Abstract
Background
The safety and efficacy of transbronchial microwave ablation (TMA) therapy in patients with malignant central airway obstruction (CAO) with respiratory failure remains unclear.
Methods
A total of 38 patients with advanced non-small cell lung cancer (NSCLC) or lung metastases with malignant endoluminal obstruction received TMA therapy under moderate sedation and high fractions of inspired oxygen (FiO2). The success rate of airway patency restoration, complication rate, and overall survival time (OS) from the initiation of TMA therapy were compared in the following two groups of patients with malignant CAO patients: the group with respiratory failure (PaO2/FiO2 ≤ 300) (RF group, n = 10) and the group without respiratory failure (PaO2/FiO2 > 300) (non-RF group, n = 28) at the time of the TMA therapy.
Results
Both the RF group and non-RF group received a median of two sessions of TMA. There was no significant difference in the percentage of patients who showed restored airway patency after the first session of TMA (90% vs. 96%), in the complication rate of TMA therapy (10% vs. 11%), or in the OS (7.1 months vs. 9.1 months) between the RF group and the non-RF group. Multivariate analysis identified no significant association between TMA therapy and the risk of death in malignant CAO patients with respiratory failure (p = 0.196).
Conclusion
TMA therapy under moderate sedation was well tolerated and effective in patients with malignant CAO, including those with respiratory failure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Malignant central airway obstruction (CAO) may occur not only in patients with advanced non-small cell lung cancer (NSCLC), but also in those with lung metastases from primary malignant tumors at other sites, and could become life-threatening, potentially causing death from suffocation, if left untreated. A number of reports suggested that some transbronchial interventions (TBIs) can relieve respiratory distress and improve the quality of life in patients with malignant CAO (Ernst et al. 2004; Ost et al. 2015b; Mudambi et al. 2017). Ablative therapies, photodynamic therapy and cryotherapy have been employed for patients with endoluminal obstruction, and stent placement has been undertaken in patients with extraluminal obstruction. Multiple modalities may be required in patients with mixed-type obstruction (Mudambi et al. 2017).
Laser ablation, electrocautery and argon plasma coagulation are commonly used ablative procedures for the treatment of endoluminal obstruction and are performed under general anesthesia using either a flexible or rigid bronchoscope. During ablative therapies, continuous suction of smoke and fraction of inspired oxygen (FiO2) of < 40% are recommended in order to minimize the risk of accidental penetrating injury and airway fire (Bolliger and Mathur 2002). Ablative therapies should be undertaken with great caution, in the operating room, for malignant CAO patients with respiratory failure who require high FiO2 values.
Microwave ablation is a thermal therapy that heats up the tissue by dielectric hysteresis. When an electrical magnetic field at a frequency of 2450 MHz is created around the monopolar antenna (electrode), polar molecules (mainly H2O) in the tissue are forced to continuously realign with the oscillating electric field, resulting in frictional heat. The tissue is coagulated by the frictional heat, but the tissue temperature rarely exceeds 100 ℃ (the boiling point of water) (Lubner et al. 2010). In addition, microwave ablation has low tissue penetrability and does not produce smoke in the airway, because the tissues are not carbonized by this procedure. The lower airway temperature decreases the risk of airway fire and the absence of smoke and relative low tissue penetrability decreases the risk of accidental penetrating injury, as compared to the case in other ablative therapies.
We speculate that in malignant CAO patients with respiratory failure, microwave ablation therapy might be safer and more effective than other ablative therapies. Although it has been reported that percutaneous microwave ablation therapy is useful for medically unresectable NSCLC patients with a solitary nodule (Wolf et al. 2008), there are no reports of the safety and efficacy of transbronchial microwave ablation (TMA) therapy for patients with malignant CAO. Therefore, we conducted this retrospective study to evaluate whether TMA therapy might be a safe and effective treatment for malignant CAO patients with respiratory failure.
Patients and methods
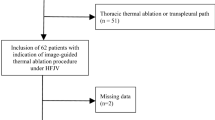
This retrospective, single-institution study was conducted with the approval of the Institutional Review Board of Kumamoto Regional Medical Center (approval date, October 2, 2020; approval number, 20–07). We retrieved the data of 60 patients of advanced NSCLC or lung metastases with symptomatic malignant CAO registered in our medical records database during the period between October 1, 2013, and March 31, 2020. Four patients did not receive any TBI because of long-standing atelectasis of the whole lung on one side (n = 2), massive hemoptysis occurring before any TBI could be undertaken (n = 1), or patient refusal for TBIs (n = 1). Of the remaining patients, 18 patients with extraluminal obstruction received metallic stent placement and 38 patients with endoluminal obstruction received TMA therapy (Fig. 1).
During TMA therapy, minimal interruptions of the procedure are needed for periodic section of smoke or adjustment of the FiO2 to minimize the risk of accidental penetrating injury/airway fire (Lubner et al. 2010). TMA therapy was performed under moderate sedation and high FiO2 values, because in patients with malignant CAO with life-threatening respiratory failure, this could save time for general anesthesia in the operating room. Before the TMA procedure was started, topical anesthesia of the oropharynx was established with 2% nebulized lidocaine. The patient lay supine on the fluoroscopic table and received supplemental oxygen via an oxygen mask (5–10 L/min), and the heart rate, electrocardiogram, and peripheral arterial oxygen saturation (SpO2) were monitored. Midazolam was administered by intravenous bolus injection at the starting dose of 3–4 mg, with additional doses of 1–2 mg administered as needed. If the patients could not lie supine because of severe dyspnea, 2% lidocaine solution for topical anesthesia of the oropharynx was administered via a bronchoscope after the patient had been moderately sedated with midazolam. A nasopharyngeal airway was temporarily placed.
TMA was performed using a microwave tissue coagulator (Microtaze® OT-110 M or AFM-712, Alfresa Pharma Co., Osaka, Japan). A flexible fiberoptic bronchoscope was introduced through the nasopharyngeal airway into the trachea. A long-roller type microwave electrode (TE-24BL) was inserted via the bronchoscope. The tip of the electrode was pressed against the surface of the endoluminal tumor. The tumor was coagulated at 50 watts until the surface of the tumor became white, and the coagulated tissue was removed with biopsy forceps. Care was taken during the procedure to ensure that the electrode did not touch the surface of the normal bronchial mucosa. The TMA was considered successful if at least 50% airway patency of was restored.
We stratified the 38 patients with malignant CAO in this study who received TMA therapy into two groups according to whether the patients had respiratory failure or not at the time of TMA therapy (presence/absence of respiratory failure was defined by the ratio of the partial pressure of oxygen in the arterial blood (PaO2) to FiO2 (PaO2/FiO2)), as follows: the group with respiratory failure (RF group: PaO2/FiO2 ≤ 300; n = 10) and the group without respiratory failure (non-RF group: PaO2/FiO2 > 300; n = 28) (Fig. 1). The endpoints of the study were the differences in the rate of successful restoration of the airway patency after the first session of TMA, the complication rate of TMA therapy, and the overall survival time (OS) between the RF group and the non-RF group. OS was defined as the period from the initiation of TMA therapy until patient death. Comorbidities at the time of TMA therapy were evaluated according to the Charlson comorbidity index (Charlson et al. 1987).
Statistical analysis was performed using the Stat View J 5.0 statistical program (SAS, Institute Inc., Berkeley, CA, USA). Analyses of the clinical numerical data and categorical variables were performed using the Mann–Whitney U-test and Fisher’s exact probability test, respectively. The log-rank test was performed to identify the clinical variables potentially associated with the OS. Variables identified as being significant with p values of < 0.10 were entered into the Cox proportional-hazards model for multivariate analysis. The hazard ratios (HRs) and corresponding 95% confidence intervals (CIs) were determined. OS was estimated by the Kaplan–Meier method. Two-tailed p values of less than 0.05 were considered as being indicative of statistically significant differences.
Results
Patient and disease characteristics
The median age of the patients was 67 years in the RF group and 73 years in the non-RF group. The percentage of patients with an Eastern Cooperative Oncology Group performance status (PS) score of 3–4 was higher in the RF group than in the non-RF group (60% vs. 21%, p = 0.045). Five patients (50%) in the RF group had respiratory failure with PaO2/FiO2 < 200. One-third of the patients in the non-RF group had lung metastases. There was no significant difference in the median value of the Charlson comorbidity index at the time of the TMA therapy, percentage of patients with brain metastasis, or proportion of patients with a previous history of anticancer therapy between the two groups (Table 1).
Efficacy
The symptoms of dyspnea on exertion, bloody sputum/hemoptysis, and stridor were the main indications for TMA therapy. The most common site of malignant CAO was the trachea/tracheal bifurcation (50%), followed in frequency by the left main bronchus (29%) and right main bronchus/truncus intermedius (21%). Airway patency was successfully restored within an hour of initiation of the first session of TMA in 36 (95%) of the 38 patients, and 30 patients (79%) showed symptomatic improvement and/or improvement of PS after the TMA therapy. Of the 38 patients, 21 patients (55%) received a second- or further- session of TMA therapy to remove the remaining coagulated tumor tissue. There was no difference in the success rate of airway patency restoration (90% vs. 96%), the median number of TMA session (2 sessions vs. 2 sessions) or the percentage of patients who showed improvement of the symptoms and/or the PS (80% vs. 79%) between the RF group and the non-RF group (Table 2).
Complications
Complications of TMA therapy occurred in 10% of patients in the RF group and 11% of patients in the non-RF group. One patient of the RF group developed endobronchial bleeding during TMA, which necessitated tracheal intubation and respiratory management. Two patients of the non-RF group showed airway patency restoration but developed airway re-occlusion within 24 h of the procedure because of bronchial epithelial detachment, as a result of the normal bronchial mucosa having been accidentally coagulated during the TMA session. The respiratory conditions in both these patients improved immediately after bronchoscopic removal of the detached epithelial tissue with biopsy forceps. The remaining one patient developed moderate endobronchial bleeding and airway patency restoration was achieved after the second session of TMA (Table 2).
Survival
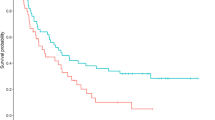
During the median follow-up period of 6.0 months (range 0.6–88.0 months) after the initiation of TMA therapy, 8 patients (80%) of the RF group and 21 patients (75%) of the non-RF group died. There was not a significant difference in the OS between the RF group and the non-RF group (7.1 months vs. 9.1 months, p = 0.059) (Fig. 2a). After the TMA therapy, 21 patients (55%) received best supportive care (BSC) alone and 17 patients (45%) received post-TMA anticancer therapy. Additional TBIs were performed, as appropriate, in nine patients who had received BSC alone. The OS was longer in the patients who received post-TMA anticancer therapy than in the patients who received BSC alone (31.0 months vs. 3.5 months, p < 0.0001). Among patients who received BSC alone, there was no difference in the OS between the RF group and the non-RF group (3.1 months vs. 3.8 months, p = 0.721) (Fig. 2b). On the other hand, among the patients who received post-TMA anticancer therapy, the OS was shorter in the RF group than in the non-RF group (7.1 months vs. 32.8 months, p = 0.011) (Fig. 2c). All the patients of the RF group had NSCLC (squamous cell carcinoma in four cases, and adenocarcinoma in one case), whereas in the non-RF group, five patients had lung metastases (the primary tumor was a thyroid cancer in three cases, renal cancer in one case and esophageal cancer in one case) and seven patients had NSCLC (squamous cell carcinoma in four cases, adenoid cystic carcinoma in two cases, and adenocarcinoma in one case).
Overall survival from the initiation of TMA therapy in malignant CAO patients with respiratory failure (RF group) and without respiratory failure (non-RF group); a all patients, b patients who received best supportive care alone, c patients who received post-TMA anticancer therapy. CAO central airway obstruction, RF respiratory failure, TMA transbronchial microwave ablation
Multivariate analysis identified restoration of the airway patency after the first session of TMA (HR 0.139, 95% CI 0.021–0.915, p = 0.040) and post-TMA anticancer therapy (HR 0.222, 95% CI 0.075–0.653, p = 0.006) as significant factors associated with a reduced risk of death, while TMA therapy under moderate sedation was not found to be associated with an increased risk of death in patients with respiratory failure (p = 0.196) (Table 3).
Discussion
Our results showed that TMA therapy under moderate sedation and higher FiO2 values was well-tolerated and effective in patients with malignant CAO, including those with respiratory failure. Airway patency restoration after the first session of TMA and post-TMA anticancer therapy were identified as important prognostic factors in the malignant CAO patients.
Airway patency restoration by TBIs can relieve the respiratory distress immediately in patients with malignant CAO. As it has been reported that TBIs have a greater impact and more useful in patients with more severe dyspnea at the baseline and that patients with more severe functional impairment are more likely to benefit from TBIs (Ost et al. 2015b), it is important to undertake TBIs as early as is safely possible, with a minimum waiting time, for patients with malignant CAO. In our study, airway patency was successfully restored within an hour of initiation of the first session of TMA in 95% of the patients, similar to the results reported previously for other TBIs (Stephens and Wood 2000).
The complication rate of TBIs is reported to be in the rage of 4–20% (Ost et al. 2015a; Chhajed et al. 2006; Han et al. 2007; Murgu et al. 2012; Mahmood et al. 2015; Stratakos 2016; Shin et al. 2018; Verma et al. 2019), and urgent or repeat procedures, presence of severe systemic comorbidities, and moderate sedation are reported associated with higher rates of complications (Ost et al. 2015a). In our study, 4 of the 38 patients (11%) developed complications after the TMA therapy; 2 patients developed endobronchial bleeding during TMA and 2 other patients developed airway re-occlusion within 24 h of the procedure by detachment of normal bronchial epithelium. Patients in whom the normal bronchial mucosa has been accidentally coagulated during TMA should be monitored carefully for at least 24 h for early detection of airway re-occlusion by detachment of normal bronchial epithelium.
In the studies listed in Table 4, all the patients with malignant CAO underwent TBIs under general anesthesia. According to the report by Murgu et al. (2012), the incidence of complications in NSCLC patients with malignant CAO and respiratory failure undergoing TBIs, including debulking, laser therapy and stent placements under general anesthesia, was 8%. As the complication rate in the RF group in our study, in which TMA was performed under moderate sedation, was 11%, we suggest that TMA therapy under moderate sedation and high FiO2 values is feasible in malignant CAO patients with respiratory failure.
TBIs have been reported to prolong the survival in patients with malignant CAO, with a median OS time of 6.6–12.4 months (Table 4), and additional radiotherapy or chemotherapy after TBI has been reported to have a significant beneficial effect on the prognosis (Chhajed et al. 2006; Verma et al. 2018; Daneshvar et al. 2019). In the present study, the median OS from the initiation of TMA therapy was 7.1 months in the RF group and 9.1 months in the non-RF group, and the efficacy of TMA therapy was not inferior to that reported for other TBIs. In addition, among the patients who received post-TMA anticancer therapy, there was a significant difference in the OS between the RF group and the non-RF group (7.1 months vs. 32.8 months, p = 0.011). The percentage of patients with lung metastases or adenoid cystic carcinoma was higher in the non-RF group than in the RF group (43% vs. 0%, p = 0.035). This could be the reason why patients in the non-RF group had a better prognosis, as patients with lung metastases with no other malignant lesions in the lung, except CAO, show a better prognosis and adenoid cystic carcinoma is a well-differentiated and slow growing tumor.
Our study had certain limitations. Firstly, the sample size was small because this was a retrospective study conducted at a single institution. This is, however, the first report to suggest that TMA therapy could be safely undertaken for malignant CAO patients with respiratory failure. Secondly, there was a potential selection bias because patients in the non-RF group possibly had a better prognosis. As the purpose of this retrospective study was to evaluate whether TMA therapy was safe and effective treatment in malignant CAO patients with respiratory failure, this bias would not have affected our results. Thirdly, we could not evaluate the change in the respiratory symptom, spirometric indices, severity of the complications of TMA therapy or the quality of life of the patients before and after TMA therapy in detail because TMA is performed as a palliative treatment in patients with life-threatening respiratory failure.
In conclusion, TMA therapy under moderate sedation and high FiO2 values was well-tolerated and effective in patients with malignant CAO, including those with respiratory failure.
References
Bolliger CT, Mathur PN (2002) ERS/ATS statement on interventional pulmonology. Eur Respir J 19(2):356–373
Charlson ME, Pompei P, Ales KL, MacKenzie CR (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40(5):373–383
Chhajed PN, Baty F, Pless M, Somandin S, Tamm M, Brutsche MH (2006) Outcome of treated advanced non-small cell lung cancer with and without central airway obstruction. Chest 130(6):1803–1807
Daneshvar C et al (2019) Prevalence and outcome of central airway obstruction in patients with lung cancer. BMJ Open Respir Res 6(1)
Ernst A, Feller-Kopman D, Becker HD, Mehta AC (2004) Central airway obstruction. Am J Respir Crit Care Med 169(12):1278–1297
Han CC, Prasetyo D, Wright GM (2007) Endobronchial palliation using Nd:YAG laser is associated with improved survival when combined with multimodal adjuvant treatments. J Thorac Oncol 2(1):59–64
Lubner MG, Brace CL, Hinshaw JL, Lee FT (2010) Microwave tumor ablation: mechanism of action, clinical results, and devices. J Vasc Interv Radiol 21(SUPPL 8):S192–S203
Mahmood K et al (2015) Therapeutic bronchoscopy improves spirometry, quality of life, and survival in central airway obstruction. Respiration 89(5):404–413
Mudambi L, Miller R, Eapen GA (2017) Malignant central airway obstruction. J Thoracic Dis 9(Suppl 10):S1087–S1110 (AME Publishing Company)
Murgu S, Langer S, Colt H (2012) Bronchoscopic intervention obviates the need for continued mechanical ventilation in patients with airway obstruction and respiratory failure from inoperable non-small-cell lung cancer. Respiration 84(1):55–61
Ost DE et al (2015a) Complications following therapeutic bronchoscopy for malignant central airway obstruction: results of the AQuIRE registry. Chest 148(2):450–471
Ost DE et al (2015b) Therapeutic bronchoscopy for malignant central airway obstruction: success rates and impact on dyspnea and quality of life. Chest 147(5):1282–1298
Shin B, Chang B, Kim H, Jeong BH (2018) Interventional bronchoscopy in malignant central airway obstruction by extra-pulmonary malignancy. BMC Pulm Med 18(1):46
Stephens KE, Wood DE (2000) Bronchoscopic management of central airway obstruction. J Thorac Cardiovasc Surg 119(2):289–296
Stratakos G et al (2016) Survival and quality of life benefit after endoscopic management of malignant central airway obstruction. J Cancer 7(7):794–802
Verma A et al (2018) Outcome of advanced lung cancer with central airway obstruction versus without central airway obstruction. ERJ Open Res 4(2):00173–02017
Verma A et al (2019) Outcome differences between recanalized malignant central airway obstruction from endoluminal disease versus extrinsic compression. Lasers Med Sci 34(5):955–962
Wolf FJ, Grand DJ, Machan JT, DiPetrillo TA, Mayo-Smith WW, Dupuy DE (2008) Microwave ablation of lung malignancies: effectiveness, CT findings, and safety in 50 patients. Radiology 247(3):871–879
Funding
No funding was received from any source for this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interests to declare.
Ethical approval
All procedures involving human participants were performed in accordance with the ethical standards laid down by the institutional and/or national research committees and were in compliance with the principle of the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kashiwabara, K., Fujii, S., Tsumura, S. et al. Efficacy and safety of transbronchial microwave ablation therapy under moderate sedation in malignant central airway obstruction patients with respiratory failure: a single-institution retrospective study. J Cancer Res Clin Oncol 147, 2751–2757 (2021). https://doi.org/10.1007/s00432-021-03560-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-021-03560-3