In an adaptive transfer experiment, our results prove that collaborative PSMA-CAR-T cells with docetaxel considerably advance curative efficaciousness against the established mouse liver metastasis model of human prostate cancer and bring about lengthen viability of recipient mice. Moreover, we indicate that collaborative docetaxel with CAR-T therapy triggered the upper infiltration of T cells in the tumor tissues, which could be ascribed to the collaborative antitumor capacity.
According to previous studies (Djeu and Wei 2012; Kodumudi et al. 2010), docetaxel modulate MDSCs by inhibiting STAT3 signaling, inducing apoptosis of murine MDSCs (Kodumudi et al. 2010). Furthermore, according to Hu, downregulation of tumor-associated MDSC capacity is mediated by ARG-1 and ins pathway inhibition (Hu et al. 2020). We establish that docetaxel treatment may trigger a decrease of quantity of MDSCs in peripheral blood, indicating advancement of the preponderantly suppressing tumor microenvironment. Generally, the collaborative effects mainly ascribed either to the decrease of MDSCs or to the gather of other stimulated immune cells regulated by the reformed microenvironment must be more explored.
Conclusion
Our data show that the collaborative of PSMA-specific CAR-T cells and the chemotherapy drug docetaxel would enormously ameliorate antitumor capacity against the customary metastatic human prostate cancer liver metastasis model and xenograft model in NPG mice. This inspection presents a novel method to strengthen the curative effectiveness of adoptive therapy containing CAR-Ts for solid tumors, which is unprecedented inclination in CAR-T therapy.
Data availability
All data are available in the main text or the supplementary materials.
Abbreviations
- ADT:
-
Androgen replacement therapy
- CAR-T:
-
Chimeric antigen receptor T cell
- CRPC:
-
Castration-resistant prostate cancer
- DTX:
-
Docetaxel
- HMGB1:
-
High mobility group protein B1
- PSMA:
-
Prostate specific membrane antigen
- MDSCs:
-
Myeloid-derived suppressor cells
- TAMs:
-
Tumor-associated macrophages
- Tregs:
-
Regulatory T cells
- RTCA:
-
Real time cell analysis
- ELISA:
-
Enzyme linked immunosorbent assay
References
Achkova D, Pule M (2018) CAR T-cell integration of multiple input signals allows for precise targeting of cancer. Cancer Discov 8:918–920
Beltran H, Morris M (2020) Docetaxel for early prostate cancer: what have we learned? Eur Urol 77:573–575
D’Aloia MM, Zizzari IG, Sacchetti B, Pierelli L, Alimandi M (2018) CAR-T cells: the long and winding road to solid tumors. Cell Death Dis 9:282
da Costa R, Passos G, Quintão N, Fernandes E, Maia J, Campos M, Calixto J (2020) Taxane-induced neurotoxicity: pathophysiology and therapeutic perspectives. Br J Pharmacol 177:3127–3146
Djeu J, Wei S (2012) Chemoimmunomodulation of MDSCs as a novel strategy for cancer therapy. Oncoimmunology 1:121–122
Gao Q, Wang S, Chen X, Cheng S, Zhang Z, Li F, Huang L, Yang Y, Zhou B, Yue D, Wang D, Cao L, Maimela N, Zhang B, Yu J, Wang L, Zhang Y (2019) Cancer-cell-secreted CXCL11 promoted CD8 T cells infiltration through docetaxel-induced-release of HMGB1 in NSCLC. J Immunother Cancer 7:42
Gorchakov AA, Kulemzin SV, Kochneva GV, Taranin AV (2019) Challenges and prospects of chimeric antigen receptor T-cell therapy for metastatic prostate cancer. Eur Urol 77:299
Hu Y, Liu J, Cui P, Liu T, Piao C, Xu X, Zhang Q, Xiao M, Lu Y, Liu X, Wang Y, Lu X (2020) Synergistic effect of adoptive immunotherapy and docetaxel inhibits tumor growth in a mouse model. Cell Immunol 348:104036
Kodumudi K, Woan K, Gilvary D, Sahakian E, Wei S, Djeu J (2010) A novel chemoimmunomodulating property of docetaxel: suppression of myeloid-derived suppressor cells in tumor bearers. Clin Cancer Res 16:4583–4594
Nabholtz J, Gligorov J (2005) Docetaxel in the treatment of breast cancer: current experience and future prospects. Expert Rev Anticancer Ther 5:613–633
Porter DL, Levine BL, Kalos M, Bagg A, June CH (2011) Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med 365:725–733
Rafiq S, Hackett C, Brentjens R (2020) Engineering strategies to overcome the current roadblocks in CAR T cell therapy. Nat Rev Clin Oncol 17:147–167
Rochigneux P, Schleinitz N, Ebbo M, Aymonier M, Pourroy B, Boissier R, Salas S, Deville J (2018) Acute myositis: an unusual and severe side effect of docetaxel: a case report and literature review. Anticancer Drugs 29:477–481
Siegel D, O’Neil M, Richards T, Dowling N, Weir H (2020) Prostate cancer incidence and survival, by stage and race/ethnicity - united states, 2001–2017. MMWR Morb Mortal Wkly Rep 69:1473–1480
Siegel R, Miller K, Fuchs H, Jemal A (2021) Cancer statistics 2021. CA Cancer J Clin 71:7–33
Verma A, Warner SL, Vankayalapati H, Bearss DJ, Sharma S (2011) Targeting Axl and Mer kinases in cancer. Mol Cancer Ther 10:1763–1773
Wen X, Zheng P, Ma Y, Ou Y, Huang W, Li S, Liu S, Zhang X, Wang Z, Zhang Q, Cheng W, Lin R, Li H, Cai Y, Hu C, Wu N, Wan L, Pan T, Rao J, Bei X, Wu W, Jin J, Yan J, Liu G (2018) Salutaxel, a conjugate of docetaxel and a muramyl dipeptide (MDP) analogue, acts as multifunctional prodrug that inhibits tumor growth and metastasis. J Med Chem 61:1519–1540
Xu J, Tian K, Zhang H, Li L, Liu H, Liu J, Zhang Q, Zheng J (2017) Chimeric antigen receptor-T cell therapy for solid tumors require new clinical regimens. Expert Rev Anticancer Ther 17:1099–1106
Xuan L, Sun B, Meng X, Liu C, Cong Y, Wu S (2020) Ototoxicity in patients with invasive ductal breast cancer who were treated with docetaxel: report of two cases. Cancer Biol Ther 21:990–993
Zhao J, Lin Q, Song Y, Liu D (2018) Universal CARs, universal T cells, and universal CAR T cells. J Hematol Oncol 11:132
Funding
This work was supported by grants from the National Science Foundation of China (81773253, 81972242), Natural Science Foundation of Jiangsu Province (BK20211057), the Youth Technology Innovation Team of Xuzhou Medical University (TD202003), the Jiangsu Provincial Key Medical Discipline, and the Project of Invigorating Health Care through Science, Technology and Education (ZDXKA2016014), the Natural Science Foundation of the Jiangsu Higher Education Institutions of China (19KJB310001, 19KJB310018, 18KJA320013), the Natural Science Key Project of Jiangsu Provincial Education Department (20KJA320006), Six talent peaks project in Jiangsu Province (WSW-064), Xuzhou Science and Technology Bureau projects grant (KC19058), the Research Foundation of Xuzhou Medical University (D2019023), the Qing Lan Project of Jiangsu Province to XG.
Author information
Authors and Affiliations
Contributions
Conceptualization, JZ and QZ; methodology, XG, JS; software, HL; validation, XZ, SS, and YM; formal analysis, YY, WZ; investigation, XZ, SS, YM, XW, CH and XH; resources, QZ and JZ; data curation, XZ, and BW; writing—original draft preparation, XZ; writing—review and editing, SS, and QZ; visualization, HX, WZ; supervision, JZ and QZ; project administration, JZ and QZ; funding acquisition, HL, SS, XG, JS, JZ and QZ. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interests
All authors have declared that there are no financial conflicts of interest with regard to this work.
Compliance with ethical standards
All procedures performed in studies involving human samples were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Consent to publish
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (DOCX 136 KB) Supplementary Fig. 1
Ex Vivo Docetaxel Existing Does Not Notably Spoil Expanding and Killing Capacity of PSMA-CAR-T Cells. A, Influence of docetaxel on the expanding of PSMA-CAR-T cells by cell counting. B, Influence of docetaxel on the cytokine release of PSMA-CAR-T cells by ELISA. C, Influence of docetaxel on the cytotoxic capacity of PSMA-CAR-T cells. PSMA-CAR-T cells were pretreated with docetaxel for 48 hours. *P<0.05; ns, not significant.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, X., Sun, S., Miao, Y. et al. Docetaxel enhances the therapeutic efficacy of PSMA-specific CAR-T cells against prostate cancer models by suppressing MDSCs. J Cancer Res Clin Oncol 148, 3511–3520 (2022). https://doi.org/10.1007/s00432-022-04248-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-022-04248-y