Abstract
Background
This study was designed to investigate the efficacy and safety of immune checkpoint inhibitors (ICIs) in advanced hepatocellular carcinoma (HCC).
Methods
Electronic databases were scanned to identify relevant trials. The primary endpoints were overall survival (OS), progression-free survival (PFS), and their prognostic factors. Stratified analyses were accomplished on ICIs agent and evaluation criteria.
Results
Totally, 3697 individuals from 40 cohorts were recruited. For patients treated with ICIs, the pooled median time to progression (TTP) was 8.0 months, median PFS 4.9 months, and median OS 12.0 months; the pooled median PFS and OS of ICIs plus anti-vascular endothelial growth factor (VEGF) agents (PFS: 6.3 months, OS: 16.4 months) were longer than those of ICIs alone. Furthermore, Child–Pugh stage (HR = 1.37, P = 0.0123) and Eastern Cooperative Oncology Group (ECOG) (HR = 1.40, P = 0.0016) were prognostic factors for PFS. Hepatitis C virus (HCV) (HR = 0.71, P = 0.0356), Alpha-fetoprotein (AFP) (HR = 1.17, P < 0.0001), Child–Pugh stage (HR = 1.58, P < 0.0001), Barcelona Clinic Liver Cancer (BCLC) stage (HR = 1.23, P = 0.0005), ECOG (HR = 1.50, P = 0.0012), portal vein invasion (HR = 1.32, P = 0.0053), extrahepatic metastasis (HR = 0.84, P = 0.0047), best response (HR = 0.58, P < 0.0001), and neutrophil-to-lymphocyte ratio (NLR) (HR = 1.23, P = 0.0451) were the prognostic factors for OS. According to both RECIST 1.1 and mRECIST, the objective response rate (ORR) and disease control rate (DCR) rate of ICIs plus anti-VEGF agents were better than those of ICIs alone. The overall rate of any grade adverse events (AEs) was 0.76 (95% CI 0.61–0.89), grade 3 or higher AEs was 0.28 (95% CI 0.15–0.42), and the rate of AEs leading to treatment discontinuation was 0.09 (95% CI 0.06–0.12).
Conclusions
The ICIs was promising in HCC with good efficacy and tolerated toxicity. Compared with ICIs monotherapy, the joint application of ICIs and anti-VEGF agents can contribute a lot more benefits to the survival of patients according to clinical practices.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC) is the third most frequent cause of cancer-related death all over the world with the incidence rising rapidly recently (Bray et al. 2018). The prognosis for patients with early stage HCC has been greatly improved with the development of surgical resection and the extensive application of locoregional therapy composed by trans-arterial chemoembolization (TACE), radiofrequency ablation (RFA), and stereotactic body radiation therapy (SBRT) (Tella et al. 2019). However, the clinical outcome of advanced HCC remains frustrating for its insensitivity to chemotherapy and limited efficacy of molecular targeted drug such as sorafenib (Gomaa and Waked 2015). Consequently, it is crucial to seek a novel approach against advanced HCC.
Fortunately, in the last decade, immune checkpoint inhibitors (ICIs) have set off a revolutionary wave in several hematological and solid tumors, including Hodgkin lymphoma, melanoma, non-small cell lung cancer (NSCLC), and triple negative breast cancer (TNBC). Accumulating evidences have demonstrated remarkable improvements in survival outcomes with ICIs-based monotherapy or combination therapy in advanced malignancies (Schachter et al. 2017; Pasello et al. 2020; Simmons et al. 2020), which shed some light on advanced HCC.
Notably enough, ICIs have been tested in advanced HCC, where promising findings were observed in phase I and II clinical trials with the programmed cell death protein 1 (PD-1) inhibitors nivolumab and pembrolizumab assessed. Nonetheless, subsequent confirmatory phase III studies on these two agents were negative, failing to report an overall survival (OS) benefit in advanced HCC patients receiving ICIs monotherapy (Rizzo et al. 2021a). At the same time, notable responses were observed in selected HCC (Finn et al. 2020; Lee et al. 2020; Yau et al. 2020), further supporting the exploration of immunotherapy and the identification of potential predictive biomarkers. On the basis of preclinical and early phase clinical studies, ICIs-based combination therapies have been studied in advanced HCC. The combination of PD-L1 inhibitor atezolizumab plus the bevacizumab has been tested in the phase III IMbrave150 clinical trial. Interestingly, after more than a decade from the publication of the landmark SHARP phase III study establishing sorafenib as the reference front-line treatment, atezolizumab plus bevacizumab improved median OS compared to sorafenib (Rizzo et al. 2021b). These recently published results have witnessed a historical step forward, with the IMbrave150 establishing the novel first-line standard. In addition, atezolizumab is also being evaluated in the COSMIC-312 phase III trial testing the association of the PD-L1 inhibitor with cabozantinib, and thus, a bigger role of ICIs is supposed to play in treating patients with advanced HCC in the near future.
To overcome the limitations of individual studies and assess the overall benefit, here, we made a comprehensively survey based on a large sample size (40 cohorts incorporating 3697 individuals) and diverse dimensions (stratified by ICIs agent and evaluation criteria) to evaluate the efficacy and safety of ICIs in advanced HCC.
Materials and methods
Data sources and literature searches
Researches were screened by a systematic electronic literature retrieval for abstracts of relevant studies in the published literature. PubMed, Cochrane Library, and EMBASE were searched and the data were updated as of November 5th, 2020. The basic search terms were used as follows: “immunotherapy”, “immune checkpoint inhibitors”, “nivolumab”, “atezolizumab”, “pembrolizumab”, “CTLA-4”, “PD-1”, “PD-L1”, “ipilimumab”, “programmed cell death ligand 1”, “programmed cell death 1”, “cytotoxic T lymphocyte-associated protein 4”, “ICIs”, “Camrelizumab”, “Toripalimab”, “Sintilimab”, “HCC”, “liver cancer”, and “Hepatocellular carcinoma”. Full-text articles were observed if abstracts did not provide enough information. Moreover, the references of related articles were reviewed for additional studies. Reviews, editorials comments, case reports, and letters to the editor were excluded. The retrieve was performed without language restriction.
Selection of studies
Initially, two investigators performed a screening of titles and abstracts, respectively, and then examined the full text of articles to acquire eligible studies. For the repetitive studies based on the same study patients, the latest or most comprehensive data were included.
Inclusion criteria
Inclusion criteria were: (1) prospective or retrospective studies to evaluate the efficacy and safety of ICIs in HCC; (2) patients pathologically or clinically confirmed as HCC; (3) the data [including any of the following outcomes: time to progression (TTP), progression-free survival (PFS), overall survival (OS), disease control rate (DCR), and objective response rate (ORR)] to evaluate the efficacy of ICIs in HCC could be obtained or calculated from the original literature.
Data extraction
Data extraction was conducted conforming to the PRISMA guidance (S1 PRISMA Checklist). Two investigators independently evaluated the quality items and differences, and then collected data from recruited studies. All eligible studies involved information as follows: publication year and region, the first author’s name, study type, number of patients, ICIs agent, and outcome measures.
Quality assessment
Quality of the included studies was assessed as reported in the literature, which consists of 20 items (Jonsson et al. 2006). The checklist examines the main domains including study design, population, intervention, outcome measures, statistical analysis, results/conclusions, competing interest, and sources of financial support.
Statistical methods
The primary endpoints were OS and/or PFS. The association between prognostic factors and efficacy of ICIs was measured by HR with the corresponding 95% CI. Stratified analyses were accomplished on ICIs agent. The secondary endpoints were best responses evaluated by RECIST 1.1 and m RECIST 1.1. Funnel plots and Egger’s test were performed to evaluate publication bias. Statistical analysis was performed with R 4.0 statistical software. Survival data were obtained based on the Kaplan–Meier curves. Heterogeneity was assessed by I-square tests and Chi-square. If P < 0.1 or I2 > 40%, remarkable heterogeneity existed. A random-effect model was adopted to calculate the pooled data when heterogeneity existed, or else, a fixed effect model was employed.
Results
Selection of study
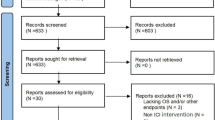
Initially, 8058 relevant articles were scrutinized intensively. Of them, 386 were filtered for duplication, and 7574 were excluded for digression after screening the titles and abstracts. Then, the full text of 98 articles was thoroughly reviewed, and 58 were filtered for reasons as follows: they were not human research, and not solid cancer, repeated study cohort, reviews or meta-analysis, and the data to evaluate the efficacy of ICIs in HCC were unavailable.
Finally, a total of 40 cohorts (detailed supplementary file in Table S1) incorporating 3697 individuals were recruited in this research. The elaborate procedure is displayed in Fig. 1.
Study characteristics
Totally, 3697 individuals in the 40 cohorts published as of November 5th, 2020 were recruited. The sample size ranged from 11 to 341. Of these studies, 22 were retrospective and 18 prospective. Meanwhile, all of these studies involved ICIs: anti-PD-(L)1 and anti-CTLA-4. HR for PFS and/or OS were used to assess the impact of probable prognostic factors on the efficacy of ICIs. Of all the adopted studies, 34 cohorts contained data for OS and 31 for PFS. The principal traits are presented in Table 1.
Data analyses
Pooled survival outcomes of ICIs in HCC
In this study, for HCC treated with ICIs, the pooled median TTP was 8.0 months (Fig. 2a), median PFS 4.9 months (Fig. 2b), and median OS 12.0 months (Fig. 2c).
Regarding ICIs-based combination therapy, seven different combination drugs were reported in recruited studies: bevacizumab, codrituzumab, apatinib, sorafenib, regorafenib, lenvatinib, and chemotherapy, of which five were anti-VEGF agents, thus constituting ICIs plus anti-VEGF agent subgroup. Stratified analyses were performed according to ICIs agent and combination therapy: the pooled median PFS of PD-(L)1 (4.7 months) was shorter than that of CTLA-4 or ICIs plus anti-VEGF agents (6.3 months) (Fig. 3a); additionally, concerning PD-(L)1, the pooled median PFS of Nivolumab (Nivo) (2.7 months) was shorter than that of Pembrolizumab (Pembro) (5.3 months) or Camrelizumab (5.4 months) (Fig. 3b); the pooled median OS of PD-(L)1 (11.4 months) was shorter than that of ICIs plus anti-VEGF agents (16.4 months) (Fig. 3c); furthermore, with regard to PD-(L)1, the pooled median OS of Nivo (9.4 months) was shorter than that of Pembro (14.7 months) (Fig. 3d). The pooled estimates for rates of PFS and OS are summarized by single-arm analysis in Table S2 and Table S3.
Subgroup analyses for PFS and OS. a Pooled PFS of ICIs plus anti-vascular endothelial growth factor (VEGF) agents, cytotoxic T lymphocyte-associated protein 4 (CTLA-4), and Programmed cell death ligand 1 (PD-(L)1); b pooled PFS of Nivolumab (Nivo), Pembrolizumab (Pembro), and Camrelizumab; c pooled OS of ICIs plus anti-VEGF agents, CTLA-4, and PD-(L)1; d pooled OS of Nivo, Pembro, and Camrelizumab
Pooled analyses of prognostic factors for PFS and OS
The pooled analyses of the relationship between PFS and/or OS and probable prognostic factors are summarized in Table 2. Child–Pugh stage (HR = 1.37, 95% CI 1.07–1.74, P = 0.0123) and ECOG (HR = 1.40, 95% CI 1.14–1.72, P = 0.0016) were the probable prognostic factors for PFS (Fig. S1). With regard to OS, the following prognostic factors possessed significance: HCV (HR = 0.71, 95% CI 0.52–0.98, P = 0.0356), AFP (HR = 1.17, 95% CI 1.10–1.25, P < 0.0001), Child–Pugh stage (HR = 1.58, 95% CI 1.33–1.87, P < 0.0001), BCLC stage (HR = 1.23, 95% CI 1.09–1.38, P = 0.0005), ECOG (HR = 1.50, 95% CI 1.17–1.93, P = 0.0012), portal vein invasion (HR = 1.32, 95% CI 1.09–1.60, P = 0.0053), extrahepatic metastasis (HR = 0.84, 95% CI 0.74–0.95, P = 0.0047), best response (HR = 0.58, 95% CI 0.52–0.64, P < 0.0001), and NLR (HR = 1.23, 95% CI 1.00–1.50, P = 0.0451) (Fig. S2).
Analyses of best response stratified by ICIs agent and evaluation criteria
Subgroup analyses were implemented according to different RECIST criteria (RECIST vs. mRECIST) and ICIs agent (ICIs vs. CTLA-4 vs. PD-(L)1), which are summarized in Table 3. With regard to ICIs alone, the ORR and DCR were 0.23 (95% CI 0.20–0.27) and 0.62 (95% CI 0.57–0.66) according to RECIST 1.1, 0.23 (95% CI 0.17–0.29) and 0.59 (95% CI 0.49–0.69) judged by mRECIST 1.1; concerning ICIs plus anti-VEGF agents, the ORR and DCR of were 0.29 (95% CI 0.22–0.37) and 0.72 (95% CI 0.61–0.82) according to RECIST 1.1, and 0.33 (95% CI 0.25–0.41) and 0.69 (95% CI 0.57–0.81) judged by mRECIST 1.1. Furthermore, the ORR and DCR of CTLA-4/PD-(L)1 plus anti-VEGF agents were also better than those of CTLA-4/PD-(L)1 alone.
Adverse events (AEs) of ICIs in HCC
The overall rate of any grade AEs was 0.76 (95% CI 0.61–0.89) (Fig. 4a), grade 3 or higher AEs was 0.28 (95% CI 0.15–0.42) (Fig. 4b), and AEs leading to treatment discontinuation was 0.09 (95% CI 0.06–0.12) (Fig. 4c). Stratified analyses of AEs were performed according to ICIs agent: the rate of any grade AEs was 0.73 (95% CI 0.43–0.95) (Fig. 4d) in Nivo and 0.74 (95% CI 0.42–0.96) (Fig. 4g) in Pembro; the rate of grade 3 or higher AEs was 0.24 (95% CI 0.03–0.56) (Fig. 4e) in Nivo and 0.39 (95% CI 0.19–0.60) (Fig. 4h) in Pembro; the rate of AEs leading to treatment discontinuation was 0.08 (95% CI 0.02–0.16) (Fig. 4f) in Nivo, 0.11 (95% CI 0.05–0.19) (Fig. 4i) in Pembro, and 0.07 (95% CI 0.05–0.10) (Fig. 4j) in Atezolizumab (Atezo).
Adverse events (AEs) of ICIs in advanced HCC. a Any grade AEs; b grade 3 or higher AEs; c AEs lead to treatment discontinuation; d any grade AEs for Nivo; e grade 3 or higher AEs for Nivo; f AEs lead to treatment discontinuation for Nivo; g any grade AEs for Pembro; h grade 3 or higher AEs for Pembro; i AEs lead to treatment discontinuation for Pembro; j AEs lead to treatment discontinuation for Atezolizumab (Atezo)
Assessment of study quality and publication bias
Quality assessment of 40 recruited studies is summarized in Table S4. No evidence of publication bias was observed via egger’s tests in the pooled analysis of ORR, DCR, CR, PR, SD, and PD (Table S5), so were the pooled analysis of OS and PFS via funnel plots (Fig. S3) and Egger’s tests (Table S6).
Discussion
HCC is the sixth most common malignancy and the fourth leading cause of cancer-related death worldwide (Llovet et al. 2018). For patients with advanced HCC, the effective therapeutic strategies are limited. Most patients are not able to benefit from chemotherapy due to the low effectiveness and serious AEs of chemotherapeutics. With the prolonged overall survival and improved quality of life, sorafenib was approved as first- line drug for advanced HCC by United States Food and Drug Administration (FDA) and China FDA (Furuse 2008; Llovet et al. 2008; Salhab and Canelo 2011). Until now, the optional drugs have expanded to regorafenib, lenvatinib, and other targeted drugs (Bruix et al. 2017; Kudo et al. 2018). Nevertheless, the expectant survival remains shorter than 1 year (El-Serag et al. 2008). In last decade, ICIs has initiated a new era for immunotherapy in oncology by monoclonal antibodies to release the anti-tumor activity of preexisting tumor-specific T-cell immunity, which inspired researchers to focus on the application of ICIs in advanced HCC.
Based on the existing studies, the pooled results of our study revealed that ICIs-based therapy is promising in advanced HCC. Additionally, compared with ICIs monotherapy, the joint application of ICIs and anti-VEGF agents has witnessed better outcomes in DCR, ORR, PFS, and OS. ICIs can effectively alleviate immune escape and enhance the anti-tumor effect mediated by T cells (Reul et al. 2019). However, there are a lot of neovascularization with special structure in tumor tissue, which makes it difficult for anti-tumor drugs and immune cells to reach the tumor site. It was documented that there were no more than 20% of patients with advanced HCC robustly responding to ICIs’ monotherapy (El-Khoueiry et al. 2017; Zhu et al. 2018). The combination of ICIs and anti-VEGF agents has a consistent vessel fortification effect in HCC and can overcome treatment resistance, as compared to monotherapies with either of the two agents (Shigeta et al. 2019). The FDA has granted the combined therapy between pembrolizumab and lenvatinib for first-line treatment of patients with HCC based on the latest interim results of the Phase 1b trial, KEYNOTE-524. Furthermore, based on the results of the phase 3 IMbrave150 study, the US FDA approved atezolizumab combined with bevacizumab (A + T) for the treatment of unresectable or metastatic HCC patients who have not received systemic treatment before (Bomze et al. 2020). Therefore, the effectiveness of a single drug is relatively limited. Combined therapy is the future development direction (Wang et al. 2020).
Currently, unlike other solid tumors, there are no recognized or validated biomarkers for HCC immunotherapy (Xu et al. 2019; Vitale et al. 2020). The pooled analysis of our study revealed that AFP, Child–Pugh stage, BCLC stage, ECOG, portal vein invasion, and neutrophil-to-lymphocyte ratio (NLR) were the independent poor prognostic factors, which implied that high AFP (Shao et al. 2019), weak physical condition (Kuo et al. 2020), poor liver functional reserve, macroscopic vascular invasion, and high inflammatory reaction have negative influences on the efficacy of ICIs.
Concerning NLR, studies have shown consistently that inflammation is associated with prognosis in solid tumors due to its effect on the immune response to the disease (Bagley et al. 2017; Cheng et al. 2016; Fouad and Aanei 2017). NLR is a marker for the general immune response to various stress stimuli (Gibney et al. 2016). It was documented that the peripheral neutrophil count measured by the NLR has been found to be directly correlated with the levels of intratumor neutrophil population (Moses and Brandau 2016) and granulocyte myeloid-derived suppressor cells (gMDSCs) (Gonda et al. 2013), which is directly associated with the anti-tumor effect of immune checkpoint inhibitors (Sacdalan et al. 2018).
On the other hand, infection with HCV, extrahepatic metastasis, and best response with CR or PR were good prognosis factors of ICIs used in advanced HCC.
Concerning ICIs used in HCC patients infected with HCV, there is a lack of data based on large clinical trials. It was documented that the HCV-specific cytotoxic T lymphocytes (CTLs) can be activated by ICIs without liver damage (Fukuda et al. 2020). However, the immunopathogenesis of HCV after the administration of ICIs has not been clarified. Due to the small number of included studies, the results need to be further confirmed by large sample research.
Extrahepatic metastases, with a diverse antigen load, may serve as a source of antigen-specific T-cell immunity, increase the immunogenicity of HCC, and enhance the anti-tumor effect of ICIs. Additionally, the tumor response to ICIs in HCC varies among different organs. This diversity of organ-specific response indicates that the immune microenvironments of different organs often differ. Different from other organs, liver sustains an immunosuppressive milieu because of a series of regulatory mechanisms including inherent tolerance, chronic HBV-mediated immunosuppression, and HCC immune escape (Pardee and Butterfield 2012). With the change of the extrahepatic immune microenvironment, the immunosuppression decreased and the immune response increased.
There were not any new specific AEs related specifically HCC and the incidence rate of grade 3 or higher AEs (leading to treatment discontinuation) was not high for patients treated with ICIs-based therapy. On the whole, the toxicity of ICIs-based therapy was tolerable for advanced HCC.
In conclusion, the ICIs-based therapeutic strategies (especially combination of ICIs and anti-VEGF agents) were promising in advanced HCC. The best strategy and time of ICIs for HCC remain a challenge to be addressed. On one hand, in the exploration of the best strategy of ICIs for HCC, we need to optimize the order of the existing drugs, to design and promote clinical research based on biomarkers, and to explore the development of other ICIs drugs and cell-based treatment schemes (such as Car-T-cell therapy); on the other hand, in choosing the best time of ICIs for HCC, we need to compare the curative effect of first-line and second-line setting on the basis of the existing outcomes, and consider perioperative immunotherapy; at the same time, the existing ICIs-based schemes need to be combined with local treatment (including TACE, HAIC, SIRT, and radiotherapy). The top priority for future research of ICIs in HCC is to find appropriate biomarkers [such as tumor mutational burden (TMB), PD-L1 expression, tumor infiltrating lymphocytes (TILs), and mismatch repair deficiency (MMR)] to screen beneficiaries (Zeng et al. 2020; Cheng et al. 2020), to explore the feasibility of ICIs combined with local therapeutics (such as radiotherapy, RFA, and TACE) (Choi et al. 2019), and to expand the application of ICIs in perioperative period for HCC and realize the transformation therapy (Tovoli et al. 2020).
Limitations
This study had some drawbacks: first, the majority of the included cohorts were single-arm trials, and multicenter randomized-controlled trials are recommended in the future; second, the recruited studies showed a high level of heterogeneity and a certain level of publication bias; finally, the ICIs served at different treatment line among included studies, which may be a possible source of bias.
Conclusions
The ICIs was promising in HCC with good efficacy and tolerated toxicity. Compared with ICIs monotherapy, the joint application of ICIs and anti-VEGF agents can contribute a lot more benefits to the survival of patients according to clinical practices.
Availability of data and materials
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
References
Bagley SJ, Kothari S, Aggarwal C et al (2017) Pretreatment neutrophilto-lymphocyte ratio as a marker of outcomes in nivolumab-treated patients with advanced non-small-cell lung cancer. Lung Cancer 106:1–7. https://doi.org/10.1016/j.lungcan.2017.01.013
Bomze D, Meirson T, Azoulay D (2020) Atezolizumab and bevacizumab in hepatocellular carcinoma. N Engl J Med 383(7):693–694. https://doi.org/10.1056/NEJMc2021840
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68(6):394–424. https://doi.org/10.3322/caac.21492
Bruix J, Qin S, Merle P et al (2017) Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 389(10064):56–66. https://doi.org/10.1016/S0140-6736(16)32453-9
Cheng H, Luo G, Lu Y et al (2016) The combination of systemic inflammation-based marker NLR and circulating regulatory T cells predicts the prognosis of resectable pancreatic cancer patients. Pancreatology 16(6):1080–1084. https://doi.org/10.1016/j.pan.2016.09.007
Cheng AL, Hsu C, Chan SL, Choo SP, Kudo M (2020) Challenges of combination therapy with immune checkpoint inhibitors for hepatocellular carcinoma. J Hepatol 72(2):307–319. https://doi.org/10.1016/j.jhep.2019.09.025
Choi C, Yoo GS, Cho WK, Park HC (2019) Optimizing radiotherapy with immune checkpoint blockade in hepatocellular carcinoma. World J Gastroenterol 25(20):2416–2429. https://doi.org/10.3748/wjg.v25.i20.2416
El-Khoueiry AB, Sangro B, Yau T et al (2017) Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 389(10088):2492–2502. https://doi.org/10.1016/S0140-6736(17)31046-2
El-Serag HB, Marrero JA, Rudolph L, Reddy KR (2008) Diagnosis and treatment of hepatocellular carcinoma. Gastroenterology 134:1752–1763. https://doi.org/10.1053/j.gastro.2008.02.090
Finn RS, Qin S, Ikeda M et al (2020) Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med 382(20):1894–1905. https://doi.org/10.1056/NEJMoa1915745
Fouad YA, Aanei C (2017) Revisiting the hallmarks of cancer. Am J Cancer Res 7(5):1016–1036
Fukuda R, Sugawara S, Kondo Y (2020) Immune checkpoint inhibitor can reduce HCV-RNA without liver damage. Intern Med 59(18):2245–2248. https://doi.org/10.2169/internalmedicine.3726-19
Furuse J (2008) Sorafenib for the treatment of unresectable hepatocellular carcinoma. Biologics 2(4):779–788
Gibney GT, Weiner LM, Atkins MB (2016) Predictive biomarkers for checkpoint inhibitor-based immunotherapy. Lancet Oncol 17(12):e542–e551. https://doi.org/10.1016/S1470-2045(16)30406-5
Gomaa AI, Waked I (2015) Recent advances in multidisciplinary management of hepatocellular carcinoma. World J Hepatol 7(4):673–687. https://doi.org/10.4254/wjh.v7.i4.673
Gonda K, Shibata M, Kanke Y, Yazawa T, Takenoshita S (2013) Circulating myeloid-derived suppressor cells (MDSC) and correlation to poor prognosis, Th2- polarization, inflammation, and nutritional damages in patients with gastric cancer. J Clin Oncol 31(15 Suppl):3063
Jonsson L, Andreasen N, Kilander L et al (2006) Patient-and proxy-reported utility in Alzheimer disease using the EuroQoL. Alzheimer Dis Assoc Disord 20:49–55. https://doi.org/10.1097/01.wad.0000201851.52707.c9
Kudo M, Finn RS, Qin S et al (2018) Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet 391(10126):1163–1173. https://doi.org/10.1016/S0140-6736(18)30207-1
Kuo HY, Chiang NJ, Chuang CH et al (2020) Impact of immune checkpoint inhibitors with or without a combination of tyrosine kinase inhibitors on organ-specific efficacy and macrovascular invasion in advanced hepatocellular carcinoma. Oncol Res Treat 43(5):211–220. https://doi.org/10.1159/000505933
Lee MS, Ryoo BY, Hsu CH et al (2020) Atezolizumab with or without bevacizumab in unresectable hepatocellular carcinoma (GO30140): an open-label, multicentre, phase 1b study. Lancet Oncol 21(6):808–820. https://doi.org/10.1016/S1470-2045(20)30156-X
Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Vlierberghe HV (2008) Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 359(4):378–390. https://doi.org/10.1056/NEJMoa0708857
Llovet JM, Montal R, Sia D, Finn RS (2018) Molecular therapies and precision medicine for hepatocellular carcinoma. Nat Rev Clin Oncol 15(10):599–616. https://doi.org/10.1038/s41571-018-0073-4
Moses K, Brandau S (2016) Human neutrophils: their role in cancer and relation to myeloid-derived suppressor cells. Semin Immunol 28(2):187–196. https://doi.org/10.1016/j.smim.2016.03.018
Pardee AD, Butterfield LH (2012) Immunotherapy of hepatocellular carcinoma: unique challenges and clinical opportunities. Oncoimmunology 1(1):48–55. https://doi.org/10.4161/onci.1.1.18344
Pasello G, Pavan A, Attili I et al (2020) Real world data in the era of immune checkpoint inhibitors (ICIs): increasing evidence and future applications in lung cancer. Cancer Treat Rev 87:102031. https://doi.org/10.1016/j.ctrv.2020.102031
Reul J, Frisch J, Engeland CE et al (2019) Tumor-specific delivery of immune checkpoint inhibitors by engineered AAV vectors. Front Oncol 9:52. https://doi.org/10.3389/fonc.2019.00052
Rizzo A, Ricci AD, Brandi G (2021a) Immune-based combinations for advanced hepatocellular carcinoma: shaping the direction of first-line therapy. Future Oncol 17(7):755–757. https://doi.org/10.2217/fon-2020-0986
Rizzo A, Ricci AD, Brandi G (2021b) Atezolizumab in advanced hepatocellular carcinoma: good things come to those who wait. Immunotherapy 13(8):637–644. https://doi.org/10.2217/imt-2021-0026
Sacdalan DB, Lucero JA, Sacdalan DL (2018) Prognostic utility of baseline neutrophil-to-lymphocyte ratio in patients receiving immune checkpoint inhibitors: a review and meta-analysis. Onco Targets Ther 11:955–965. https://doi.org/10.2147/OTT.S153290
Salhab M, Canelo R (2011) An overview of evidence-based management of hepatocellular carcinoma: a meta-analysis. J Cancer Res Ther 7(4):463–475. https://doi.org/10.4103/0973-1482.92023
Schachter J, Ribas A, Long GV et al (2017) Pembrolizumab versus ipilimumab for advanced melanoma: final overall survival results of a multicentre, randomised, open-label phase 3 study (KEYNOTE-006). Lancet 390(10105):1853–1862. https://doi.org/10.1016/S0140-6736(17)31601-X
Shao YY, Liu TH, Hsu C et al (2019) Early alpha-foetoprotein response associated with treatment efficacy of immune checkpoint inhibitors for advanced hepatocellular carcinoma. Liver Int 39(11):2184–2189. https://doi.org/10.1111/liv.14210
Shigeta K, Datta M, Hato T et al (2019) Duan programmed death receptor-1 and vascular endothelial growth factor receptor-2 blockade promotes vascular normalization and enhances antitumor immune responses in hepatocellular carcinoma. Hepatology 71:1247–1261. https://doi.org/10.1002/hep.30889
Simmons CE, Brezden-Masley C, McCarthy J, McLeod D, Joy AA (2020) Positive progress: current and evolving role of immune checkpoint inhibitors in metastatic triple-negative breast cancer. Ther Adv Med Oncol 12:1758835920909091. https://doi.org/10.1177/1758835920909091
Tella SH, Mahipal A, Kommalapati A, Jin Z (2019) Evaluating the safety and efficacy of nivolumab in patients with advanced hepatocellular carcinoma: evidence to date. Onco Targets Ther 12:10335–10342. https://doi.org/10.2147/OTT.S214870
Tovoli F, De Lorenzo S, Trevisani F (2020) Immunotherapy with checkpoint inhibitors for hepatocellular carcinoma: where are we now? Vaccines (basel) 8(4):578. https://doi.org/10.3390/vaccines8040578
Vitale A, Trevisani F, Farinati F, Cillo U (2020) Treatment of hepatocellular carcinoma in the precision medicine era: from treatment stage migration to therapeutic hierarchy. Hepatology. https://doi.org/10.1002/hep.31187
Wang Y, Jiang M, Zhu J et al (2020) The safety and efficacy of lenvatinib combined with immune checkpoint inhibitors therapy for advanced hepatocellular carcinoma. Biomed Pharmacother 132:110797. https://doi.org/10.1016/j.biopha.2020.110797
Xu W, Liu K, Chen M et al (2019) Immunotherapy for hepatocellular carcinoma: recent advances and future perspectives. Ther Adv Med Oncol 11:1758835919862692. https://doi.org/10.1177/1758835919862692
Yau T, Kang YK, Kim TY et al (2020) Efficacy and safety of nivolumab plus ipilimumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib: the CheckMate 040 randomized clinical trial. JAMA Oncol 6(11):e204564. https://doi.org/10.1001/jamaoncol.2020.4564
Zeng Z, Yang B, Liao ZY (2020) Current progress and prospect of immune checkpoint inhibitors in hepatocellular carcinoma. Oncol Lett 20(4):45. https://doi.org/10.3892/ol.2020.11909
Zhu AX, Finn RS, Edeline J et al (2018) Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol 19(7):940–952. https://doi.org/10.1016/S1470-2045(18)30351-6
Funding
None.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by QW, NL, BM, and RW. The first draft of the manuscript was written by QW, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, R., Lin, N., Mao, B. et al. The efficacy of immune checkpoint inhibitors in advanced hepatocellular carcinoma: a meta-analysis based on 40 cohorts incorporating 3697 individuals. J Cancer Res Clin Oncol 148, 1195–1210 (2022). https://doi.org/10.1007/s00432-021-03716-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-021-03716-1