Abstract
Introduction
While some clinical studies have shown that PD-1 and PD-L1 can also be an effective neoadjuvant treatment for early-stage non-small cell lung cancer (NSCLC), no evidence has been available for the use of the PD-1 inhibitor sintilimab combined with chemotherapy as a neoadjuvant treatment for potentially resectable NSCLC in the Chinese population.
Methods
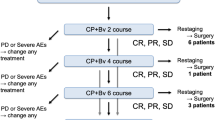
This prospective, single-center, single-arm, phase 2 clinical trial (registration number: NCT04326153) included treatment-naive patients with potentially resectable NSCLC (stage IIIA/IIIB) who received sintilimab plus nab-paclitaxel and carboplatin for two to three cycles before systematic nodal dissection 30 to 45 days after neoadjuvant treatment. After surgery, patients needed to complete two cycles of adjuvant chemoimmunotherapy (sintilimab + nab-paclitaxel + carboplatin). The primary endpoint was disease-free survival rate at 24 months, whereas secondary endpoints included major pathological response (MPR) and pathologic complete response (pCR) rates, the proportion of patients who achieved tumor downstaging, overall survival, objective response rate (ORR), and adverse effects. PD-L1 status before and after treatment was also determined.
Results
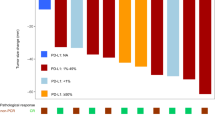
Among the 20 patients who received neoadjuvant chemoimmunotherapy, 16 underwent radical resection. The disease control rate and ORR were 90% and 70%, respectively. Among the 16 patients who underwent surgery, 10 (62.5%) and 5 (31.25%) achieved MPR and pCR, respectively. Squamous cell NSCLC exhibited superior response rates compared to adenocarcinoma (pCR 35.7% vs. 0%). Moreover, 14 patients (70%) experienced grade 1 or 2 neoadjuvant treatment-related adverse events (TRAEs), whereas 6 (30%) experienced grade 3 TRAEs. Bronchopleural fistula (BPF) was found in the current study as an adverse reaction of concern. The rate of BPF was 20% (4/20), of which three patients were in grade 1–2, and one patient died. The occurrence of BPF had no significant correlation with basic disease history, nutritional status, anemia, hypoalbuminemia, surgical procedure, pathological remission, and PD-L1 expression. However, during neoadjuvant treatment, no adverse events prompted dose reduction, treatment discontinuation, surgery delay, or death. Although PD-L1 expression may change after chemoimmunotherapy, no regular pattern was noted. PD-L1 expression, neither at baseline nor after neoadjuvant chemoimmunotherapy, was associated with pathological remission.
Conclusions
The current study found similar ORR, slightly lower MPR and pCR rates, and lower grade 3 TRAEs among patients with potentially resectable stage IIIA/IIIB NSCLC compared to the NADIM trial, as well as a greater ORR, MPR rate, pCR rate, and grade 3 TRAEs compared to Gao’s study involving sintilimab for Chinese patients with resectable stage IA–IIIB NSCLC. Though neoadjuvant chemoimmunotherapy had been found to promote a high risk of BPF for patients with stage IIIA/IIIB disease, it offered greater potential for radical cure. Therefore, the current study suggests that neoadjuvant chemoimmunotherapy can be a safe approach in increasing the efficiency of treatment and hopefully improving the prognosis of patients with potentially resectable locally advanced NSCLC.




Similar content being viewed by others
Availability of data and material
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability (software application or custom code)
Not applicable.
Abbreviations
- AE:
-
Adverse events
- CT:
-
Computed tomography
- CTCAE:
-
Common Terminology Criteria for Adverse Events
- BPF:
-
Bronchopleural fistula
- DFS:
-
Disease-free survival
- ECT:
-
Emission computed tomography
- ICI:
-
Immune checkpoint inhibitors
- irAE:
-
Immune-related adverse events
- MDT:
-
Multi-disciplinary team
- MPR:
-
Major pathological response
- MRI:
-
Magnetic resonance imaging
- NSCLC:
-
Non-small cell lung cancer
- ORR:
-
Objective response rate
- OS:
-
Overall survival
- pCR:
-
Pathologic complete response
- PD:
-
Progression disease
- PR:
-
Partial response
- RECIST:
-
Response Evaluation Criteria in Solid Tumors
- SD:
-
Stable disease
- SLD:
-
Sum of the lesion diameters
- TRAE:
-
Treatment-related adverse events
References
Ahmadzada T, Kao S, Reid G et al (2018) An update on predictive biomarkers for treatment selection in non-small cell lung cancer. J Clin Med 20167(6):153
Boku N, Ryu MH, Kato K et al (2019) Safety and efficacy of nivolumab in combination with S-1/capecitabine plus oxaliplatin in patients with previously untreated, unresectable, advanced, or recurrent gastric/gastroesophageal junction cancer: interim results of a randomized, phase II trial (ATTRACTION-4). Ann Oncol 30(2):250–258
Bray F, Ferlay J, Soerjomataram I et al (2020) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries (vol 68, pg 394, 2018). Ca-a Cancer J Clin 70(4):313–313
Cascone T, William WN, Weissferdt A et al (2019) Neoadjuvant nivolumab (N) or nivolumab plus ipilimumab (NI) for resectable non-small cell lung cancer (NSCLC): clinical and correlative results from the NEOSTAR study. J Clin Oncol 37(15_suppl):8504–8504
Chaft JE, Hellmann MD, Velez MJ et al (2017) Initial experience with lung cancer resection after treatment with T-cell checkpoint inhibitors. Ann Thorac Surg 104(3):e217–e218
Chatwal MS, Tanvetyanon T (2018) Combination chemotherapy and immunotherapy in metastatic non-small cell lung cancer: a setback for personalized medicine? Transl Lung Cancer Res 7:S208–S210
Forde PM, Chaft JE, Smith KN et al (2018) Neoadjuvant PD-1 blockade in resectable lung cancer. N Engl J Med 378(21):1976–1986
Gandhi L, Rodríguez-Abreu D, Gadgeel S et al (2018) Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med 378(22):2078–2092
Gao S, Li N, Gao S et al (2020) Neoadjuvant PD-1 inhibitor (Sintilimab) in NSCLC. J Thorac Oncol 15(5):816–826
Gentzler RD, Riley DO, Martin LW (2020) Striving toward improved outcomes for surgically resectable non-small cell lung cancer: the promise and challenges of neoadjuvant immunotherapy. Curr Oncol Rep 22(11):109
Hellmann MD, Chaft JE, William WN Jr et al (2014) Pathological response after neoadjuvant chemotherapy in resectable non-small-cell lung cancers: proposal for the use of major pathological response as a surrogate endpoint. Lancet Oncol 15(1):e42-50
Helmink BA, Reddy SM, Gao J et al (2020) B cells and tertiary lymphoid structures promote immunotherapy response. Nature 577:549–555
Hu XF, Duan L, Jiang GN et al (2013) A clinical risk model for the evaluation of bronchopleural fistula in non-small cell lung cancer after pneumonectomy. Ann Thorac Surg 96(2):419–424
Jichen QV, Chen G, Jiang G et al (2009) Risk factor comparison and clinical analysis of early and late bronchopleural fistula after non-small cell lung cancer surgery. Ann Thorac Surg 88(5):1589–1593
Li S, Fan J, Liu J et al (2016) Neoadjuvant therapy and risk of bronchopleural fistula after lung cancer surgery: a systematic meta-analysis of 14 912 patients. Jpn J Clin Oncol 46(6):534–546
Liang H, Liu Z, Cai X et al (2019) PD- (L)1 inhibitors vs. chemotherapy vs. their combination in front-line treatment for NSCLC: an indirect comparison. Int J Cancer 145:3011–3021
Mayekar MK, Bivona TG (2017) Current landscape of targeted therapy in lung cancer. Clin Pharmacol Ther 102(5):757–764
Mazzella A, Pardolesi A, Maisonneuve P et al (2017) Bronchopleural fistula after pneumonectomy: risk factors and management, focusing on open window thoracostomy. J Thorac Cardiovasc Surg S0022–5223(17):31189–31193
McKenna RJ Jr, Houck W, Fuller CB (2006) Video-assisted thoracic surgery lobectomy: experience with 1100 cases. Ann Thorac Surg 81:421–425 (discussion 425–426)
Niki M, Yokoi T, Kurata T et al (2017) New prognostic biomarkers and therapeutic effect of bevacizumab for patients with non-small-cell lung cancer. Lung Cancer(Auckl). 2017; 8: 91–99
NSCLC Meta-analysis Collaborative Group (2014) Preoperative chemotherapy for non-small-cell lung cancer: a systematic review and meta-analysis of individual participant data. Lancet 383(9928):1561–1571
Onaitis MW, Petersen RP, Balderson SS et al (2006) Thoracoscopic lobectomy is a safe and versatile procedure: experience with 500 consecutive patients. Ann Surg 244:420–425
Pataer A, Kalhor N, Correa AM et al (2012) Histopathologic response criteria predict survival of patients with resected lung cancer after neoadjuvant chemotherapy. J Thorac Oncol 7(5):825–832
Pforr A, Pages PB, Baste JM et al (2016) A predictive score for bronchopleural fistula established using the French database epithor. Ann Thorac Surg 101(1):287–293
Provencio M, Nadal E, Insa A et al (2020) Neoadjuvant chemotherapy and nivolumab in resectable non-small-cell lung cancer (NADIM): an open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol 21(11):1413–1422
Ramnath N, Dilling TJ, Harris LJ et al (2013) Treatment of stage III non-small cell lung cancer diagnosis and management of lung cancer, 3rd ed: American college of chest physicians evidence-based clinical practice guidelines. Chest 143(5):E314–E340
Shu CA, Gainor JF, Awad MM et al (2020) Neoadjuvant atezolizumab and chemotherapy in patients with resectable non-small cell lung cancer: an open-label, multicentre single-arm, phase 2 trial. Lancet Oncol 21(6):786–795
Socinski MA, Obasaju C, Gandara D et al (2016) Clinicopathologic features of advanced squamous NSCLC. J Thorac Oncol 11(9):1411–1422
Socinski MA, Jotte RM, Cappuzzo F et al (2018) Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med 378:2288–2301
Topalian SL, Taube JM, Pardoll DM (2020) Neoadjuvant checkpoint blockade for cancer immunotherapy. Science 367:eaax0182
William WN Jr, Pataer A, Kalhor N et al (2013) Computed tomography RECIST assessment of histopathologic response and prediction of survival in patients with resectable non-small-cell lung cancer after neoadjuvant chemotherapy. J Thorac Oncol 8(2):222–228
Xu J-M, Jia R, Wang Y et al (2018) A first-in-human phase 1a trial of sintilimab (IBI308), a monoclonal antibody targeting programmed death-1 (PD-1), in Chinese patients with advanced solid tumors. J Clin Oncol 36(15):e15125
Zhong WZ, Chen KN, Chen C et al (2019) Erlotinib versus gemcitabine plus cisplatin as neoadjuvant treatment of stage IIIA-N2 EGFR-mutant non-small-cell lung cancer (EMERGING-CTONG 1103): a randomized phase II study. J Clin Oncol 37(25):2235–2245
Funding
None.
Author information
Authors and Affiliations
Contributions
CS: neoadjuvant data management (efficiency) and writing-original. YL: postoperative data management. PZ: postoperative data management. XW: neoadjuvant data management (AE) and writing-original. YX: data statistics and writing-original. XL: postoperative data management. XM: pathological effect interpretation. SQ: data curation. YG: data curation. GS: surgery management. ZY: writing—editing. KM: project administration and writing—editing.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest or competing interests.
Ethics approval
All included patients gave their oral and written informed consent. The study was approved by the Ethics Committee (full name: Regional Ethics Committee of Jilin University First Hospital) (reference number K2019158).
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sun, C., Liu, Y., Zhang, P. et al. Interim analysis of the efficiency and safety of neoadjuvant PD-1 inhibitor (sintilimab) combined with chemotherapy (nab-paclitaxel and carboplatin) in potentially resectable stage IIIA/IIIB non-small cell lung cancer: a single-arm, phase 2 trial. J Cancer Res Clin Oncol 149, 819–831 (2023). https://doi.org/10.1007/s00432-021-03896-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-021-03896-w