Abstract
Purpose
To evaluate the long-term local control, failure patterns, and toxicities after individualized clinical target volume (CTV) delineation in unilateral nasopharyngeal carcinoma (NPC) treated with intensity-modulated radiotherapy (IMRT).
Methods
Unilateral NPC was defined as a nasopharyngeal mass confined to one side of the nasopharynx and did not exceed the midline. From November 2003 to December 2017, 95 patients were retrospectively included. All patients received IMRT. The CTVs were determined based on the distance from the gross tumor. The contralateral para-pharyngeal space and skull base orifices were spared from irradiation.
Results
There were three local recurrences and eight regional recurrences in 10 patients during an 84-month follow-up. All local recurrences were within PGTVnx, and all in-field recurrences. No recurrences were found in traditional high-risk areas including contralateral the para-pharyngeal space and skull base orifices. The 10-year local-recurrence-free survival, regional-recurrence-free survival and overall survival were 96.2%, 90.5% and 84.7%, respectively. The dosimetry parameters of the tumor-contralateral organs were all lower than the values of the tumor-ipsilateral side (P < 0.05). The late toxicities occurred mainly in the tumor-ipsilateral organs, including radiation-induced temporal lobe injury, impaired visuality, hearing loss and subcutaneous fibrosis.
Conclusion
Individualized CTV delineation in unilateral NPC could yield excellent long-term local control with limited out-of-field recurrences, reduced dose to tumor- contralateral organs and mild late toxicities, which is worthy of further exploration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Intensity-modulated radiation therapy (IMRT) is the standard radiotherapy technique for nasopharyngeal carcinoma (NPC). In the era of IMRT, the prognosis of NPC has significantly improved, mainly due to the markedly improved local control rate (LCR), with the 5-year LCR exceeding 90% reported by several cancer centers (Chen et al. 2019; Wu et al. 2017, 2021; Au et al. 2018). Local recurrence patterns were mainly in-field recurrences reported in various literature, with the gross tumor volume (GTV) in-field recurrence being most common, and the out-of-field recurrence rarely observed (Chen et al. 2019; Wu et al. 2017, 2021; Au et al. 2018). However, it should be noted that although the incidence and severity of xerostomia have significantly decreased in the IMRT setting, cured NPC patients still have some radiation-related sequelae, such as hearing loss, visual loss, radiation-induced temporal lobe injury (TLI), endocrine dysfunction and subcutaneous fibrosis (McDowell Lachlan et al. 2018; Zeng et al. 2015). Therefore, on the basis of the existing target delineation standards, it is worthy to discuss and try to reduce the irradiation range and radiation-related toxicities or side effects in NPC patients.
In the last 20 years, the target delineation principle of NPC is mainly based on a fixed anatomical position, and the prophylactic irradiation range has not fundamentally changed (Lai et al. 2011; Lee et al. 2002). The CTV range usually needs to include the traditional high-risk areas in the era of conventional radiotherapy, including the posterior maxillary sinus, inferior sphenoid sinus, partial clivus, para-pharyngeal space, and the skull base orifice. In 2017, specialists on NPC treatment from multiple regions reviewed and discussed the previous clinical and pathological evidence for NPC, and developed an international guideline on target delineation for NPC (Lee Anne et al. 2018). In this guideline, the 5 + 5 mm principle was established, but most experts still recommended that some traditional high-risk areas be prophylactically irradiated regardless of tumor stage.
Scholars have been exploring the optimization of the target volume to reduce the long-term adverse effects of NPC patients. In 2019, Sanford Nina et al. (2019) proposed an individualized CTV delineation based on tumor invasion trends, without requiring all traditional high-risk areas to be included. Long-term follow-up results showed good efficacy and no out-of-field recurrence. Since 2003, we have been exploring the individual CTV delineation in NPC with a focus on the unilateral NPC population. The principle of CTV delineation was to set the CTVs based on the distance of the gross tumor, without requiring the inclusion of specific high-risk structures. We then investigated the safety and feasibility of the contralateral para-pharyngeal, skull base and even contralateral nasopharyngeal mucosa after sparing from delineation. Here, we report a 10-year investigation in this field.
Methods and materials
Patient characteristics
From November 2003 to December 2017, the total number of initially treated NPC patients treated in our research group was 517, among which 101 (19.5%) patients were unilateral NPC that were admitted to our research group at the Sun Yat-sen University Cancer Center (SYSUCC). Finally, 95 cases that were followed up for more than 2 years were included in long-term analysis. Unilateral NPC was defined as a nasopharyngeal mass confined to one side of nasopharynx and did not exceed the midline detected by electronic nasopharyngoscope (ENS) and magnetic resonance imaging (MRI) (Fig. 1A). The inclusion criteria for patients were: (1) initial pathologically confirmed unilateral NPC; (2) > 18 years old; (3) treated with IMRT; (4) the performance status (PS) was 0 or 1; (5) no distant metastasis; and (6) informed consent related to treatment signed. Exclusion criteria: (1) metachronous or synchronous malignancy; (2) pregnancy or lactation; and (3) mental disorders. All patients were staged according to the 7th American Joint Committee on Cancer (AJCC) staging classifications. The study conformed to the ethical guidelines of the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of SYSUCC with the approved number of B2019-169-01.
Treatment
All patients received IMRT. Sixty-three patients received a one-course radiotherapy IMRT and 32 patients received an adaptive re-planning IMRT (Xie et al. 2019). Six, six and eight patients with stage I, II and III diseases, respectively, received radiotherapy alone. Six, 32 and 10 patients with stage II, III and IV diseases, respectively, received concurrent chemo-radiotherapy (CCRT). Sixteen and 11 patients with stage III and IV disease, respectively, received induction chemotherapy (IC) plus CCRT.
Radiation therapy
Radiotherapy positioning
All patients were immobilized using a head-and-neck thermoplastic mask in a supine position, followed by computed tomography (CT) simulation using 3-mm slices scanned from the top of the head to 2-cm sub-clavicle. Patients were selected to undergo re-planning if they were evaluated as being in partial remission in tumor according to Response Evaluation Criteria in Solid Tumors V 1.1 (RECIST V1.1) or had weight loss of more than 10% (≥ CTCAE grade 2). For patients treated with re-planning IMRT, a second CT simulation was performed at 22 fractions of radiotherapy.
Definition of target volumes
The definition of target volumes and organs at risk (OARs) followed the International Commission on Radiation Units and Measurement (ICRU) Reports No. 50 and No. 62. The principles of target volume delineation were referred to the norms of SYSUCC (Miao et al. 2020) and were revised according to the method applied in our research group (Xie et al. 2019; Wang et al. 2019). The CTV range was set according to the distance from gross tumor based on the experience of surgical resection margin for squamous cell carcinoma of head and neck (HNSCC). From November 2003 to May 2013, GTVnasopharynx (nx) was defined as all gross disease detected by clinical, imaging and endoscopic examination. GTV retropharyngeal lymph node (rpn)/neck lymph node (nd) was defined as the metastatic retropharyngeal lymph nodes/neck lymph nodes detected by clinical and imaging examination. The high-risk CTV (CTV1) was defined as subclinical disease consisting of 10 mm margin surrounding GTVnx (not including all nasopharyngeal mucosa); CTVrpn/nd was defined as having a 0.5−1.0 cm margin surrounding the GTVrpn/nd. The low-risk CTV (CTV2) was defined as having a 0.5−1.0 cm margin surrounding CTV1, including all nasopharyngeal mucosa within contralateral pharyngobasilar fascia, and including the bilateral prophylactically irradiated lymph drainage areas (bilateral retropharyngeal lymph node area, levels IIa, IIb, III, and Va were routinely covered for all N0 patients, whereas ipsilateral levels IV and Vb were also included for N1-3 patients). The illustration of target volume delineation is shown in Fig. 1B. From June 2013 to present, CTV2 was defined as having a 0.5−1.0 cm margin surrounding CTV1, not including all nasopharyngeal mucosa within contralateral pharyngobasilar fascia (Fig. 1C), including the bilateral prophylactically irradiated lymph drainage areas. In addition, the delineation of other target volumes, including the bilateral prophylactically irradiated lymph drainage areas, remained the same as mentioned above.
In addition to anatomical modifications, we modified GTV in the re-planning according to the shrinkage of the tumor. The detailed description of target delineation of re-planning IMRT is shown below and in our previous study (Xie et al. 2019). Briefly, for re-planning, GTVnx/rpn/nd-II was defined as all detected gross disease after 22 fractions. CTV1/rpn1/nd1-II maintained the extent of the originally irradiated region of CTV1s-I, including the area in which the tumor disappeared/dissolved after 22 fractions of radiotherapy. CTV2-II was not delineated and was given no dose prescriptions.
For the delineation of OARs, the temporal lobe, brain stem, spinal cord, optic chiasma, optic nerve, lens, pituitary gland, oral cavity, oropharynx, hypopharynx, parotid gland, submandibular gland, thyroid gland, middle ear, temporomandibular joint and mandible were delineated according to Radiation Therapy Oncology Group (RTOG) 0225 and the practice of our hospital (Sun et al. 2014). According to the standards of our hospital and the requirements of radiotherapy quality control and quality assurance, planning target volume (PTV) were created by expanding 3−5 mm from all target volumes from the head-to-foot, front-to-back, and left-to-right directions around the target volumes mentioned above to compensate for geometric uncertainties and patient movements, such as PGTVnx, PGTVrpn/nd, PCTV1, PCTVnd, and PCTV2.
Dose prescription
From November 2003 to May 2013, the dose prescriptions of one-course IMRT were: PGTVnx/rpn 68 Gy/30 fractions (F), PGTVnd 62−66 Gy/30 F, PCTV1 60 Gy/30 F and PCTV2 50−54 Gy/30 F. From June 2013 to December 2017, the dose prescriptions of re-planning IMRT were: PGTVnx/rpn 68−69.5 Gy/31–32 F, PGTVnd 68−69.5 Gy/31−32 F, PCTV1 60−61 Gy/31−32 F, PCTV2 45−47 Gy/25−26 F (Xie et al. 2019). Dose constraints and evaluation of OARs for patients with one-course IMRT and patients with re-planning IMRT were referred to our hospital and RTOG 0225 guidelines and our previous studies (Xie et al. 2019; Wang et al. 2019), respectively. Dose constraints for the contralateral unaffected OARs: according to the distance between OARs and the target volumes, stricter constraints were imposed on the basis of the standard limits to reduce dose to the unaffected organs.
Chemotherapy
Seventy-five patients received chemotherapy. Among them, 48 patients received concurrent chemotherapy, including cisplatin weekly regimen (30 mg/m)2, 4−6 times in total and cisplatin three-week regimen (80 mg/m)2, 2−3 times in total. A total of 27 patients received IC combined with concurrent chemotherapy: IC regimen was TPF (docetaxel 60 mg/m2, cisplatin 60 mg/m2, fluorouracil 500 mg/m2 continuous venous perfusion for 120 h), 3−4 times in total. Concurrent chemotherapy was a weekly regimen containing platinum, including cisplatin (30 mg/m2), nedaplatin (30 mg/m2), 4–6 times in total.
Evaluation of toxicities
The Common Terminology Criteria for Adverse Events (version 4.0) were used to evaluate treatment-related acute toxicities, and the Late Radiation Morbidity Scoring Criteria of RTOG was used to evaluate radiotherapy-related toxicities. Acute toxicities were defined as those occurring either during the course of IMRT and chemotherapy or within 3 months of its completion, while late toxicities were the toxicities occurred at least 3 months after radiotherapy.
Evaluation and follow-up
All patients received detailed examinations before and after treatment including medical history, physical examination, hematological examination, chest X-ray, abdominal B-ultrasound, emission computed tomography (ECT) on bone, ENS, and head-and-neck enhanced MRI or positron emission tomography–computed tomography (PET/CT). Local recurrence was confirmed by endoscopic biopsy and/or MRI. Distant metastases were clinically confirmed by clinical symptoms, physical examination, and imaging examinations and pathological biopsies if necessary. Patients were followed up once every 3 months within 3 years after radiotherapy, once every 6 months after 3 years, and once every 5 years after 5 years.
Treatment of recurrent/metastasized patients
Whenever possible, salvage treatments (including re-IMRT, surgery, and chemotherapy) were provided for patients who developed relapse or persistent disease (persistent disease is defined as tumor residue exists at least 6 months after treatment). The treatment for recurrent/metastasized patients was determined by multidisciplinary consultation. For recurrent patients, operable patients received surgical resection, and inoperable patients received re-irradiation. Systemic treatment is the main treatment for recurrent/metastasized patients who are not suitable for surgery or re-irradiation. The detailed methods for surgical and re-irradiation refer to the literatures (Liu et al. 2021; Ng et al. 2021).
Survival analysis
Our primary endpoints were local-recurrence-free survival (LRFS) and patterns of failure, which were classified as ‘in-field” if 95% recurrent tumor volume was within the PGTV, PCTV1 or PCTV2, “marginal” if 20−95% recurrent tumor volume was within the PGTV, PCTV1 or PCTV2 or “out-of-field” if < 20% recurrent tumor volume was within the PGTV, PCTV1 or PCTV2 (Clifford et al. 2003; Dawson et al. 2000). If the recurrent tumor was both within 20–95% of PCTV1 and 95% of PCTV2, all such cases were defined as PCTV2 in-field recurrence in this study. The recurrence patterns were determined by re-delineating the tumor volume detected by MRI at the time of recurrence in the original plan evaluation system, and the relationship between the recurrence area and the original dose coverage was analyzed.
Our secondary endpoints were overall survival (OS), regional relapse-free survival (RRFS), distant metastasis-free survival (DMFS) and progression-free survival (PFS). LRFS was calculated as the duration from the date of pathological diagnosis to the date of local recurrence. OS was calculated as the duration from the date of pathological diagnosis to the date of death or the last follow-up. RRFS was calculated as the duration from the date of pathological diagnosis to the date of regional recurrence. PFS was calculated as the duration from the date of disease progresses to the date of death or the last follow-up.
Statistical analysis
Continuous data followed a normal distribution were analyzed using a t test, while Wilcoxon rank-sum test was used for non-normal distribution data when comparing two groups. Chi-square test was used for categorical data, and the Fisher’s exact probability method was applied when the sample size of the fourfold table is less than forty or the frequency of at least one of the fourfold tables is less than one. LRFS, OS, RRFS, DMFS and PFS were estimated by Kaplan–Meier survival analysis. Statistical analysis and the survival curve were generated by R Studio version 1.2.1335. Bilateral test P < 0.05 was considered statistically significant.
Results
Patient characteristics
From November 2003 to December 2017, 95 unilateral NPC patients were included in the analysis. The median age of the patients was 46 years (21−77 years). Of the 95 cases, 50 had a primary tumor located right of the nasopharynx, while 45 had a primary tumor left of the nasopharynx. Seventy-seven patients (81.1%) had stage III–IV, 89.6% of whom received chemotherapy. Detailed data are shown in Table 1.
Survival results
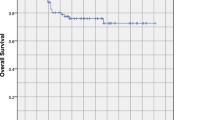
As of August 31, 2020, with a median follow-up of 84 months (24–199 months), three patients had local recurrence, seven patients had regional recurrence, nine patients developed distant metastasis, and a total of 12 patients died (three died of local–regional recurrence, seven died of distant metastasis, one died of colon cancer, and one died of unknown cause). The 5-year LRFS, RRFS, OS, PFS and DMFS of the whole patients were 97.9% [95% confidence interval (CI): 95.0−100%), 92.4% (95% CI: 87.1−98%), 88.1% (95% CI: 81.4−95.4%), 82.1% (95% CI 74.4−90.5%) and 89.5% (95% CI 83.1−96.3%); 10-year LRFS, RRFS, OS, PFS and DMFS were 96.2% (95% CI 91.9−100%), 90.5% (95% CI 84.3−97.2%), 84.7% (95% CI 77.0−93.3%), 77.4% (95% CI 68.8−87.1%) and 87.8% (95% CI 80.8−95.3%) (Fig. 2).
Failure patterns
All local failures were located in the high-dose GTV irradiation area. Among them, one patient was staged as T4, with local residue after radiotherapy, progressed 12 months later, and eventually died of local progression (Fig. 3A-1, A-2). The other two patients were all staged as T3. One patient had recurrence of the nasopharyngeal lateral wall in tumor-ipsilateral side 66 months after treatment, and was still free of recurrence and metastasis after re-radiotherapy (Fig. 3B-1, B-2). The other case developed recurrence with tumor invasion to the longus cephalus in tumor-ipsilateral side and multiple bone metastases 25 months after radiotherapy. After re-radiotherapy and systemic treatment, the patient was still alive with bone metastasis at the end of follow-up and had survived for 49 months since diagnosis (Fig. 3C1, C-2).
Among the seven regional recurrence cases, one patient diagnosed as T1N2 had lymph node in lower parotid recurrence determined as out-of-field recurrence. Before treatment, this patient had significant extracapsular invasion in level-II lymph nodes on the tumor relapsed side. One patient diagnosed as T3N3b had PGTVnd in-field recurrence in the level-II region in the tumor-ipsilateral side accompanied with contralateral level IV region PCTV2 in-field recurrence. The remaining patients had PGTVnd in-field recurrence. The detailed illustration and description of failure patterns are shown in Table 2.
Dosimetric comparison of OARs
The dosimetry of the OARs was included in the analysis. The mean dose (Dmean), minimum dose (Dmin) and maximum dose (Dmax) of the tumor-contralateral OARs were all significantly lower than the values of the tumor-ipsilateral side (P < 0.05). Furthermore, absolute dose reduction percentage of organs demonstrated a higher dose on the tumor-ipsilateral side relative to tumor-contralateral side. See Table 3 for details. Additionally, Fig. 4 illustrated an advanced-staged patient with the primary tumor located left of the pharyngeal recess, showing that the doses to the contralateral temporomandibular joint, mandible (5000 cGy dose line) and contralateral chiasm (3000 cGy dose line) were significantly lower than those to the ipsilateral tumor.
Target delineation and isodose curve of an advanced-staged NPC patient with the primary tumor locating in the left side pharyngeal recess. As shown in the figure, when the CTV did not delineate the contralateral nasopharyngeal mucosa and parapharyngeal structures, the doses to the contralateral temporomandibular joint, mandible (5000 cGy dose line) and contralateral chiasm (3000 cGy dose line) were significantly lower than those to the ipsilateral tumor. A Target delineation of GTVnx CTV1 and CTV2. B showing isodose lines. Red line: GTVnx, pink line: CTV1, blue line: CTV2
Toxicities
The most common acute toxicity involved grades I−II reactions. Grade III reactions were mainly oropharyngeal mucositis (15.8%) and leukopenia (12.6%), while grade IV mucositis and grade IV leukopenia occurred only in 1 case, respectively. The detailed acute toxicities are shown in Table 4.
By the last follow-up, for late toxicities, 94 patients (98.9%) had grade I–II xerostomia. For late toxicities that could be distinguished as tumor-ipsilateral side and tumor-contralateral side, significantly higher incidence of impaired hearing on tumor-ipsilateral side (10/95) was observed than that on the tumor-contralateral side (1/95) (P = 0.005). Furthermore, the incidence of MRI-confirmed TLI on the tumor-ipsilateral side (7/95) was marginally significantly higher than that on the tumor-contralateral side (1/95) (P = 0.065), among which only one patient with TLI on the tumor-ipsilateral side had clinical symptoms of dizziness and headache. Additionally, the occurrences of impaired visuality (1/95 vs 0/95, P > 0.999) and subcutaneous fibrosis (5/95 vs 1/95, P = 0.211) were more common on the tumor-ipsilateral side than on the tumor-contralateral side, though not reached significant difference. As shown in Table 5, there was no significant difference in toxicity and side effects before and after 2013 when the treatment concept changed.
Discussion
Unilateral NPC accounts for approximately 10% of all cases of NPC (Sun et al. 2016). In the current specification for target volume delineation of NPC, bilateral tissue and structures, such as the para-pharyngeal space and skull base orifices, are still required to be included in the prophylactic irradiation range for this type of patient (Lee Anne et al. 2018). However, unilateral NPC is relatively far away from the contralateral para-pharyngeal space and contralateral skull base, and jumping invasion is rare. Therefore, it may not be necessary to include contralateral para-pharyngeal structures and skull base orifices in the target volume. Therefore, we explored the feasibility of sparing contralateral para-pharyngeal space and skull base orifices from irradiation. The long-term follow-up results of this group showed that the 10-year results achieved 96.2%, 90.5% and 84.7% for LRFS, RRFS and OS, respectively, reaching a satisfied efficacy.
For unilateral NPC, individualized CTV delineation method was adopted. In this study, the CTV range was set according to the distance from gross tumor based on the experience of surgical resection margin for squamous cell carcinoma of head and neck (HNSCC), and all traditional high-risk structures were not required to be included. From 2003 to 2013, a 1 cm margin surrounding GTVnx was set for CTV1 and a 0.5–1.0 cm margin surrounding CTV1 was set for CTV2, but all nasopharyngeal mucosa needed to be included. After 10 years of investigation, there was no recurrence of contralateral para-pharyngeal structure. After 2013, we further set a 0.5−1.0 cm margin surrounding CTV1 for CTV2, without requiring to inclusion of all contralateral nasopharyngeal mucosa. After further narrowing the CTV, no recurrence in contralateral structures and contralateral un-irradiated nasopharyngeal mucosa was observed. However, patients treated with further narrowed CTV had a shorter median follow-up period of 66 months (range: 24–116 months) versus 189.5 months (range: 28–199 months). An individual CTV delineation study from Harvard Medical School did not require the inclusion of contralateral para-pharyngeal space in CTV for unilateral NPC, and the long-term follow-up showed no recurrence in the contralateral para-pharyngeal space (Sanford Nina et al. 2019).
In the present study, the determined irradiation range was about 10 + 5 mm from the gross tumor, but further study would be necessary to determine whether it could be further reduced on this basis. The CTV has been delineated according to the principle of 5 + 5 mm in HNSCC (Grégoire et al. 2018), and this principle was also recommended in the international guideline for NPC (Lee Anne et al. 2018). However, for future CTV delineation, whether we can completely learn from the experience of HNSCC and only delineate 5 + 5 mm in NPC without including specific high-risk structures is worthy of further exploration and attempt. Surgical resection is an important treatment method for locally recurrent NPC patients. For patients who can be surgically resected, the resection range was generally 0.5−1.0 cm around the gross tumor (Liu et al. 2021). Therefore, this also provided a basis and reference for our future CTV optimization of NPC.
It should be noted that the contralateral structures would still receive a certain irradiation dose even though the contralateral structures were not delineated in the CTV under IMRT technology. Meanwhile, routine prophylactic retropharyngeal region irradiation was required regardless of retropharyngeal lymph node metastasis (Lee Anne et al. 2018). This could also lead to a dose of exposure to nearby traditional high-risk areas. However, this was mainly attributed to the limitations of existing IMRT technology. Moreover, the scattering doses to adjacent structures were obviously lower than the values to those delineated in the CTV (Fig. 4). Dosimetry analysis also showed that the dose to OARs on the tumor-contralateral side was significantly lower than those on the tumor-ipsilateral side with an average Dmean reduction percentage of 7.18–33.02% of the above-mentioned organs.
The recorded late toxicities were mainly on the tumor-ipsilateral side, which is in accordance with lower doses in the organs in the tumor-ipsilateral side compared with those on the tumor-contralateral side. However, no statistical difference was found in TLI impaired visuality and subcutaneous fibrosis except for impaired hearing. Although we narrowed the target volume again after 2013, there was no significant difference in toxicity and side effects before and after 2013. This may be due to the fact that this was a retrospective study and there was perhaps insufficient evaluation of toxic and side effects. Therefore, the toxicities and side effects of patients in the two eras were not further distinguished and compared.
In this study, 6 patients with stage II and 8 patients with stage III received radiotherapy alone, which did not conform to the chemotherapy and radiotherapy combined regimen recommended by the guideline (National Comprehensive Cancer Network 2018). This deviation from guidelines was attributed to: (i) before 2009, the Chinese 1992 stage was mainly used for NPC staging in mainland China. Five of the eight stage III patients were treated from 2003 to 2009, whose initial stage was stage II according to the Chinese 1992 stage; (ii) for stage II NPC, there have been controversies in our center about whether combined chemotherapy is necessary on the basis of radiotherapy. Mai et al. conducted a phase III clinical study on NPC of stage II CCRT compared with radiotherapy alone in the conventional radiotherapy setting, and found that the CCRT could bring survival benefits to stage II patients (Chen et al. 2011; Li, et al. 2019). In the IMRT setting, several retrospective studies at our center concluded that CRT did not bring a survival benefit to stage II patients or even some stage III patients with low tumor burden (Zhang et al. 2015; Su et al. 2012); (iii) other 3 stage III patients did not receive chemotherapy; one was a 77-year-old elderly patient, and the other two patients refused chemotherapy.
Our study also had some limitations. First, it was a single sample retrospective study without a control group to compare efficacy and toxicity. Second, as a preliminary exploration, the CTV2 of the earlier 24 patients included the contralateral pharyngobasilar fascia, while the other 71 patients did not (with the confidence from the accumulation of experience). However, the uniform principle of the CTV range setting according to the distance from gross tumor was based on the experience of surgical resection margin HNSCC has not changed. Third, treatment heterogeneity included the introduction of IC and re-planning IMRT, but a different treatment regimen had no effect on the irradiation range. Finally, deficiencies in the long-term toxicity evaluation methods were due to the retrospective collection of data, making it difficult to fully reflect the quality-of-life benefits brought by the CTV reduction in this study.
In conclusion, our invasion distance-based CTV delineation was feasible for unilateral NPC based on a 10-year experience. We did not observe failure in the reduced-irradiated area. By narrowing the irradiation volume and sparing the contralateral para-pharyngeal space, even nasopharyngeal mucosal from irradiation, the dose to the contralateral normal tissues was significantly reduced, thereby reducing the toxicities. This method deserves to be verified and explored by more scholars to make up for the deficiencies of this study.
Availability of data and material
The datasets used and/or analyzed during the current study were uploaded onto the Research Data Deposit public platform (www.researchdata.org.cn), with the RDD approval number RDDB2020001032.
Abbreviations
- IMRT:
-
Intensity-modulated radiotherapy
- NPC:
-
Nasopharyngeal carcinoma
- LCR:
-
Local control rate
- GTV:
-
Gross tumor volume
- CTV:
-
Clinical target volume
- TLI:
-
Temporal lobe injury
- SYSUCC:
-
Sun Yat-sen University Cancer Center
- ENS:
-
Electronic nasopharyngoscope
- MRI:
-
Magnetic resonance imaging
- PS:
-
Performance status
- AJCC:
-
American Joint Committee on Cancer
- CCRT:
-
Concurrent chemo-radiotherapy
- IC:
-
Induction chemotherapy
- CT:
-
Computed tomography
- OARs:
-
Organs at risk
- ICRU:
-
The International Commission on Radiation Units and Measurement
- CTV1:
-
High-risk clinical target volume
- CTV2:
-
Low-risk clinical target volume
- RTOG:
-
Radiation Therapy Oncology Group
- PTV:
-
Planning target volume
- ECT:
-
Emission computed tomography
- PET/CT:
-
Positron emission tomography-computed tomography
- LRFS:
-
Local-recurrence-free survival
- OS:
-
Overall survival
- RRFS:
-
Regional relapse-free survival
- DMFS:
-
Distant metastasis-free survival
- PFS:
-
Progression-free survival
- Dmean:
-
Mean dose
- Dmin:
-
Minimum dose
- Dmax:
-
Maximum dose
References
Au KH, Ngan Roger KC, Ng Alice WY et al (2018) Treatment outcomes of nasopharyngeal carcinoma in modern era after intensity modulated radiotherapy (IMRT) in Hong Kong: A report of 3328 patients (HKNPCSG 1301 study). Oral Oncol 77:16–21
Chen Q-Y, Wen Y-F, Guo L et al (2011) Concurrent chemoradiotherapy vs radiotherapy alone in stage II nasopharyngeal carcinoma: phase III randomized trial. J Natl Cancer Inst 103:1761–1770
Chen L, Zhang Y, Lai S-Z et al (2019) 10-year results of therapeutic ratio by intensity-modulated radiotherapy versus two-dimensional radiotherapy in patients with nasopharyngeal carcinoma. Oncologist 24:e38–e45
Clifford CKS, Ozyigit G, Tran BN et al (2003) Patterns of failure in patients receiving definitive and postoperative IMRT for head-and-neck cancer. Int J Radiat Oncol Biol Phys 55:312–321
Dawson LA, Anzai Y, Marsh L et al (2000) Patterns of local-regional recurrence following parotid-sparing conformal and segmental intensity-modulated radiotherapy for head and neck cancer. Int J Radiat Oncol Biol Phys 46:1117–1126
Grégoire V, Evans M, Le Q-T et al (2018) Delineation of the primary tumour Clinical Target Volumes (CTV-P) in laryngeal, hypopharyngeal, oropharyngeal and oral cavity squamous cell carcinoma: AIRO, CACA, DAHANCA, EORTC, GEORCC, GORTEC, HKNPCSG, HNCIG, IAG-KHT, LPRHHT, NCIC CTG, NCRI, NRG Oncology, PHNS, SBRT, SOMERA, SRO, SSHNO TROG Consensus Guidelines. Radiother Oncol 126:3–24
Lai S-Z, Li W-F, Chen L et al (2011) How does intensity-modulated radiotherapy versus conventional two-dimensional radiotherapy influence the treatment results in nasopharyngeal carcinoma patients? Int J Radiat Oncol Biol Phys 80:661–668
Lee N, Xia P, Quivey Jeanne M et al (2002) Intensity-modulated radiotherapy in the treatment of nasopharyngeal carcinoma: an update of the UCSF experience. Int J Radiat Oncol Biol Phys 53:12–22
Lee Anne W, Ng WT, Pan JJ et al (2018) International guideline for the delineation of the clinical target volumes (CTV) for nasopharyngeal carcinoma. Radiother Oncol 126:25–36
Li X-Y, Chen Q-Y, Sun X-S et al (2019) Ten-year outcomes of survival and toxicity for a phase III randomised trial of concurrent chemoradiotherapy versus radiotherapy alone in stage II nasopharyngeal carcinoma. Eur J Cancer 110:24–31
Liu Y-P, Wen Y-H, Tang J et al (2021) Endoscopic surgery compared with intensity-modulated radiotherapy in resectable locally recurrent nasopharyngeal carcinoma: a multicentre, open-label, randomised, controlled, phase 3 trial. Lancet Oncol 22:381–390
McDowell Lachlan J, Rock K, Xu W et al (2018) Long-Term late toxicity, quality of life, and emotional distress in patients with nasopharyngeal carcinoma treated with intensity modulated radiation therapy. Int J Radiat Oncol Biol Phys 102:340–352
Miao J, Di M, Chen B et al (2020) A prospective 10-year observational study of reduction of radiation therapy clinical target volume and dose in early-stage nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys 107:672–682
National Comprehensive Cancer Network (2018) (NCCN) Clinical Practice Guidelines in Oncology. Head and Neck Cancer, Version 1. 2018. https://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf. Accessed 15 Feb 2018
Ng WT, Soong YL, Ahn YC et al (2021) International recommendations on reirradiation by intensity modulated radiation therapy for locally recurrent nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys 110:682–695
Sanford Nina N, Jackson L, Lam Miranda B et al (2019) Individualization of clinical target volume delineation based on stepwise spread of nasopharyngeal carcinoma: outcome of more than a decade of clinical experience. Int J Radiat Oncol Biol Phys 103:654–668
Su S-F, Han F, Zhao C et al (2012) Long-term outcomes of early-stage nasopharyngeal carcinoma patients treated with intensity-modulated radiotherapy alone. Int J Radiat Oncol Biol Phys 82:327–333
Sun Y, Yu X-L, Luo W et al (2014) Recommendation for a contouring method and atlas of organs at risk in nasopharyngeal carcinoma patients receiving intensity-modulated radiotherapy. Radiother Oncol 110:390–397
Sun Y, Yu X-L, Zhang G et al (2016) Reduction of clinical target volume in patients with lateralized cancer of the nasopharynx and without contralateral lymph node metastasis receiving intensity-modulated radiotherapy. Head Neck 38(S1):E468–E472
Wang L, Wu Z, Xie D et al (2019) Reduction of target volume and the corresponding dose for the tumor regression field after induction chemotherapy in locoregionally advanced nasopharyngeal carcinoma. Cancer Res Treat 51:685–695
Wu L-R, Liu Y-T, Jiang N et al (2017) Ten-year survival outcomes for patients with nasopharyngeal carcinoma receiving intensity-modulated radiotherapy: an analysis of 614 patients from a single center. Oral Oncol 69:26–32
Wu Z, Wang L, Xie D-H et al (2021) Failure patterns and prognostic factors for cervical node-negative nasopharyngeal carcinoma in the intensity-modulated radiotherapy era. Asia Pac J Clin Oncol 17:330–337
Xie D, Cheng W, Lv S et al (2019) Target delineation and dose prescription of adaptive replanning intensity-modulated radiotherapy for nasopharyngeal carcinoma. Cancer Commun (lond) 39:18
Zeng L, Huang S-M, Tian Y-M et al (2015) Normal tissue complication probability model for radiation-induced temporal lobe injury after intensity-modulated radiation therapy for nasopharyngeal carcinoma. Radiology 276:243–249
Zhang F, Zhang Y, Li W-F et al (2015) Efficacy of concurren chemotherapy for intermediate risk NPC in the intensity-modulated radiotherapy era: a propensity-matched analysis. Sci Rep 5:17378
Acknowledgements
We thank International Science Editing (http://www.internationalscienceediting.com) for editing this manuscript.
Funding
The authors declare that they had no funding for this study.
Author information
Authors and Affiliations
Contributions
DHX, ZW, WZL, YS, and YQH designed the study. DHX, ZW, YS, TXL, JM, CZ, NJC, WQC, LW, and SWL delineated the targets and performed radiation treatment DHX, ZW, WZL, WQC, YLT, LW, SWL, FFL, and SMH acquired data DHX, ZW, WZL, analyzed and interpreted the data. DHX, ZW, WZL, and YS wrote the manuscript, and revised the manuscript. All authors have reviewed the manuscript and approved the final version.
Corresponding authors
Ethics declarations
Conflicts of interest/Competing interests
The authors declare that they have no competing interests.
Ethics approval
Retrospective ethical approval was obtained from the institutional ethics committee of the Sun Yat‑sen University Cancer Center, with the approval number B2019-169–01. Written informed treatment consents were required from all patients with respect to chemotherapy and/or radiotherapy.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xie, DH., Wu, Z., Li, WZ. et al. Individualized clinical target volume delineation and efficacy analysis in unilateral nasopharyngeal carcinoma treated with intensity-modulated radiotherapy (IMRT): 10-year summary. J Cancer Res Clin Oncol 148, 1931–1942 (2022). https://doi.org/10.1007/s00432-022-03974-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-022-03974-7