Abstract
Purpose
Osimertinib is the standard treatment for advanced non-small cell lung cancer (NSCLC) patients with T790M mutation after the failure of first-/second-generation epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKI). We comprehensively analyzed factors that affect the therapeutic efficacy of the osimertinib treatment in NSCLC patients.
Methods
351 NSCLC patients with T790M mutation receiving osimertinib treatment were included. We investigated the value of different factors in predicting the clinical outcomes of the osimertinib therapy, including progression-free survival (PFS), overall survival (OS) and objective response rate (ORR). Logistic and COX regression were used to identify prognosticators.
Results
In osimertinib therapy, EGFR mutation status (19Del/L858R) at initial diagnosis and the therapeutic choice of prior EGFR-TKI agent was not associated with patients’ prognosis. Notably, the PFS of the prior EGFR-TKI was independently related to ORR (OR, 95% CI 0.98, 0.96–1.00, p = 0.030), PFS (HR, 95% CI 0.98, 0.97–1.00, p = 0.009) and OS (HR, 95% CI 0.96, 0.93–0.98, p < 0.001) of osimertinib treatment. Among distinct organ metastases, only bone metastasis was related to the efficacy of osimertinib, in terms of ORR (OR, 95% CI 1.97, 1.27–3.06, p = 0.002), PFS (HR, 95% CI 1.55, 1.18–2.03, p = 0.001) and OS (HR, 95% CI 1.81, 1.27–2.59, p = 0.001). However, the therapeutic efficacy of osimertinib was not further impacted by the accumulation of metastatic organs. A performance status score of 2–4 was also an adverse prognosticator for the osimertinib therapy.
Conclusion
PFS of the prior EGFR-TKI treatment, performance status score and bone metastasis were independent prognosticators of the osimertinib treatment. These findings may facilitate clinicians in the decision-making of osimertinib.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lung cancer ranks first in incidence and mortality rates among various cancer types worldwide (Siegel et al. 2018). Non-small cell lung cancer (NSCLC) accounts for approximately 80–85% of all lung cancer cases (Molina et al. 2008; Shi et al. 2014). At initial diagnosis, more than 50% of primary NSCLC patients were identified as the metastatic stage with a 5-year survival rate of merely about 4% (Miller et al. 2016).
In recent decades, epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI) has significantly improved the survival outcomes of NSCLC patients with sensitive EGFR mutations (19del and L858R) compared with traditional chemotherapy (Inoue et al. 2013). However, most patients inevitably developed progression or resistance with a median progression-free survival (PFS) of 9–13 months after receiving the first- (e.g., gefitinib, erlotinib, icotinib) or second-generation (e.g., afatinib) EGFR-TKI (Mok et al. 2009). Among the many mechanisms that lead to the acquired resistance to prior first- or second-generation EGFR-TKI, The EGFR exon 20 Thr790Met (T790M) mutation is a major one, accounting for 48–62% (Campo et al. 2016; Oxnard et al. 2011; Sequist et al. 2011; Yu et al. 2013).
Osimertinib (AZD9291), as an irreversible third-generation EGFR-TKI, is capable to overcome the acquired T790M-mediated resistance by binding to the EGFR kinase at the cysteine-797 residue of the ATP-binding site (Cross et al. 2014). According to the remarkable outcomes of clinical trials, the NCCN guidelines recommend osimertinib as the standard treatment for NSCLC patients with acquired EGFR-T790M mutation who failed in first- or second-generation EGFR-TKIs. However, real-world studies investigating the clinical prognostic factors of osimertinib in NSCLC patients with acquired T790M mutation remain limited, especially in Asian populations. Previous studies have reported that age (Kato et al. 2019; Ono et al. 2019), gender (Peng et al. 2021), performance status (Kato et al. 2019; Ono et al. 2019), smoking history (Liu et al. 2020), EGFR 19 Del (Igawa et al. 2019; Ono et al. 2019; Peng et al. 2021), the extrathoracic metastasis status(Chen et al. 2020), central nervous system (CNS) metastasis (Peng et al. 2021) and pleural effusion (Masuhiro et al. 2018) were potential prognostic factors of osimertinib treatment. However, at present, the prognostic effect of prior EGFR-TKI treatment on sequential osimertinib is uncertain. In addition, the exact influence of patients’ metastatic modalities on the therapeutic efficacy of osimertinib treatment remains unclear.
The purpose of our study is to comprehensively investigate the prognostic value of different clinical factors in a real-world Chinese NSCLC cohort with acquired T790M mutation receiving osimertinib treatment, with special concerns on the prior EGFR TKI treatment and metastatic modalities.
Materials and methods
Study population and data collection
A total of 351 NSCLC patients with acquired T790M mutation receiving osimertinib treatment at West China Hospital from 2016 to 2021 were included in the current study. All patients performed re-biopsy (fluid or tissue biopsy) after the failure of the first- or second-generation EGFR-TKI treatment including gefitinib, icotinib, erlotinib, and afatinib and were confirmed with T790M mutation. We retrospectively collected clinical factors from all cases including age, sex, smoking history, performance status score, laterality, location, clinical stage, metastasis site, surgical history, primary EGFR mutation types, prior EGFR-TKI treatment and sequential osimertinib (Table 1). This retrospective study was approved by the ethics committee of West China Hospital.
Response assessments and study endpoints
Clinical stage was evaluated according to the 8th edition of the American Joint Committee on Cancer (AJCC) tumor-node-metastasis (TNM) classification system. The tumor response to osimertinib was assessed based on the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1. The primary endpoints of this study were PFS, which was defined as the time from the initiation of osimertinib treatment to the date of disease progression or the last follow-up visit; overall survival (OS), which referred to the time between osimertinib initiation to death from any cause or the last follow-up visit. Our secondary endpoint was the objective response rate (ORR). The ORR referred to complete remission (CR) or partial remission (PR).
Statistical methods
R software (4.0.0) was utilized for all analyses of this study. The Chi-square test or Fisher’s exact test was conducted to compare the difference in categorical and continuous variables, as well as the therapeutic responses of osimertinib between distinct subgroups. The Kaplan–Meier method was employed to analyze the survival outcomes. The log-rank test was performed for the comparison of survival curves in different subgroups. Univariate and multivariate logistic or COX regression were carried out to identify the prognostic factors associated with ORR/PFS/OS of osimertinib. All tests were two-sided and a p value less than 0.05 was considered to be statistically significant.
Results
Baseline characteristics of patients
The baseline characteristics of the total cohort and patients of distinct response groups are shown in Table 1. The median age was 58 (IQR: 51–68), 40.5% (142/351) of all patients were male, 24.5% (86/351) had a smoking history, 5.4% (19/351) had a performance status score of 2–4 and 16.5% (58/351) had a surgical treatment. Prior to osimertinib treatment, T3–4, N2–3 and IVB stage were diagnosed in 70.4% (247/351), 65.2% (229/351) and 65.5% (230/351) of patients, respectively. The primary EGFR mutations types at initial diagnosis were 19 Del (55.6%) and L858R (34.8%), while the rest 9.7% of patients harbored other/unknown mutations. Before osimertinib initiation, 54.4%, 26.2%, 16.8% and 2.6% of all patients received gefitinib, icotinib, erlotinib and afatinib therapy, respectively.
Overall therapeutic outcomes of osimertinib
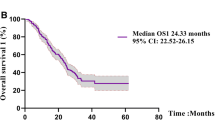
The median follow-up time was 21.3 Mo (95% CI 17.0–25.7 Mo) for the whole cohort. Overall, among the total 351 patients receiving the sequential osimertinib treatment, the best response of PR, stable disease (SD) and progressive disease (PD) was achieved in 180 (51.3%), 136 (38.7%) and 35 (10.0%) cases, respectively. No CR was confirmed among the total cohort. The overall ORR was 51.3%. At the end of follow-up, disease progression and death occurred in 227 (64.7%) and 130 (37.0%) patients, respectively. The median PFS and OS for the total cohort were 12.7 Mo (95% CI 11.5–13.9 Mo) and 25.9 Mo (95% CI 22.5–31.5 Mo), respectively.
The impact of prior EGFR-TKI treatment and primary EGFR mutation on the osimertinib therapy
Previous studies revealed that the different EGFR mutations have distinct prognostic values, with EGFR 19 Del being the one with favorable clinical outcomes (Igawa et al. 2019; Ono et al. 2019; Peng et al. 2021). In the current study, however, EGFR 19 Del showed no effect in predicting either the PFS, OS or the ORR (Figs. 1A, 2A, 3AI; Table 2) of the sequential osimertinib therapy. Nevertheless, patients with EGFR 19 Del harbored a relatively longer PFS in the prior EGFR-TKI therapy compared to cases with other mutations (mean PFS: 17.1 Mo vs. 14.8Mo, p = 0.058).
Kaplan–Meier curves showing the progression-free survival of the sequential osimertinib treatment in patients with distinct clinical subgroups. A Patients with different primary EGFR mutations; B Patients with different prior EGFR-TKI therapies; C Patients with different PFS of the prior EGFR-TKI treatment; D Patients with different age groups; E Patients with different genders; F Patients with or without smoking history; G Patients with different PS; H Patients with different T stages; I Patients with different N stages; J Patients with different M stages; K Patients with different stages; L Patients with or without surgical treatment. EGFR-TKI: epidermal growth factor receptor tyrosine kinase inhibitors; PS: performance status score; PFS: progression-free survival
Kaplan–Meier curves showing the overall survival of the sequential osimertinib treatment in patients with distinct clinical subgroups. A Patients with different primary EGFR mutations; B Patients with different prior EGFR-TKI therapies; C Patients with different PFS of the prior EGFR-TKI treatment; D Patients with different age groups; E Patients with different genders; F Patients with or without smoking history; G Patients with different PS; H Patients with different T stages; I Patients with different N stages; J Patients with different M stages; K Patients with different stages; L Patients with or without surgical treatment. EGFR-TKI: epidermal growth factor receptor tyrosine kinase inhibitors; PS: performance status score; PFS: progression-free survival
We also investigated the potential impact of prior EGFR-TKI treatment on the sequential osimertinib therapy. As shown in Figs. 1B, 2B, 3AII, the distinct therapeutic choice of prior EGFR-TKI was not a predictor of the therapeutic efficacy of osimertinib treatment. However, the PFS of the prior EGFR-TKI agent was significantly associated with patients’ PFS, OS and ORR in osimertinib therapy whether as a continuous variable or a dichotomous variable according to the median value (13 Mo) of their PFS of the prior EGFR-TKI treatment (Figs. 1C, 2C, 3AIII; Table 2).
The significance of pretreatment baseline clinical factors in predicting osimertinib therapy
Next, we explored if different pretreatment clinical factors could impact the therapeutic efficacy of osimertinib treatment. Patients with a higher performance status score (PS2–4 vs. 0–1: 17.2% vs. 52.7%, p = 0.033, Fig. 3AVII) and stage IVB (Stage IVB vs. IVA: 47.4% vs. 58.7%, p = 0.045, Fig. 3AXI) harbored a lower chance of having an objective response in the osimertinib treatment (Table 2A). Other clinical factors had no influence on osimertinib therapy.
The results of Kaplan–Meier curves (Figs. 1D–L, 2D–L) and univariate COX regression (Table 2A) revealed that PS2–4 (HR and 95% CI PFS: 3.30, 2.00–5.47; OS: 2.84, 1.68–4.80) and Stage IVB (HR and 95% CI PFS: 1.61, 1.21–2.13; OS: 2.20, 1.47–3.29) was accompanied with rapid disease progression or death in the osimertinib treatment. In addition, compared to those with M1a disease, patients with M1b (HR, 95% CI 1.66, 1.02–2.71) and M1c (HR, 95% CI 1.87, 1.34–2.60) NSCLC harbored a higher chance of developing disease progression, while the presence of M1c was also related to shorter OS (HR and 95% CI 2.35, 1.48–3.74).
The effect of diverse metastatic modalities on the efficacy of osimertinib therapy
The exact prognostic values of various metastatic modalities in the treatment efficacy of osimertinib therapy remain unclear. Here we comprehensively compared the prognosis of osimertinib in patients with different metastatic modes. Univariate analysis showed that bone metastasis was a predictor of lower ORR (OR and 95% CI 2.08, 1.36–3.19). Besides, bone metastasis (HR and 95% CI PFS: 1.63, 1.25–2.13; OS: 1.85, 1.30–2.63), as well as hepatic metastasis (HR and 95% CI PFS: 1.51, 1.01–2.26; OS: 1.90, 1.20–2.99), was associated with unfavorable PFS and OS (Fig. 4). Pleural effusion was accompanied by more unfavorable OS (HR and 95% CI 1.50, 1.05–2.14) but not PFS. On the contrary, the presence or absence of metastases in other organs or pericardial effusion was not related to the clinical outcomes of the sequential osimertinib treatment.
In addition, we divided all patients into distinct groups based on the number of extrathoracic organ metastasis they had and compared the therapeutic efficacy of the subsequent osimertinib therapy among all groups. The presence of extrathoracic organ metastasis was obviously associated with shorter median PFS and OS for patients receiving sequential osimertinib therapy (Figure S1). However, the prognosis of patients with different number of extrathoracic organ metastasis (metastatic burden) was comparable (Figure S1), indicating that the therapeutic efficacy of osimertinib was not obviously impacted with the accumulating of metastatic organs.
Multivariate analyses identifying independent prognosticator of osimertinib therapy
Multivariate logistic and COX regression was carried out to identify the independent risk factors of osimertinib treatment (Table 2). Factors with predictive ability in univariate analysis (Table 2A) as well as previously reported prognostic factors including age, gender, smoking history and primary EGFR mutation were included into the multivariate analysis. Considering that bone metastasis, M stage and stage were closely related to one another, we only included bone metastasis into the multivariate analysis. The results were consistent in different analyses regarding OR, PFS and OS as an endpoint, respectively. PFS of prior EGFR-TKI, performance status score and bone metastasis were strongly associated with a shorter PFS and OS and a lower ORR.
Discussion
To the best of our knowledge, our research is currently the largest real-world study of the Chinese population investigating the prognosis of osimertinib treatment in NSCLC patients with acquired T790M mutation resistant to prior first-/second-generation EGFR-TKI therapy. The median PFS of our study was 12.7 Mo, consistent with the outcomes of the AURA extension randomized trial (12.3 Mo) (Yang et al. 2017a, b) and real-world studies from two Chinese and one French cohort (12–12.4 Mo) (Auliac et al. 2019; Chen et al. 2020; Peng et al. 2021). The median OS of the present study was 25.9 Mo which was also in line with the AURA III clinical trial (26.8 Mo) (Mok et al. 2017). Similar to other real-world researches (40.5–58.8%), the ORR of the current study was 51.3% (Huang et al. 2021; Igawa et al. 2019; Peng et al. 2021; Yang et al. 2021). In aggregate, the current study verified the favorable clinical efficacy of osimertinib treatment in a large real-world Chinese cohort.
As a novel inhibitor targeting the EGFR signaling, the therapeutic efficacy of the third-generation EGFR-TKI, osimertinib, may be potentially affected by the initial first/second-generation EGFR-TKI treatment. However, little research regarding this issue had been carried out. Only one small-sample study with 27 NSCLC patients from Japan reported that the PFS of initial EGFR-TKIs was significantly associated with the PFS of osimertinib treatment (Yoshimura et al. 2019). In the current study, we systematically reported the prognostic effects of relevant factors in the prior EGFR-TKI therapy on the sequential osimertinib treatment, including prior EGFR-TKI agents, PFS of prior EGFR-TKI and primary EGFR mutation. Our analyses supported different therapeutic choices of prior EGFR-TKI (gefitinib/erlotinib/icotinib/afatinib) are not predictors of osimertinib treatment. Huang et al. (2021) also reported a similar finding that the therapeutic efficacy of osimertinib was not significantly different among patients treated with various first-line EGFR-TKIs, including gefitinib, erlotinib and afatinib group (10.9 Mo vs. 10.0 Mo vs. 6.7 Mo, p = 0.534). Furthermore, in the present study, we observed the significant relationship between the PFS of prior EGFR-TKI treatment and the PFS/OS/ORR of the sequential osimertinib therapy. As far as we know, this is the first Chinese real-world study to report the effect of PFS of prior EGFR-TKI on the efficacy of osimertinib treatment in NSCLC patients with acquired T790M mutation.
Previous studies have demonstrated that different primary EGFR mutation types had distinct prognostic values. EGFR 19 Del was considered as a favorable prognostic factor in first- or second-generation EGFR-TKI therapy (Yang et al. 2017a, b; Zhang et al. 2014). However, the prognostic role of primary EGFR mutation types in the sequential osimertinib treatment remains controversial. Ono et al. (2019) identified EGFR genotype as an independent predictor of PFS of osimertinib in 47 T790M-positive NSCLC patients (HR: 2.83, 95% CI 1.32–6.06, p = 0.007). In addition, a prospective observational study indicated that the response rate of osimertinib in patients with 19 Del was obviously more favorable than L858R (69.7% vs. 38.9%, p = 0.033), so were the median PFS (8.0 Mo vs. 5.2 Mo, p = 0.045) and median OS (19.8 Mo vs. 12.9 Mo, p = 0.0015) (Igawa et al. 2019). However, on the contrary, in the current study, EGFR 19 Del did not significantly influence the efficacy of osimertinib therapy. There are a number of studies that support our findings (Huang et al. 2021; Kishikawa et al. 2020; Masuhiro et al. 2018; Yoshimura et al. 2019). Interestingly, one study reported that against the EGFR L858R mutation, the presence of EGFR 19 Del mutation was associated with shorter OS but not PFS (Peng et al. 2021). Taking together, these findings implied that the exact significance of the primary EGFR mutation on the osimertinib treatment is controversial and more validations are warranted in future studies.
Previous researches indicated that osimertinib has a superior ability to penetrate the blood–brain barrier than first-/second-generation EGFR-TKI, which could consequently result in sustained tumor remission of brain metastasis, indicating favorable intracranial effect (Ballard et al. 2016; Goss et al. 2016; Mok et al. 2017; Yang et al. 2017a, b). In our study, we found that brain metastasis was not correlated with either PFS, OS or ORR of osimertinib therapy. Similarly, several studies also draw the same conclusion that brain or CNS metastasis was not a prognostic feature of osimertinib treatment (Huang et al. 2021; Kishikawa et al. 2020; Li et al. 2021; Masuhiro et al. 2018; Peng et al. 2021). We speculate that the reason for the above findings may be that the good intracranial efficacy of osimertinib can control brain metastasis and minimize the survival difference between patients with and without brain metastasis.
Apart from brain metastasis, we also comprehensively explored the potential impact of different metastatic modalities on the therapeutic efficacy of the osimertinib treatment. Our research revealed that bone metastasis was a remarkable predictor of lower ORR and inferior PFS/OS of the osimertinib treatment, which might indicate that the effect of osimertinib was diminished in treating cases with bone metastasis. Gu et al. (2020) reported a similar result that osimertinib had an effective control on the primary disease but poor effect on bone metastasis in a 65-year-old lung adenocarcinoma patient with T790M mutation resistance to gefitinib.
In addition, we also investigated the value of extrathoracic organ metastasis in predicting the PFS and OS of the osimertinib treatment. We found that the presence of extrathoracic organ metastasis was strongly related to the unfavorable prognosis of the osimertinib therapy, which was consistent with another Chinese real-world study (Chen et al. 2020). Moreover, our study first investigated if patients’ prognosis in osimertinib therapy gets worse with the accumulation of extrathoracic organ metastases. However, the prognosis of patients with different number of extrathoracic metastasis receiving osimertinib was comparable, suggesting that the therapeutic efficacy of osimertinib was not obviously influenced by metastatic burden. This finding implied that, in clinical practice of osimertinib use, patients with high metastatic burden shared comparable clinical outcomes to cases with low metastatic burden, and, therefore, should not be treated with more aggressive therapeutic schemes.
In this study, we also analyzed the prognostic effects of a variety of baseline clinical factors on the efficacy of osimertinib treatment. Multivariate analysis showed that a higher performance status score (PS ≥ 2) was related to a shorter PFS/OS and a lower ORR of osimertinib, which was consistent with previous studies(Huang et al. 2021; Kato et al. 2019; Li et al. 2021; Ono et al. 2019). In contrast, clinical characteristics such as age, sex and smoking history had no significant impact on the prognosis of osimertinib therapy. Though a number of studies had similar findings (Huang et al. 2021; Kishikawa et al. 2020; Li et al. 2021; Masuhiro et al. 2018), several researches identified age (Kato et al. 2019; Ono et al. 2019), sex (Peng et al. 2021) and smoking history (Liu et al. 2020) as independent prognostic factors of osimertinib therapy. Thus, there is a need of more evidence to identify the prognostic value of pretreatment clinical characteristics on osimertinib therapy.
There existed several limitations in our study. First, selection bias was not avoidable because of the retrospective design of the current study. Second, the data of our cohort were collected from a single institution. Third, there is a lack of biological markers for prognostic prediction of osimertinib. Previous studies have reported higher TMB value (Xing et al. 2019), germline BCL2L11 deletion polymorphism (Li et al. 2021), T790M relative mutation purity quartiles (Zheng et al. 2020), act-EGFR MAF and T790M/act-EGFR MAF ratio (Del Re et al. 2018) might be potentially associated with clinical outcomes of osimertinib therapy. Therefore, verifications on prognosticators of osimertinib from prospective multi-center research data with larger sample sizes and biological prognostic markers are needed in the future.
Conclusions
By analyzing data of a large Chinese cohort, our research exhibited the therapeutic efficacy of osimertinib in NSCLC patients with acquired T790M mutation from the real-world perspective. We comprehensively investigated the prognostic factors affecting the therapeutic efficacy of osimertinib treatment. PFS of the prior EGFR-TKI treatment, performance status score and bone metastasis were independent prognosticators of the osimertinib treatment. Our findings may facilitate and guide clinicians in decision-making of osimertinib therapy in NSCLC patients.
References
Auliac JB, Pérol M, Planchard D, Monnet I, Wislez M, Doubre H, Guisier F, Pichon E, Greillier L, Mastroianni B, Decroisette C, Schott R, Le Moulec S, Arrondeau J, Cortot AB, Gerinière L, Renault A, Daniel C, Falchero L, Chouaid C (2019) Real-life efficacy of osimertinib in pretreated patients with advanced non-small cell lung cancer harboring EGFR T790M mutation. Lung Cancer 127:96–102. https://doi.org/10.1016/j.lungcan.2018.11.037
Ballard P, Yates JW, Yang Z, Kim DW, Yang JC, Cantarini M, Pickup K, Jordan A, Hickey M, Grist M, Box M, Johnström P, Varnäs K, Malmquist J, Thress KS, Jänne PA, Cross D (2016) Preclinical comparison of osimertinib with other EGFR-TKIs in EGFR-mutant NSCLC brain metastases models, and early evidence of clinical brain metastases activity. Clin Cancer Res 22(20):5130–5140. https://doi.org/10.1158/1078-0432.Ccr-16-0399
Campo M, Gerber D, Gainor JF, Heist RS, Temel JS, Shaw AT, Fidias P, Muzikansky A, Engelman JA, Sequist LV (2016) Acquired resistance to first-line afatinib and the challenges of prearranged progression biopsies. J Thorac Oncol 11(11):2022–2026. https://doi.org/10.1016/j.jtho.2016.06.032
Chen Y, Wang S, Zhang B, Zhao Y, Zhang L, Hu M, Zhang W, Han B (2020) Clinical factors affecting the response to osimertinib in non-small cell lung cancer patients with an acquired epidermal growth factor receptor T790M mutation: a long-term survival analysis. Target Oncol 15(3):337–345. https://doi.org/10.1007/s11523-020-00724-y
Cross DA, Ashton SE, Ghiorghiu S, Eberlein C, Nebhan CA, Spitzler PJ, Orme JP, Finlay MR, Ward RA, Mellor MJ, Hughes G, Rahi A, Jacobs VN, Red Brewer M, Ichihara E, Sun J, Jin H, Ballard P, Al-Kadhimi K, Rowlinson R, Klinowska T, Richmond GH, Cantarini M, Kim DW, Ranson MR, Pao W (2014) AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated resistance to EGFR inhibitors in lung cancer. Cancer Discov 4(9):1046–1061. https://doi.org/10.1158/2159-8290.Cd-14-0337
Del Re M, Bordi P, Rofi E, Restante G, Valleggi S, Minari R, Crucitta S, Arrigoni E, Chella A, Morganti R, Tiseo M, Petrini I, Danesi R (2018) The amount of activating EGFR mutations in circulating cell-free DNA is a marker to monitor osimertinib response. Br J Cancer 119(10):1252–1258. https://doi.org/10.1038/s41416-018-0238-z
Goss G, Tsai CM, Shepherd FA, Bazhenova L, Lee JS, Chang GC, Crino L, Satouchi M, Chu Q, Hida T, Han JY, Juan O, Dunphy F, Nishio M, Kang JH, Majem M, Mann H, Cantarini M, Ghiorghiu S, Mitsudomi T (2016) Osimertinib for pretreated EGFR Thr790Met-positive advanced non-small-cell lung cancer (AURA2): a multicentre, open-label, single-arm, phase 2 study. Lancet Oncol 17(12):1643–1652. https://doi.org/10.1016/s1470-2045(16)30508-3
Gu H, Sun L, Dou Z, Kong C, Zu J, Xiao J, Jiang T, Li N (2020) Analysis of lung adenocarcinoma with bone metastasis: a case report. Transl Lung Cancer Res 9(2):389–392. https://doi.org/10.21037/tlcr.2020.03.11
Huang YH, Tseng JS, Hsu KH, Chen KC, Su KY, Yu SL, Chen JJW, Yang TY, Chang GC (2021) The impact of different first-line EGFR-TKIs on the clinical outcome of sequential osimertinib treatment in advanced NSCLC with secondary T790M. Sci Rep 11(1):12084. https://doi.org/10.1038/s41598-021-91657-7
Igawa S, Ono T, Kasajima M, Ishihara M, Hiyoshi Y, Kusuhara S, Nishinarita N, Fukui T, Kubota M, Sasaki J, Hisashi M, Yokoba M, Katagiri M, Naoki K (2019) Impact of EGFR genotype on the efficacy of osimertinib in EGFR tyrosine kinase inhibitor-resistant patients with non-small cell lung cancer: a prospective observational study. Cancer Manag Res 11:4883–4892. https://doi.org/10.2147/cmar.S207170
Inoue A, Kobayashi K, Maemondo M, Sugawara S, Oizumi S, Isobe H, Gemma A, Harada M, Yoshizawa H, Kinoshita I, Fujita Y, Okinaga S, Hirano H, Yoshimori K, Harada T, Saijo Y, Hagiwara K, Morita S, Nukiwa T (2013) Updated overall survival results from a randomized phase III trial comparing gefitinib with carboplatin-paclitaxel for chemo-naïve non-small cell lung cancer with sensitive EGFR gene mutations (NEJ002). Ann Oncol 24(1):54–59. https://doi.org/10.1093/annonc/mds214
Kato Y, Hosomi Y, Watanabe K, Yomota M, Kawai S, Okuma Y, Kubota K, Seike M, Gemma A, Okamura T (2019) Impact of clinical features on the efficacy of osimertinib therapy in patients with T790M-positive non-small cell lung cancer and acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors. J Thorac Dis 11(6):2350–2360. https://doi.org/10.21037/jtd.2019.06.03
Kishikawa T, Kasai T, Okada M, Nakachi I, Soda S, Arai R, Takigami A, Sata M (2020) Osimertinib, a third-generation EGFR tyrosine kinase inhibitor: a retrospective multicenter study of its real-world efficacy and safety in advanced/recurrent non-small cell lung carcinoma. Thorac Cancer 11(4):935–942. https://doi.org/10.1111/1759-7714.13378
Li X, Zhang D, Li B, Zou B, Wang S, Fan B, Li W, Yu J, Wang L (2021) Clinical implications of germline BCL2L11 deletion polymorphism in pretreated advanced NSCLC patients with osimertinib therapy. Lung Cancer 151:39–43. https://doi.org/10.1016/j.lungcan.2020.12.002
Liu J, Li X, Shao Y, Guo X, He J (2020) The efficacy and safety of osimertinib in treating nonsmall cell lung cancer: a PRISMA-compliant systematic review and meta-analysis. Medicine (baltimore) 99(34):e21826. https://doi.org/10.1097/md.0000000000021826
Masuhiro K, Shiroyama T, Suzuki H, Takata SO, Nasu S, Takada H, Morita S, Tanaka A, Morishita N, Okamoto N, Hirashima T (2018) Impact of pleural effusion on outcomes of patients receiving osimertinib for NSCLC harboring EGFR T790M. Anticancer Res 38(6):3567–3571. https://doi.org/10.21873/anticanres.12629
Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, Stein KD, Alteri R, Jemal A (2016) Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin 66(4):271–289. https://doi.org/10.3322/caac.21349
Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, Sunpaweravong P, Han B, Margono B, Ichinose Y, Nishiwaki Y, Ohe Y, Yang JJ, Chewaskulyong B, Jiang H, Duffield EL, Watkins CL, Armour AA, Fukuoka M (2009) Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 361(10):947–957. https://doi.org/10.1056/NEJMoa0810699
Mok TS, Wu YL, Ahn MJ, Garassino MC, Kim HR, Ramalingam SS, Shepherd FA, He Y, Akamatsu H, Theelen WS, Lee CK, Sebastian M, Templeton A, Mann H, Marotti M, Ghiorghiu S, Papadimitrakopoulou VA (2017) Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med 376(7):629–640. https://doi.org/10.1056/NEJMoa1612674
Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA (2008) Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc 83(5):584–594. https://doi.org/10.4065/83.5.584
Ono T, Igawa S, Ozawa T, Kasajima M, Ishihara M, Hiyoshi Y, Kusuhara S, Nishinarita N, Fukui T, Kubota M, Sasaki J, Hisashi M, Katagiri M, Naoki K (2019) Evaluation of osimertinib efficacy according to body surface area and body mass index in patients with non-small cell lung cancer harboring an EGFR mutation: a rospective observational study. Thorac Cancer 10(4):880–889. https://doi.org/10.1111/1759-7714.13018
Oxnard GR, Arcila ME, Sima CS, Riely GJ, Chmielecki J, Kris MG, Pao W, Ladanyi M, Miller VA (2011) Acquired resistance to EGFR tyrosine kinase inhibitors in EGFR-mutant lung cancer: distinct natural history of patients with tumors harboring the T790M mutation. Clin Cancer Res 17(6):1616–1622. https://doi.org/10.1158/1078-0432.Ccr-10-2692
Peng D, Shan D, Dai C, Li J, Wang Z, Huang Z, Peng R, Zhao P, Ma X (2021) Real-world data on osimertinib in chinese patients with pretreated, EGFR T790M mutation positive, advanced non-small cell lung cancer: a retrospective study. Cancer Manag Res 13:2033–2039. https://doi.org/10.2147/cmar.S287466
Sequist LV, Waltman BA, Dias-Santagata D, Digumarthy S, Turke AB, Fidias P, Bergethon K, Shaw AT, Gettinger S, Cosper AK, Akhavanfard S, Heist RS, Temel J, Christensen JG, Wain JC, Lynch TJ, Vernovsky K, Mark EJ, Lanuti M, Iafrate AJ, Mino-Kenudson M, Engelman JA (2011) Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med 3(75):75ra26. https://doi.org/10.1126/scitranslmed.3002003
Shi Y, Au JS, Thongprasert S, Srinivasan S, Tsai CM, Khoa MT, Heeroma K, Itoh Y, Cornelio G, Yang PC (2014) A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology (PIONEER). J Thorac Oncol 9(2):154–162. https://doi.org/10.1097/jto.0000000000000033
Siegel RL, Miller KD, Jemal A (2018) Cancer statistics, 2018. CA Cancer J Clin 68(1):7–30. https://doi.org/10.3322/caac.21442
Xing P, Han X, Wang S, Liu Y, Yang S, Hao X, Wang Y, Liu P, Li J, Wang L, Chang L, Guan Y, Zhang Z, Wu D, Yao J, Yi X, Shi Y (2019) Co-mutational assessment of circulating tumour DNA (ctDNA) during osimertinib treatment for T790M mutant lung cancer. J Cell Mol Med 23(10):6812–6821. https://doi.org/10.1111/jcmm.14565
Yang JC, Ahn MJ, Kim DW, Ramalingam SS, Sequist LV, Su WC, Kim SW, Kim JH, Planchard D, Felip E, Blackhall F, Haggstrom D, Yoh K, Novello S, Gold K, Hirashima T, Lin CC, Mann H, Cantarini M, Ghiorghiu S, Jänne PA (2017a) Osimertinib in pretreated T790M-positive advanced non-small-cell lung cancer: AURA study phase II extension component. J Clin Oncol 35(12):1288–1296. https://doi.org/10.1200/jco.2016.70.3223
Yang JJ, Zhou Q, Yan HH, Zhang XC, Chen HJ, Tu HY, Wang Z, Xu CR, Su J, Wang BC, Jiang BY, Bai XY, Zhong WZ, Yang XN, Wu YL (2017b) A phase III randomised controlled trial of erlotinib vs gefitinib in advanced non-small cell lung cancer with EGFR mutations. Br J Cancer 116(5):568–574. https://doi.org/10.1038/bjc.2016.456
Yang Y, Guo Y, Wang R, Li J, Zhu H, Guo RTW (2021) Effect of osimertinib in treating patients with first-generation EGFR-TKI-resistant advanced non-small cell lung cancer and prognostic analysis. J Buon 26(1):51–57
Yoshimura A, Yamada T, Okura N, Takeda T, Hirose K, Kubota Y, Shiotsu S, Hiranuma O, Chihara Y, Tamiya N, Kaneko Y, Uchino J, Takayama K (2019) Clinical characteristics of osimertinib responder in non-small cell lung cancer patients with EGFR-T790M mutation. Cancers (basel) 11(3):365. https://doi.org/10.3390/cancers11030365
Yu HA, Arcila ME, Rekhtman N, Sima CS, Zakowski MF, Pao W, Kris MG, Miller VA, Ladanyi M, Riely GJ (2013) Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res 19(8):2240–2247. https://doi.org/10.1158/1078-0432.Ccr-12-2246
Zhang Y, Sheng J, Kang S, Fang W, Yan Y, Hu Z, Hong S, Wu X, Qin T, Liang W, Zhang L (2014) Patients with exon 19 deletion were associated with longer progression-free survival compared to those with L858R mutation after first-line EGFR-TKIs for advanced non-small cell lung cancer: a meta-analysis. PLoS ONE 9(9):e107161. https://doi.org/10.1371/journal.pone.0107161
Zheng Q, Hong S, Huang Y, Zhao H, Yang Y, Hou X, Zhao Y, Ma Y, Zhou T, Zhang Y, Fang W, Zhang L (2020) EGFR T790M relative mutation purity predicts osimertinib treatment efficacy in non-small cell lung cancer patients. Clin Transl Med 9(1):17. https://doi.org/10.1186/s40169-020-0269-y
Funding
This work was supported by the National Natural Science Foundation of China [Grant numbers NSFC30900360].
Author information
Authors and Affiliations
Contributions
Conceptualization, methodology, and formal analysis: XT, YL, and YG; data curation and investigation: XT, YL, WQ, WY, and TP; supervision: ZY and YG; writing—original draft: XT and YL; writing—review and editing: XT, YL, YG, and ZY; final approval of the manuscript: all authors.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
This retrospective study was approved by the ethics committee of West China Hospital.
Consent to participate
Written informed consent was waived given the nature of the study.
Consent for publication
Informed consent was obtained from the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
432_2021_3797_MOESM1_ESM.pdf
Supplementary Fig. S1. Kaplan–Meier curves showing the progression-free survival (A) and overall survival (B) of patients with different number of metastatic extrathoracic organs (PDF 255 KB)
Rights and permissions
About this article
Cite this article
Tang, X., Li, Y., Qian, Wl. et al. A comprehensive prognostic analysis of osimertinib treatment in advanced non-small cell lung cancer patients with acquired EGFR-T790M mutation: a real-world study. J Cancer Res Clin Oncol 148, 2475–2486 (2022). https://doi.org/10.1007/s00432-021-03797-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-021-03797-y