Abstract
Background/aims
A proliferation-inducing ligand (APRIL, also known as TNFSF13, CD256) is a member of the tumor necrosis factor (TNF) superfamily and involved in a diverse set of diseases. In this work, we explored the potential associations and underlying mechanism in patients suffered from gastric cancer between the expression of APRIL and H. pylori infection.
Methods
We analyzed APRIL expression levels in 200 GC tissue samples by immunohistochemistry staining. H. pylori infection was detected by modified Giemsa staining. The biological effects of APRIL on human GC cells in vitro and in vivo were tested by CCK-8 assay, colony formation, flow cytometry detection, transwell migration assay, matrigel invasion assay, and tumor xenograft assay in animals.
Results
APRIL reactivity was positively correlated with H. pylori infection in vitro and vivo. It turned out that the decrease of miR-145 expression was dose-dependent and time-dependent on H. pylori infection and in consistent with APRIL expression. MiR-145 significantly attenuated the effect of H. pylori infection on APRIL gene expression in SGC7901 and BGC823 cell lines. Furthermore, APRIL overexpression promoted the proliferation, migration, invasion, and transfer of GC cells and decreased apoptosis, while APRIL knockdown suppressed these effects. We confirmed that APRIL activated the canonical NF-κB pathway through phosphorylation of AKT.
Conclusion
The expression of APRIL, which promoted the proliferation, migration, invasion, viability, and metastasis of GC cells, was upregulated in human H. pylori-infected GC through miR-145. Besides, APRIL-induced gastric tumorigenicity via activating NF-κB pathway. These results may provide a framework for the deeper analysis of APRIL in GC risk and prognosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric cancer (GC) is the fifth most common malignancy and the third leading cause of cancer death worldwide, with an estimated incidence of almost one million new cases in 2012 (952,000 cases, 6.8% of the total) (Ferlay et al. (2015); Torre et al. 2015). The rates are generally about twice as high in men as in women and vary widely across countries (Torre et al. 2015), which could be due to differences in dietary patterns, food storage, the availability of fresh produce, and the prevalence of Helicobacter pylori (H. pylori) infection (Torre et al. 2015; Parkin 2006). Among these risk factors, H. pylori, has been recognized as a type 1 carcinogen for gastric cancer by the International Agency for Research on Cancer (IARC 2006; Wong et al. 2004; McColl 2010; Plummer et al. 2015; Li et al. 2011). Numerous epidemiological data suggest that about 90% of new cases of noncardia GC worldwide are associated with the H. pylori (Plummer et al. 2015). Furthermore, the risk of GC is highest among patients in whom H. pylori infection induces inflammation of both the antral and fundic mucosa and causes mucosal atrophy and intestinal metaplasia (Eid and Moss 2002). However, the exact molecular mechanism for how H. pylori infection causes GC remains unclear.
A proliferation-inducing ligand (APRIL, also known as TNFSF13, CD256) is a member of the tumor necrosis factor (TNF) superfamily and involved in the development of several human diseases, including autoimmunity and cancer (Schwaller et al. 2015; Litinskiy et al. 2002; Castigli et al. 2004; Dillon et al. 2006). Human APRIL gene is located on chromosome 17p13 and contains 6 exons transcribed as 3 alternatively spliced mRNA of 1.8, 2.1 and 2.4 kb, encoding a 250-amino-acid protein (Dillon et al. 2006).
MicroRNAs (miRNAs) represent a class of small non-coding endogenous RNAs with a length of 18–22 nucleotides that predominantly bind to 3′untranslated region of mRNAs, resulting in negative regulation of protein translation and mRNA degradation at the post-transcriptional level (Lin et al. 2017; Kloosterman and Plasterk 2006; Zhao and Srivastava 2007). Recently, it has been showed that miRNAs play a critical role in the the initiation and progression of carcinomas (Xi 2013). MiR-145 was proved to be underexpressed in breast (2.5-fold) (Sachdeva and Mo 2010), colon (5-fold) (Schepeler et al. 2008), bladder (20-fold) (Ichimi et al. 2009), and gastric cancer (32.35-fold) (Gao et al. 2013).
In our previous studies, we demonstrated that APRIL was significantly upregulated in GC and high levels of APRIL was correlated with resistance to cisplatin, highlighting that APRIL might play an important role in GC development (Ni et al. 2012; Zhi et al. 2015). In this work, we were interested in and determined to explore the potential associations and underlying mechanism in patients suffered from gastric cancer between the expression of APRIL and H. pylori infection. Our findings will expand our understanding of H. pylori-associated GC’s pathogenesis and provide references of research and application for APRIL-targeting drugs.
Materials and methods
Patients
A total of 200 patients who had been diagnosed with GC at the First Affiliated Hospital of Nanjing Medical University between Sep 2013 and Jan 2015 were invited to participate in this work, including 124 (62%) males and 76 (38%) females, with a median age of 67 years (range = 35–86 years). All subjects were of Chinese origin. The diagnosis was based on the criteria of American Joint Committee on Cancer. Clinicopathologic characteristics of these patients are listed in Table 1. Gastric cancer tissue samples were used for tissue microarray construction, as described by Kononen et al. (Kononen et al. 1998). Each case signed the informed consent approved by the Ethical Committee of the Affiliated Hospital of Nanjing Medical University. The acquisition of tissue specimens and study protocol were performed in strict accordance with the regulations of the Nanjing Medical University Institutional Review Board (Table 1).
Cells and cell culture
The human gastric cancer AGS (ATCC, USA) and SGC7901 (CBTCCCAS, Shanghai, China) cell lines were cultured in RMPI-1640 (Life Technologies, Gibco BRL, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FBS; Invitrogen), penicillin/streptomycin (1:100; Sigma, St.Louis, MO), and 4 mM glutamine (Life Technologies, Gibco BRL) in a humidified atmosphere that contained 5% CO2 at 37 °C.
Quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA was extracted from frozen tissues and cell lines using Trizol Reagent (Invitrogen, USA), according to the manufacturer’s protocol, and reverse transcribed into cDNA using Primescript RT Reagent (Takara, Japan). Real-time PCR was performed using a 7500 Real-time PCR System (Applied Biosystems) with SYBR Premix Ex Taq Kit (Takara). The following primers were used: APRIL, forward: 5′-ATTAACGCCACCTCCAAG-3′, reverse: 5′-CAGCAGATAAACTCCAGCAT-3′; β-actin, forward: 5′-AGAGCCTCGCCTTTGCCGATCC-3′, reverse: 5′-CTGGGCCTCGTCGCCCACATA-3′; miR-145, forward: 5′-ATCGTCCAGTTTTCCCAGG-3′, reverse: 5′-CGCCTCCACACACTCACC-3′; U6 primers, forward: 5′-ATTGGAACGATACAGAGAAGATT-3′, reverse: 5′-GGAACGCTTCACGAATTTG-3′. TaqMan probes (Genepharma, Shanghai, China) were used to quantify miR-145, and miR-145 expression levels were normalized to snRNA U6. All procedures were performed in triplicate.
Immunoblotting
Cell extracts were collected in a lysis buffer (50 mM Tris–HCl [pH 7.4], 150 mM NaCl, 1% Triton X-100, 0.1% SDS, 1 mM EDTA and protease inhibitor cocktail). The cellular protein was size-fractionated by SDS–polyacrylamide gel electrophoresis and transferred to PVDF membranes (Bio-Rad Laboratories). After blocking with PBS containing 5% BSA, the membrane was incubated with the appropriate primary antibody at 4 °C overnight, followed by incubation with HRP-conjugated anti-mouse or antirabbit IgG at room temperature for 2 h. The protein bands were detected using an enhanced chemiluminescence (ECL) detection system following the manufacturer’s instructions. The following primary antibodies were used: anti-APRIL, anti-MDM2 (Abcam), anti-Bcl-2, anti-Bcl-xl, anti-Bax, anti-p53, anti-pAKT, anti-AKT, anti-IκBα, anti-p65, anti-GAPDH and anti-H3 (Cell signaling technology). All procedures were performed in triplicate.
H. pylori culture and co-culture with GC cells
Experiments were performed with a cytotoxic (CagA+ and VacA+) reference strain of H. pylori 26695 (ATCC). H. pylori bacteria were grown under microaerophilic conditions on Columbia agar plates (bioMe´rieux, Marcy l’Etoile, France) that contained 100 U/ml H. pylori selective supplement (Oxoid, Basingstoke, United Kingdom) at 37uC using an anaerobic chamber (BBL Campy Pouch System, Becton Dickinson Microbiology Systems) for 48–72 h, harvested, and resuspended in antibiotic-free RPMI 1640 medium (Life Technologies, Gibco BRL) supplemented with 2% fetal calf serum (FCS). The bacterial densities were adjusted by optical density (OD) measurement at 660 nm, in which 1 OD660 = 16,108 colony-forming units (CFU)/ ml. H. pylori was then incubated with BGC823 or SGC7901 cells at a cell-to-bacterium ratio of 1:50 or 1:100 for up to 12 or 24 h in the medium.
Modified giemsa staining of H. pylori in gastric mucous tissues
Modified Giemsa staining was used to detect H. pylori in gastric mucous tissues according to a previous report (Xi 2013).
Immunohistochemistry and assessment
All specimens were fixed in 4% formalin and embedded in paraffin. MaxVisionTM techniques (MaixinBio, China) were used for IHC analysis, according to the manufacturer’s instructions. After blocking the endogenous peroxides and proteins, 4 μm sections were incubated overnight at 4 °C with diluted primary antibodies specific for APRIL (Abcam). Next, the slides were incubated with an HRP-Polymer-conjugated secondary antibody at 37 °C for 1 h. The slides were then stained with a 3,3-diaminobenzidine solution for 3 min and counterstained with hematoxylin. Tumor slides were examined in a blinded manner. Three fields were selected for examination, and the percentage of positive tumor cells and cell-staining intensity was determined.
Establishment of C57BL/6 mice model colonized by H. pylori
Twelve C57BL/6 mice (Vitalriver, Nanjing, China) were randomly divided into two groups: H. pylori treatment group and control group, each containing six mice. Mice in the H. pylori treatment group were orally inoculated with H. pylori 26,695 resuspended in 2 ml phosphate-buffered saline (PBS) that contained 5 × 108 CFU/ml. H. pylori treatment was performed three times at the first week. Before inoculation, these mice were fasted for 24 h and slowly pretreated with 2 ml of 5% NaHCO3 intragastrically 30 min before inoculation. The animals in the control group were administered PBS accordingly. Three months after inoculation, gastric mucous tissues in the antrum were harvested for further assay, including pathological diagnosis with hematoxylin–eosin staining using the Visual Analog Scale of the Updated Sydney System (Kang et al. 2012), Warthin–Starry stains (Jonkers et al. 1997) with cultivation and rapid urease test for H. pylori detection, and Western blot to assess APRIL expression. Care of experimental animals was in accordance with institutional animal care and use committee guidelines.
Construction of recombinant plasmids and lentivirus production
The full-length ORF of APRIL (753 bp, NM_003808.3) was amplified from cDNA of GC cells SGC7901. The primers were as follows: forward, 5′-AGAGAATTCATGCCAGCCTCATCTCCTTT-3′, reverse, 5′-AGAGGATCCTCACAGTTTCACAAACCCCA-3′. The PCR product was inserted into the expression vector pcDNA3.1/myc-His (−) B (Invitrogen). To construct the lentivirus production containing APRIL, the ORF of APRIL was subcloned into the pLenti-CMV-GFP vector (GenePharma). The synthesized DNA fragments encoding the short hairpin RNA (shRNA) used for the knockdown of endogenous APRIL were inserted into the pGPU6/GFP/Neo vector (GenePharma). The sequences of the shRNAs were as follows: APRIL-shRNA, 5′-GCTGGAGTTTATCTGCTGTATCTCGAGATACAGCAGATAAACTCCAGC-3′, shRNA-NC,5′-TTCTCCGAACGTGTCACGTCTCGAGACGTGACACGTTCGGAGAA-3′. All plasmids were verified by sequencing.
Cell proliferation and assay
Cells ( 2000 cells/well) were seeded into 96-well plates in 100 μl complete medium. The Cell Counting Kit-8 (Dojindo Labs) was used to measure cell viability according to the manufacturer’s instructions. The plates were incubated for 7 days. The number of viable cells was assessed by measurement of the absorbance at 450 nm.
Colony formation
GC cells transfected with the vectors containing APRIL (or the empty vector as a control) were cultured in 6-well plates (3000 cells/well). The plates were incubated at 37 °C in a humidified atmosphere of 5% CO2 for 2 weeks. Colonies composed of 50 or more cells were scored as survivors. Proliferating clonies were stained with crystal violet and counted. All procedures were performed in triplicate.
Apoptosis analysis by flow cytometry
Cells were seeded in 6-well plates at a density of 5 × 105 cells/well in RPMI 1640 medium supplemented with 10% FBS for 24 h. Then, the cells were stained with a solution containing 5 μl/ml Alexa Flour 647 (AF-647) and 10 μl/ml 7-amino-actinomycin D (7-AAD) in the dark for 30 min and analyzed using a FACSCalibur flow cytometer (Becton Dickinson) with the Cell Quest software.
GC cells migration and invasion assays
Cell migration and invasion assays were performed using a chamber 6.5 mm in diameter with an 8 μm pore size (Corning, USA). The upper chambers were seeded with 1 × 104 GC cells. Subsequently, the different groups of conditioned medium were added to the lower chamber. For the invasion assay, the top chamber was coated with 100 μl of 1 mg/ml Matrigel (BD, USA). Cells were incubated at 37 °C for 48 h, and then cells in the upper chamber were removed using cotton swabs. Cells migrating into or invading the bottom of the membrane were stained with 0.1% crystal violet for 20 min at 37 °C, followed by washing with PBS. Four random fields from each membrane were photographed and counted for statistical analysis.
Tumorigenicity in vivo
Nude mice (BALB/c nude mice) were purchased from the Department of Laboratory Animal Centre of Nanjing Medical University. SGC7901 cells (2 × 106 cells in 100 μl of PBS) and AGS cells (1 × 107 cells in 200 μl of PBS) with differential APRIL expression were subcutaneously injected into 4-week-old male nude mice. Bidimensional tumor measurements were obtained with vernier calipers every 4 days, and the mice were euthanised after about 3 weeks. The volume of the implanted tumor was calculated using the formula: tumor volume = length × width2 × 0.5. Care of experimental animals was in accordance with institutional animal care and use committee guidelines.
Statistical analysis
Data are expressed as mean ± standard deviation (SD) values. Clinicopathological findings were compared using unpaired t-tests or Pearson × 2 tests. Analysis of variance (ANOVA) was used to compare the control and treated groups. p values < 0.05 were considered to be statistically significant. Statistical analysis was performed using the SPSS software (Version 15.0).
Results
APRIL expression in GC tissues was associated with H. pylori infection
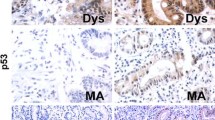
We analyzed APRIL expression levels in 200 GC patients by immunohistochemistry. H. pylori infection was performed by modified Giemsa staining. As we published previously, APRIL was expressed in tumor cells protein, while normal mucosal cells rarely expressed APRIL (Kloosterman and Plasterk 2006). Moreover, APRIL reactivity in gastric cancer tissues was obviously correlated with H. pylori infection (p = 0.001; Table 2), implying that APRIL was in relation to H. pylori infection-related gastric diseases. Two typical cases were validated in Fig. 1.
APRIL expression and H. pylori observation in human GC tissue by immunohistochemistry and Giemsa staining. a, b Representative examples of APRIL immunohistochemistry in H. pylori (−) and H. pylori (+) gastric tumor tissue arrays (magnification: 50×). High positive (+++) APRIL immunoreactivity was identified (d, magnification: 200×) in the carcinoma with H. pylori detection (f, yellow arrow, magnification: 400×). Low positive (–) APRIL reactivity was detected (c, magnification: 200×) in the carcinoma without H. pylori detection (e, magnification: 400×)
APRIL was upregulated in human H. pylori infected GC in vitro and in vivo
To demonstrate whether APRIL expression is correlated with H. pylori, two SGC7901 and BGC823 gastric cancer cell lines were treated with H. pylori at three different MOIs (Multiplicity of Infection, MOI) of 0, 50, and 100 and APRIL expression were recorded at different time point. As shown in Fig. 2b and d, H. pylori infection led to a bacterial popolation-dependent increase in APRIL protein and mRNA levels. In SGC7901 cells, both protein and mRNA levels were increased by approximated fourfold and sevenfold at MOI 50 and 100. While in BGC823 cells, the rise was fivefold and 10-fold. We also demonstrated the levels were increased four- to sixfold at 12 h and seven- to ninefold at 24 h in two kinds of cell lines (Fig. 2a and c).
The expression of APRIL and H. pylori infection in vitro and in vivo experiments. We co-cultured SGC7901 and BGC823 gastric cancer cells with H. pylori and b-actin. Western blot and RT-PCR were used to analyze APRIL protein and mRNA expression at different time points (untreated, 12 and 24 h) (a, c) and with different concentration of H. pylori (untreated, 1:50 and 1:100) (b, d). The data are expressed as the mean 6 SE of three independent experiments. p < 0.05 was considered significant. e and f represented gastric tissue sections from C57BL/6 mice with hematoxylin–eosin staining. Yellow arrow showed sub-mucosal bleeding. g and h represented the tissue by immunohistochemistry to show APRIL expression. i and j represented the tissue with Warthin–Starry staining to detect H. pylori. Blue arrow showed H. pylori detection. Data are mean ± standard error (*p < 0.05)
We further performed in vivo analysis to examine the correlation between APRIL expression and H. pylori infection. C57BL/6 mice were divided into H. pylori infection group (treatment group, Fig. 2j) and none H. pylori infection group (control group, Fig. 2i). In treatment group, we found sub-mucosal bleeding (Fig. 2f) and strong positive APRIL immunoreactivity (Fig. 2h) in gastric tissue of mice. The result was further confirmed through Western blot (Fig. 2k). Nothing was discovered in control group (Fig. 2e and g).
H. pylori upregulated APRIL expression through miR-145
To determine whether miR-145 suppressed tumor growth in H. pylori-related GC, we used real-time PCR to analyze miR-145 levels in H. pylori (+) GC tissues and H. pylori (−) GC tissues. As shown in Fig. 3a, average miR-145 expression levels were significantly downregulated in the H. pylori (+) GC tissues with comparison to control specimens. Then we examined miR-145 expression with three different MOIs and at three different time points of H. pylori in SCG7901 and BGC823 cells. It turned out that the decrease of miR-145 expression was dose-dependent (Fig. 3b and d) and time-dependent (Fig. 3c and e) on H. pylori infection and in consistent with APRIL expression. These data support the hypothesis that H. pylori infection decreased the level of miR-145 in GC.
The expression of miR-145 in H. pylori (+) GC tissues and H. pylori (−) GC tissues. APRIL is the direct target of miR-145 in H. pylori-related GC. a Relative expression of miR-145 in H. pylori positive and negative tissues. miR-145 expression levels were detected by real-time PCR. b, d Relative expression of miR-145 with three different MOIs of H.pylori in SCG7901 and BGC823 cells. c, e Relative expression of miR-145 at different time points in SCG7901 and BGC823 cells. f, g miR-145 abated the level of APRIL in SGC7901 and BGC823 cells.. The expression of APRIL was analyzed by Western blot and normalized to β-actin. Data are mean ± standard error (*p < 0.05)
In our previous study, we confirmed that miR-145 was significantly downregulated in GC and GC cell lines and the level of miR-145 was inversely correlated with APRIL mRNA (Zhi et al. 2015). In this research, we performed Western blot to further show that miR-145 significantly attenuated the effect of H. pylori infection on APRIL gene expression in SGC7901 and BGC823 cell lines (Fig. 3f, g).
APRIL promoted the proliferation of GC cells and decreased apoptosis in vitro
To analyze the role of APRIL in cell proliferation and viability, we chose two human GC cell lines (SGC7901 and AGS) that one with APRIL knockdown (SGC7901-shAPRIL) and one APRIL overexpression (AGS-APRIL) to perform the following studies. Western blot analysis revealed that APRIL expression was successfully modulated in these cell lines (Fig. 4a, b). A CCK8 assay with cells culture for 1, 2, 3, 4, 5, 6, and 7 days identified that knockdown of APRIL remarkably inhibited cell growth of SGC7901 cells (Fig. 4c) and overexpression of APRIL remarkably promoted the proliferation of AGS cells (Fig. 4d). The conclusion was confirmed by a colony formation assay (Fig. 4e, f).
APRIL contributed to proliferation and prevented apoptosis of GC cells. a, b APRIL gene expression was displayed by Western blot in SGC7901-NC, SGC7901-shAPRIL, AGS-NC, and AGS-APRIL cells. c, d The proliferation of SGC7901-NC, SGC7901-shAPRIL, AGS-NC, and AGS-APRIL cells were determined using a CCK8 assay and presented as a growth curve. e, f The proliferation of SGC7901-NC, SGC7901-shAPRIL, AGS-NC, and AGS-APRIL cells were determined by a plate clone assay. Representative images and histograms about numbers of colonies were shown. g Representative scatter diagrams descripting apoptosis in SGC7901-NC, SGC7901-shAPRIL, AGS-NC, and AGS-APRIL cells. Apoptotic cells were calculated by flow cytometry and presented by a histogram. Data are mean ± standard error (*p < 0.05)
Next, we detected the viability of APRIL-inhibited GC cells via flow cytometry. As shown in Fig. 4g, the proportion of apoptotic cells was significantly increased in APRIL knockdown GC cell line and decreased in APRIL overexpression GC cell line.
APRIL induces migration, invasion, and metastatis of GC cells
To assess the effect of APRIL on GC cells, we used a transwell migration and matrigel invasion assay. As compared with control cells, knockdown of APRIL significantly inhibited SGC7901 cells migration and invasiveness. By contrast, the AGS cells migration and invasiveness were significantly boosted by overexpression of APRIL (Fig. 5a, b). In mice infused with luciferase, higher concentrations of GC cells were seen in lungs. As shown in Fig. 5c and d, signal intensity luciferase expression in pulmonary tissues was in consistent with APRIL gene expression. Pulmonary specimens with hematoxylin–eosin staining could confirm the GC cells metastasis.
APRIL positively regulated the migration, invasion and transfer of GC cells. a, b Transwell migration and Matrigel invasion assays with SGC7901 and AGS cells were performed in each group. Representative images were shown. c, d induction of luciferase expression in the lung and represented pulmonary tissue sections from nude mice with hematoxylin–eosin staining. e Photographs of tumors derived from the different groups of nude mice. f, g The graph is representative of tumor growth 21 days after inoculation. Tumor volume and the weight were calculated, and all date are shown as mean ± SD. Data are mean ± standard error (*p < 0.05)
We also used in vivo experiments to prove the positive effect of APRIL on tumorigenicity. Transfected cells were injected into the flanks of nude mice to form ectopic tumors. After 21 days, we observed impaired tumor growth in the SGC7901-NC group than in the APRIL-knockdown group (SGC-7901 cells). Similar phenomenon was observed in AGS cells, that tumor in NC group grow slower than in APRIL-overexpression group (Fig. 5e, f). Average tumor weight was next calculated (Fig. 5g).
APRIL-induced gastric tumorigenicity via activating NF-κB pathway
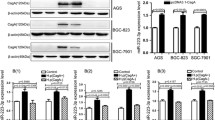
The above results elucidated that APRIL is important in the development of GC. However, the underlying mechanism is not clear. By Western Blot, we found and verified that APRIL could induce gastric tumorigenicity via NF-κB pathway. Our study showed that APRIL significantly promoted the phosphorylation of AKT and upregulated total p65 (Fig. 6a). Besides, we confirmed that APRIL overexpression induced nuclear translocation of p65, and then regulated the expression of genes in the nucleus (Fig. 6b). We confirmed that LY294002 attenuated the expression of pAKT and nuclear translocation of p65 (Fig. 6c). NF-κB-regulated gene products, such as Bcl-2, Bcl-xl, MDM2, were positively correlated with APRIL overexpression. Whereas, Bax、p53 is negative correlated with APRIL overexpression. Bcl-2/Bax and Bcl-xl/Bax were positively correlated with APRIL (p < 0.05) (Fig. 6d, e).
Activation of NF-κB pathway in GC cells. a Expression levels of pAKT, AKT, IκBα and p65 were analyzed by Western blotting. b, c p65 extracted from cytoplasma and nuclear was detected by Western blotting. d Expression of Bcl-2, Bcl-xl, Bax, p53 and MDM2 were analyzed by Western blotting. e The density of each band was measured and ratios of Bcl-2/Bax and Bcl-xl/Bax were calculated. Data are mean ± standard error (*p < 0.05)
Discussion
Gastric cancer is one of the leading causes of cancer-related deaths with a high incidence (Torre et al. 2015; Siegel et al. 2013; Bertuccio et al. 2009), particularly in East Asia (Crew and Neugut 2006). Most of patients were H. pylori related (Wroblewski et al. 2010; Torres et al. 2013). Because of the lack of specific early symptoms and biologic tumor markers, the early diagnosis of H. pylori-related gastric cancer is typically hard, resulting in the static and inferior survival rate (Davis and Sano 2001; Nishiyama and Eguchi 2009; Correa 2004). APRIL is a new ligand of tumor-necrosis factor (TNF) family (Dillon et al. 2006). To our knowledge, there are currently a mass of citations in the literature related to APRIL. However, the biology of APRIL involved in H. pylori-related gastric cancer has received less attention.
In this study, some significant relationship between APRIL and H. pylori-related gastric cancer was found, which influenced the risk and prognosis of the GC development. Human H. pylori-infected GC upregulated the expression of APRIL, which induced the proliferation, migration, invasion and transfer of GC cells and decreased apoptosis. Previous studies have shown high levels of APRIL mRNA are detectable in transformed cell lines, and human cancers of colon, thyroid and lymphoid tissues in vivo (Hahne et al. 1998; Kern et al. 2015; Moreaux et al. 2005; Novak et al. 2002). Besides, nearly half of the malignant glioblastoma cell lines that tested overexpressed APRIL (Deshayes et al. 2004) and analysis of the human EST database indicates pancreatic, ovarian, and uterine adenocarcinomas also express APRIL (Kalled et al. 2005). Guihua Wang et al. (Wang et al. 2013) further reported that knockdown of APRIL in colorectal cancer cells in vitro and in BALB/c nude mice in vivo inhibited malignancy, tumor growth and metastasis in the liver. These significant findings, as well as our outcomes, arrived at the same conclusion.
MicroRNAs (miRNAs, miRs) are endogenous short non-coding RNA molecules, with similar length to 17–25 nucleotides, which have been frequently observed to be dysregulated in various types of human cancer (Xi 2013; Bartel 2004; Calin and Croce 2006; Zhang et al. 2007). Since miRNAs were discovered, many studies have confirmed that they could promote the expression of oncogenes and inhibit the expression of tumor suppressor genes (Zhang et al. 2007). MiR-145 is an ideal cancer biomarker and therapeutic monitoring indicator (Zhang et al. 2007). MiR‑145 was identified as a tumor‑suppressive miRNA, which is downregulated in several types of cancer, including prostate, bladder, breast, lung, and ovarian cancer (Wu et al. 2013; Cho et al. 2009). Our study further indicated that miR-145 suppressed tumor growth in H. pylori-related GC. We also showed that APRIL activated the canonical NF-κB pathway through phosphorylation of AKT. As far as we know, the association was not documented in gastric cancer field.
References
Bartel DP (2004) MicroRNAs_genomics, biogenesis, mechanism, and function. Cell 116:281–297
Bertuccio P, Chatenoud L, Levi F, Praud D, Ferlay J, Negri E, Malvezzi M, La Vecchia C (2009) Recent patterns in gastric cancer: a global overview. Int J Cancer 125:666–673
Calin GA, Croce CM (2006) MicroRNA signatures in human cancers. Nat Rev Cancer 6:857–866
Castigli E, Scott S, Dedeoglu F, Bryce P, Jabara H, Bhan AK, Mizoguchi E, Geha RS (2004) Impaired IgA class switching in APRIL-deficient mice. ProcNatlAcadSci U S A 101:3903–3908
Cho WC, Chow AS, Au JS (2009) Restoration of tumour suppressor hsa-miR-145 inhibits cancer cell growth in lung adenocarcinoma patients with epidermal growth factor receptor mutation. Eur J Cancer 45:2196–2206
Correa P (2004) Is gastric cancer preventable? Gut 53:1217–1219
Crew KD, Neugut AI (2006) Epidemiology of gastric cancer. World J Gastroenterol 12:354–362
Davis PA, Sano T (2001) The difference in gastric cancer between Japan, USA and Europe_What are the facts_What are the suggestions. Crit Rev OncolHematol 40:77–94
Deshayes F, Lapree G, Portier A, Richard Y, Pencalet P, Mahieu-Caputo D, Horellou P, Tsapis A (2004) Abnormal production of the TNF-homologue APRIL increases the proliferation of human malignant glioblastoma cell lines via a specific receptor. Oncogene 23:3005–3012
Dillon SR, Gross JA, Ansell SM, Novak AJ (2006) An APRIL to remember: novel TNF ligands as therapeutic targets. Nat Rev Drug Discov 5:235–246
Eid R, Moss SF (2002) Helicobacter pylori infection and the development of gastric cancer. N Engl J Med 346:65–67
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F (2015) Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136:E359–E386
Gao P, Xing A-Y, Zhou G-Y, Zhang T-G, Zhang J-P, Gao C, Li H, Shi D-B (2013) The molecular mechanism of microRNA-145 to suppress invasion–metastasis cascade in gastric cancer. Oncogene 32(4):491–501
Hahne M, Kataoka T, Schröter M, Hofmann K, Irmler M, Bodmer JL, Schneider P, Bodmer JL, Schneider P, Bornand T, Holler N, French LE, Sordat B, Rimoldi D, Tschopp J (1998) APRIL, a new ligand of the tumor necrosis factor family, stimulates tumor cell growth. J Exp Med 188:1185–1190
IARC Working Group (2006) Schistosomes, liver flukes and Helicobacter pylori IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Lyon, 7–14 June 1994. IARC MonogrEvalCarcinog Risks Hum 61:1–241
Ichimi T, Enokida H, Okuno Y, Kunimoto R, Chiyomaru T, Kawamoto K, Kawahara K, Toki K, Kawakami K, Nishiyama K, Tsujimoto G, Nakagawa M, Seki N (2009) Identification of novel microRNA targets based on microRNA signatures in bladder cancer. Int J Cancer 125(2):345–352
Jonkers DSE, de Bruine A, Arends JW, Stockbrügger R (1997) Evaluation of immunohistochemistry for the detection of Helicobacter pylori in gastric mucosal biopsies. J Infect 35:149–154
Kalled SL, Ambrose C, Hsu YM (2005) The biochemistry and biology of BAFF, APRIL and their receptors. CurrDirAutoimmun 8:206–242
Kloosterman WP, Plasterk RHA (2006) The diverse functions of microRNAs in animal development and disease. Dev Cell 11(4):441–450
Kang SY, Han JH, Ahn MS, Lee HW, Jeong SH, Park JS, Cho YK, Han SU, Kim YB, Kim JH, Sheen SS, Lim HY, Choi JH (2012) Helicobacter pylori infection as an independent prognostic factor for locally advanced gastric cancer patients treated with adjuvant chemotherapy after curative resection. Int J Cancer 130:948–958
Kern CCJ, Billard C, Tang R, Rouillard D, Stenou V, Defrance T, Ajchenbaum-Cymbalista F, Slimonin PY, Feldblum S, Kolb JP (2015) Involvement of BAFF andAPRILin the resistance to apoptosis of B-CLL through an autocrine pathway. Blood 103:679–688
Kononen JBL, Kallioniemi A, Bärlund M, Schraml P, Leighton S, Torhorst J, Mihatsch MJ, Sauter G, Kallioniemi OP (1998) Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med 4:844–847
Li Z, Chen J, Chan KW, Qiao L, Wong BC (2011) A possible role of cIAP2 in Helicobacter pylori-associated gastric cancer. CancerLett 313:192–200
Lin DC, Lin JB, Chen Z, Chen R, Wan CY, Lin SW, Ruan QS, Li HY, Wu SY (2017) Independent and combined effects of environmental factors and miR-126, miR-143 and miR-145 on the risk of coronary heart disease. J Geriatri Cardiol 14:688–695
Litinskiy MB, Nardelli B, Hilbert DM, He B, Schaffer A, Casali P, Cerutti A (2002) DCs induce CD40-independent immunoglobulin class switching through BLyS and APRIL. Nat Immunol 3:822–829
McColl KE (2010) Clinical practice. Helicobacter pylori infection. N Engl J Med 362:1597–1604
Moreaux J, Cremer FW, Reme T, Raab M, Mahtouk K, Kaukel P, Pantesco V, De Vos J, Jourdan E, Jauch A, Legouffe E, Moos M, Fiol G, Goldschmidt H, Rossi JF, Hose D, Klein B (2005) The level of TACI gene expression in myeloma cells is associated with a signature of microenvironment dependence versus a plasmablastic signature. Blood 106:1021–1030
Ni SZ, Cao HY, Chen Z, Zhu Y, Xu ZK (2012) siRNA interference with a proliferation-inducing ligand gene in the Sgr-7901 gastric carcinoma cell line. Asian Pac J Cancer Prev 13:1511–1514
Nishiyama M, Eguchi H (2009) Pharmacokinetics and pharmacogenomics in gastric cancer chemotherapy. Adv Drug Deliv Rev 61:402–407
Novak AJ, Bram RJ, Kay NE, Jelinek DF (2002) Aberrant expression of B lymphocyte stimulator by B chronic lymphocytic leukemia cells: a mechanism for survival. Blood 100:2973–2979
Parkin DM (2006) The global health burden of infection-associated cancers in the year 2002. Int J Cancer 118:3030–3044
Plummer M, Franceschi S, Vignat J, Forman D, de Martel C (2015) Global burden of gastric cancer attributable to Helicobacter pylori. Int J Cancer 136:487–490
Sachdeva M, Mo Y-Y (2010) MicroRNA-145 Suppresses cell invasion and metastasis by directly targeting Mucin 1. Cancer Res 70 (1):378–387
Schepeler T, Reinert JT, Ostenfeld MS, Christensen LL, Silahtaroglu AN, Dyrskjøt L, Wiuf C, Sørensen FJ, Kruhøffer M, Laurberg S, Kauppinen S, Ørntoft TF, Andersen CL (2008) Diagnostic and prognostic microRNAs in stage II colon cancer. Cancer Res 68(15):6416–6424
Schwaller JSP, Mhawech-Fauceglia P, McKee T, Myit S, Matthes T, Tschopp J, Donze O, Le Gal FA, Huard B (2015) Neutrophil-derived APRIL concentrated in tumor lesions by proteoglycans correlates with human B cell lymphoma aggressiveness. Blood 109:331–338
Siegel R, Naishadham D, Jemal A (2013) Cancer statistics, 2013. CA Cancer J Clin 63:11–30
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A (2015) Global cancer statistics, 2012. CA Cancer J Clin 65:87–108
Torres J, Correa P, Ferreccio C, Hernandez-Suarez G, Herrero R, Cavazza-Porro M, Dominguez R, Morgan D (2013) Gastric cancer incidence and mortality is associated with altitude in the mountainous regions of Pacific Latin America. Cancer Causes Control 24:249–256
Wang G, Wang F, Ding W, Wang J, Jing R, Li H, Wang X, Wang Y, Ju S, Wang H (2013) APRIL induces tumorigenesis and metastasis of colorectal cancer cells via activation of the PI3K/Akt pathway. PLoS ONE 8:e55298
Wong BCLS, Wong WM, Chen JS, Zheng TT, Feng RE, Lai KC, Hu WH, Yuen ST, Leung SY, Fong DY, Ho J, Ching CK, Chen JS, China Gastric Cancer Study Group (2004) Helicobacter pylori eradication to prevent gastric cancer in a high-risk region of China. JAMA 291:187–194
Wroblewski LE, Peek RM Jr, Wilson KT (2010) Helicobacter pylori and gastric cancer: factors that modulate disease risk. ClinMicrobiol Rev 23:713–739
Wu H, Xiao Z, Wang K, Liu W, Hao Q (2013) MiR-145 is downregulated in human ovarian cancer and modulates cell growth and invasion by targeting p70S6K1 and MUC1. BiochemBiophys Res Commun 441:693–700
Xi JJ (2013) MicroRNAs in cancer. Cancer Treat Res 158:119–137
Zhang B, Pan X, Cobb GP, Anderson TA (2007) microRNAs as oncogenes and tumor suppressors. Dev Biol 302:1–12
Zhao Y, Srivastava D (2007) A developmental view of microRNA function. Trends Biochem Sci 32(4):189–197
Zhi X, Tao J, Xiang G, Cao H, Liu Z, Yang K, Lv C, Ni S (2015) APRIL induces cisplatin resistance in gastric cancer cells via activation of the NF-kappaB pathway. Cell PhysiolBiochem 35:571–585
Acknowledgements
This work was partially supported by the National Natural Science Foundation of China (81272712, 30901421), the National Natural Science Foundation Project of International Cooperation(NSFC-NIH, 812111519), the Program for Development of Innovative Research Team in the First Affiliated Hospital of NJMU, the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD, JX10231801), and the translational research of early diagnosis and comprehensive treatment in pancreatic cancer (The research Special Fund For public welfare industry of health, 201202007).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declared that they have no financial or non-financial competing interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, Q., Ni, Y., Zhi, X. et al. Involvement of APRIL in Helicobacter pylori-related gastric cancer. J Cancer Res Clin Oncol 147, 1685–1697 (2021). https://doi.org/10.1007/s00432-021-03574-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-021-03574-x