Abstract
Purpose
To perform a systematic review of the current level of evidence on post-operative management following brain metastasectomy (namely: adjuvant stereotactic radiosurgery, whole brain radiotherapy or observation), and to propose a GRADE-based dedicated recommendation to inform Radiation Oncologists’ clinical practice.
Methods
A panel of expert Radiation Oncologists from the Italian Association of Radiotherapy and Clinical Oncology had defined the search question per the PICO methodology. Electronic databases were independently screened; the Preferred Reporting Items for Systematic Reviews and Meta-Analyses was adopted. The individual and pooled hazard ratios with 95% confidence intervals (CI), as well as the pooled risk ratio (RR) were calculated using a fixed- or random-effects model.
Results
Eight full-texts were retrieved: six retrospective studies and two randomized clinical trials. Outcomes of benefit and damage were analyzed for SRS + observation (PICO A) and SRS + WBRT. SRS allowed for increased rates of local control when compared to both observation and WBRT, while evidence was less conclusive for distant brain control, leptomeningeal disease control and overall survival. In the SRS, the incidence of severe radionecrosis was higher as compared to WBRT, despite neurocognitive deterioration rates were lower. Overall, SRS seems to favorably compare with observation and whole brain RT, despite the level of evidence for the recommendation was low and very low, respectively.
Conclusion
Despite low level of evidence, the panel concluded that the risk/benefit ratio probably favors adjuvant SRS as compared to the observation and whole brain RT as adjuvant treatments following brain metastasectomy (5 votes/5 participants, 100% attendance).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
At present, the prevalence of patients with brain metastases (BMs) is roughly 20–40%, the incidence has increased given technological development and therapeutic advances (Owonikoko et al. 2014). The number of BMs, patient’s age and Karnofsky performance status (KPS), primary tumor histology and extracranial disease control have been used to identify those patients who may benefit from intensive treatment (Gaspar et al. 1997; Sperduto et al. 2010). Neurosurgical resection is the gold standard of treatment for patients with a single large (> 2.1 cm) BM and good prognosis (Level of Evidence 1 +) (Patchell et al. (1990; Vecht et al. 1993). Nevertheless, the European Association of Neuro-Oncology (EANO) has underlined a general lack of evidence for the optimal post-surgical management (Soffietti et al. 2017). Adjuvant whole brain radiotherapy (WBRT) has been questioned after the publication of data showing a detrimental effect on cognitive function, as well as a limited benefit on survival (Chang et al. 2009; Brown et al. 2016). Some data suggested that WBRT could be withhold in favor of close observation (McPherson et al. (2010). The use of radiosurgery following metastasectomy has been increasingly investigated as a potentially safe and effective treatment for reducing loco-regional recurrence. However, concerns exist regarding the risk of radionecrosis and steroids dependency to control chronic edema, which have not been fully investigated (Ling et al. 2015; Minniti et al. 2013). Moreover, the optimal dose and fractionation have not been clarified, as well as the effects on quality of life and cognitive function. In this systematic review, we extensively considered the term “radiosurgery” as encompassing both Stereotactic Radiosurgery—SRS (single fraction) and Fractionated Stereotactic Radiotherapy—FSRT (3–5 fractions).
The Italian Association of Radiotherapy and Clinical Oncology (AIRO) has promoted the development of a dedicated task force to assess the current level of evidence on post-surgical management of BMs, according to GRADE (Grading of Recommendations Assessment, Development and Evaluation) methodology. This effort is a part of a wider project aiming to address clinical-relevant questions in the field of Radiation Oncology with a well-established and reproducible methodology.
Materials and methods
Clinical question design and identification of outcomes
The clinical question was developed by a panel of experts of the AIRO according to the PICO model (Santos et al. 2017), and resulted as following: “Patients treated with brain metastasectomy: stereotactic radiosurgery vs other treatment options (i.e., no adjuvant therapy, whole brain RT)”. In detail, no restrictions were applied according to the primary tumor of origin, nor additional criteria on dose, fractionation, timing of adjuvant therapies were defined for the intervention. Only studies presenting a direct comparison between intervention (SRS) and a control arm were considered for the purpose of the analysis. Outcomes of benefit and harm were proposed and rated by the Panel with a score ranging from 1 to 10. According to the GRADE methodology, only the items reaching a median score ≥ 7/10 were considered as clinically relevant and were included in the analysis (Table 1).
Search strategy and selection of evidence
A systematic literature search was performed by two independent clinicians (CR and SV) who had received a dedicated training on bibliographic research and GRADE Grading of Recommendations Assessment, Development and Evaluation) methodology. Pubmed NCBI, the Cochrane Library and Embase were screened without date restrictions up to March 2019. Free text, Boolean operators, truncation and proximity operators and Medical Subject Headings (MeSH) were used. All types of works were initially considered for the analysis. The full search strategy is provided in Supplementary Material (Appendix I).
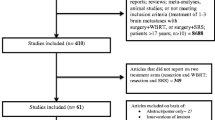
Findings were independently screened and selected by the same Authors in compliance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) methodology (Fig. 1).
Evidence evaluation
The quality of evidence for publications included in the statistical analysis was assessed according to study limitations, inconsistency of results, indirectness of evidence, imprecision and reporting bias (Andrews et al. 2013). Discrepancies between the two Authors were resolved following the consultation of a third Author (SA). After articles reviewing, the authors agreed on splitting the original PICO into two distinct questions:
-
PICO question A: Patients treated with brain metastasectomy: radiosurgery vs observation
-
PICO question B: Patients treated with brain metastasectomy: radiosurgery vs WBRT.
Information on study design, patients’-, disease- and treatment-related characteristics, and results for the outcomes of interest were collected.
Statistical analysis
Data analysis was performed using Review Manager (RevMan) version 5.3. The individual and pooled hazard ratios (HR) with 95% confidence intervals (CI), as well as the pooled risk ratio (RR) were calculated using a fixed- or random-effects model. In case individual data were unavailable, survival probabilities were extracted by study’s reported numbers and estimations from Kaplan–Meier curves. A significant two-way p value for comparison was defined as p < 0.05. Statistical heterogeneity among studies was examined using both the Cochrane Q statistic (significant for p < 0.1) and the I2 value (significant heterogeneity if > 50%).
Results
Overall, of the originally identified studies (n = 848), eight met the inclusion criteria(Brown et al. 2016; Vuong et al. 2013; Lee et al. 2013; Mahajan et al. 2017; Chung et al. 2017; Scheitler-Ring et al. 2016; Patel et al. 2014; Hwang et al. 2010) and were included in the analysis. Of these, two were randomized clinical trials (Brown et al. 2016; Mahajan et al. 2017), while the remainder was represented by retrospective series (Vuong et al. 2013; Lee et al. 2013; Chung et al. 2017; Scheitler-Ring et al. 2016; Patel et al. 2014; Hwang et al. 2010). The median number of enrolled patients was 130 (interquartile range, IQR: 60–199); publication years ranged from 2010 to 2017. Here follows the report of the findings per single study, as sorted per the PICO (A vs. B) and per individual outcome of interest.
PICO A: selected evidence
When considering the first PICO, we noted a general lack of absolute and/or relative measures of the effect in currently available literature.
The work by Vuong et al. (2013) is a retrospective study aiming to compare the cost-effectiveness of neurosurgery and radiosurgery in the management of BMs. Inclusion criteria were: number of BMs ≤ 10 and maximum diameter of each lesion ≤ 3 cm. A propensity score matching (n = 98) was performed to account for selection bias. This study is biased with indirectness.
Lee et al. (2013) published a retrospective longitudinal study of 157 consecutive patients having brain metastasectomy. Primary hematologic histology and patients who received WBRT prior to surgery were excluded. Inclusion criteria were: histological diagnosis, solid and/or cystic BM, good prognosis, controlled extracranial disease, seizures not responding to medical treatment or critically increased intracranial pressure. Nearly 70% of patients had adjuvant WBRT, 10.8% radiosurgery and 19.7% observation. OS, tumor control and KPS were evaluated.
Mahajan et al. (2017) conducted a monocenter, randomized, phase III trial to assess whether radiosurgery to the surgical cavity improved local recurrence as compared with observation. Patients enrolled had: age ≥ 3 years, KPS ≥ 70, complete resection of 1–3 BM, post-operative cavity diameter ≤ 4 cm. Patients with extracranial disease suitable for further therapies and patients receiving concomitant systemic treatment were not excluded. Patients were assigned (1:1) to either radiosurgery or observation; allocation was done with stratification factors and a block size of four. The primary endpoint was local recurrence within the surgical cavity. Distant brain metastatic progression and OS were secondary outcomes. There was a risk of performance bias as both patients and medical doctors were not blinded to the treatment modality, excepted for neuroradiologist. Moreover, a transferability concern exists, as recruited patients were enrolled at a single tertiary-care cancer center. We also detected a high risk of imprecision.
PICO A: outcomes of benefit
Local control (LC)
Mahajan et al. (2017) documented a statistically significant reduction in the rate of local recurrence with adjuvant radiosurgery: the 12-month local progression free survival was 72% (95% CI 60–87%, 15 events) vs. 43% in the observation arm (95% CI 31–59%, 31 events). The HR was 0.46 (95% CI 0.24–0.88%); a competing risk analysis showed comparable results (HR = 0.41; 95% CI 0.21–0.80, p = 0.0097). Lee et al. (2013) considered local recurrence as a secondary endpoint, despite data are provided for the observation arm only (20.8%, 5 events/24 patients).
Distant brain control (DBC)
Two studies reported on DBC for the PICO of interest. Mahajan et al. (2017) did not identify any difference in the development of distant BMs between the two study cohorts. The probability of being free of distant BMs 12 months after surgery was 33% (95% CI 22–49%) in observation arm and 42% (95% CI 30–58%) in radiosurgery group, with an HR of 0.81 (CI 0.51–1.27, p = 0.35). In the retrospective study by Lee et al. (2013), 4/23 patients had distant brain progression without adjuvant treatment (17.4%, p = 0.129).
Intracranial control (ICC)
Vuong et al. (2013) reported a rate of ICC of 10.4 months for patients treated with surgery followed by observation. None of the other included studies evaluated ICC among clinical endpoints.
LMD control
Mahajan et al. (2017) analyzed leptomeningeal progression, despite it was not formally included among the study endpoints. No difference was recorded between the treatment arms (HR 1.4, 95% CI 0.6–3.4, p = 0.46).
Overall survival
Mahajan et al.(Mahajan et al. 2017) did not find any significant difference in OS between the two groups: the median OS was 18 months (95% CI 13 months to NR) in the observation group and 17 months (95% CI 13–22 months) in radiosurgery arm (HR 1.29, 95% CI 0.84–1.98, p = 0.24). The number of events were 39 and 46, respectively. Lee et al. (2013) observed the OS was longer in patients who did not receive adjuvant treatment as compared to the radiosurgery group (15.5 vs. 12.2 months). Nevertheless, the study does not provide the HR; therefore, the effect of the intervention is not fully assessable. Finally, Vuong et al. (2013) report the mean OS of 13 months for the observation group.
Quality of life
Quality of life was not considered as an endpoint in any of the studies included in the analysis. Therefore, no data could be retrieved for the evaluation of this outcome.
PICO A: outcomes of harm
Due to the paucity of data, it was not possible to provide a quantitative estimate of the analyzed outcomes of harm and benefit for PICO A.
PICO A: evidence-to-decision framework
The Evidence-to-Decision Framework (EDT), with stakeholders’ synthetical and detailed judgements and final recommendation for PICO A are provided in Tables 2 and 3, respectively.
PICO B: selected evidence
The study by Brown et al. (2016) is a randomized, multicenter, phase III trial aiming to verify the superiority of SRS over WBRT on 194 patients with resected BM. This study randomized 1:1 adult patients, with a single BM surgical resected and post-operative surgical bed ≤ 5 cm, to receive SRS (12–20 Gy) or WBRT (30 Gy/10 or 37.5 Gy/15 fractions). The risk of imprecision and the risk of detection are serous as both patients and investigators were not blinded to treatment allocation. End-points were OS and cognitive deterioration free survival.
Chung et al. (2017) published a non-randomized, mono-institutional, retrospective study aimed at comparing SRS and/or local RT vs. WBRT vs. WBRT + boost to the tumor bed. Fifty-three patients with one to four non-small-cell-lung-cancer BMs treated with primary resection were included. SRS dose of 14–15 Gy was delivered with Gamma Knife, local RT treatment consisted in 25–40 Gy/10–15 fractions or 45 Gy/25 fractions, WBRT most commonly used regimen was 25 Gy/10 fractions and the boost dose was 15 Gy/5 fractions. Due to its retrospective design, limited sample size, heterogeneity in prescription doses, technique and modality, this study has a very serious risks of bias, imprecision and indirectness.
The work of Scheitler-Ring et al. (2016) is a retrospective, mono-institutional study designed to assess the non-inferiority of radiosurgery compared to WBRT in 83 patients with a single BM post-metastasectomy. SRS dose was 15 Gy; if the surgical cavity was > 3 cm FSRT dose was 21 Gy/3 fractions. WBRT dose was 30 Gy/10–12 fractions. End points: LC, distant brain recurrence, OS and adverse events. The risk of bias is very high and the risk of imprecision is also very serious as allocation was chosen by physicians.
Patel et al. (2014) investigated the non-inferiority of adjuvant radiosurgery over WBRT in 136 consecutive patients with BM from any primary tumor histology. This is a retrospective study, the adjuvant treatment was chosen based on physicians’ preference and year of presentation. Patients received SRS up to 15–21 Gy unless, if the surgical cavity was > 40 mm they had FSRT. WBRT doses were: 30 Gy/10 fractions or 37.5 Gy/15 fractions. End points: LC, distant brain control, leptomeningeal disease, OS, and radiologically confirmed leukoencephalopathy. This study had some limitations: no randomization (a higher percentage of patients with multiple BMs in the WBRT group), and a very serious risk of imprecision and indirectness.
Hwang et al. (2010) aimed to assess whether OS with SRS was non-inferior to WBRT. The SRS dose of 15–20 Gy was delivered with Gamma Knife. This is a non-randomized, retrospective study with very serious risk of selection bias as patients in the WBRT group were more likely to have worse prognosis (all patients with more than three metastases were treated with WBRT), the risk of imprecision is also very serious.
PICO B: outcomes of benefit
Local control
Brown et al. (2016) reported a statistically significant benefit in LC in WBRT arm compared to radiosurgery (p = 0.00016). The 6-month LC rate was 69.4% in radiosurgery group vs. 92.5% in WBRT and the 1-year LC rate was 61.8% vs. 87.1%, respectively. Chung et al. (2017) outlined the rate of local failure was comparable in WBRT vs. radiosurgery arm (10.3% vs. 9.1%, p = 0.41). Scheitler-Ring et al. (2016) reported no statistical difference in local recurrence rate (33%—radiosurgery vs. 55%—WBRT, p = 0.09). LC rates at 6 months and 1 year were similar among groups: 91.4% and 65.2% in radiosurgery compared to 75.9% and 64.3% in WBRT. According to Patel et al. (2014), there was no difference in LC: the local failure was 15% in radiosurgery vs. 24% in WBRT; and the 1-year LC rates were 83% vs. 74% (p = 0.31).
Distant brain control
Brown et al. (2016) demonstrated the 6-month and 1-year DBC rates were statistically significant in favor of WBRT: 64.7% and 72.1% for radiosurgery vs. 94.6% and 89.2% in WBRT arm (p = 0.00045). An advantage in DBC was observed for WBRT over radiosurgery also by Patel et al. (2014) (70% vs. 48%, p = 0.03, after correcting for the number of BMs). Conversely, Scheitler-Ring et al. (2016) did not identify any statistically significant difference in DBC control at 6, 12 and 24 months.
Intracranial control
Brown et al. (2016) found the median intracranial free survival was 27.5 months in WBRT vs. 6.4 months in the radiosurgery group (HR 2·45, 95% CI 1·62–3·72, p < 0·0001). The 6- and 12-month ICC rate were also significantly improved: 80.8% and 72.1% for WBRT vs. 55.1% and 36.6% for radiosurgery (p < 0.0001). Chung et al. (2017) reported, the rate of intracranial failure was statistically lower in WBRT arm compared to radiosurgery (34% vs. 66.7% p = 0.006). Patel et al. (2014) showed that the risk of global intracranial progressive disease was higher in patients treated with radiosurgery compared to WBRT (p = 0.03).
Leptomeningeal disease control
The LMD control rate at 6 months and 1 year were comparable between WBRT and radiosurgery according to Brown et al. (2016): the 6-month and 1-year LMD control rate were 93.9% and 92.8%—radiosurgery vs. 96.8% and 94.6%—WBRT (p = 0.62). Patel et al. (2014) found the 18-month risk of LMD was significantly higher in radiosurgery group: 31% vs. 13%—WBRT, (p = 0.045), also the cumulative incidence of LMD was more pronounced in radiosurgery arm (HR 5.67, 95% CI 1.5–21.51, p = 0.011).
Overall survival (OS)
Brown et al. (2016) described the median OS among groups was comparable (HR 1.07, 95% CI 0.76–1.5, p = 0.70). Chung et al. (2017) confirmed by the 1-year OS rate did not vary: 79.3%—WBRT vs. 78.6%—radiosurgery (p = 0.667). In the study lead by Scheitler-Ring et al. (2016) the median OS was comparable: 14.4 months in SRS vs. 6.7 months in WBRT group. Radiosurgery arm showed a better survival trend but it was not statistically significant (p = 0.062). Nevertheless, the 6-month OS rate was statistically improved in radiosurgery over WBRT group (p = 0.0034). Patel et al. (2014) reported comparable 1-year OS rate: 55%—radiosurgery vs. 56%—WBRT (p = 0.64). Hwang et al. (2010) did not find any difference is OS within group of treatment: the median OS was 15 months in radiosurgery vs. 6.8 months in WBRT (p = 0.08).
Quality of life (QoL)
Brown et al. (2016) demonstrated that patients in the radiosurgery cohort had better QoL defined as stable or improved functional independence (HR 0·56, 95% CI 0·32–0·96, p = 0·034).
PICO B: outcomes of harm
Cognitive deterioration
Brown et al. (2016) reported patients treated with radiosurgery experienced a statistically significant longer interval from cognitive deterioration compared to WBRT: 3.7 months vs. 3.0 months (HR 0·47, 95% CI 0.35–0.63), p < 0·0001.
Morbidity (Grade ≥ 3)
The outcome was not analyzed in the selected studies.
Mortality
The study by Brown et al. (2016) did not find any significant difference in mortality between the two groups (7%—radiosurgery vs. 11%—WBRT).
Symptomatic radionecrosis (Grade 2–3)
Patel et al. (2014) proved that the incidence of symptomatic radionecrosis (Grade 3–4) was 13% in radiosurgery vs. no events in WBRT group (p = 0.001).
Neurological damage (Grade ≥ 3)
Brown et al. (2016) proved the cumulative frequency of severe neurological damage was similar between groups (12%—radiosurgery vs. 18%—WBRT). Patel et al. (2014), demonstrated in radiosurgery group the 1-year rate of leukoencephalopathy damage was 7% compared to 47% in WBRT group (p = 0.001). The risk of toxicity according to Scheitler-Ring et al. (2016) published data was not statistically significant even if the percentage of patients experienced adverse events post-radiosurgery was higher (22% vs. 8.7%, p = 0.152).
The forest plots for the analyzed outcomes of PICO B are provided in Figs. 2 and 3.
PICO B: evidence-to-decision framework
The EDT with stakeholders’ synthetical and detailed judgements and final recommendation for PICO B are provided in Tables 4 and 5, respectively.
Conclusions and discussion
We aimed to systematically review literature data evidences on adjuvant treatment post brain metastasectomy and to elaborate guidelines based on the GRADE system to uniform clinical practice. Overall, the panel of experts ranked the quality of evidence available for PICO A as low for the outcomes of benefit, while outcomes of harm could not be evaluated. Despite strong limitations, the panel concluded the risk/benefit ratio probably favors adjuvant radiosurgery over observation. Even if cost-effectiveness was not recorded by literature data, it was estimated that the use of adjuvant radiosurgery would require a moderate amount of additional technological resources and expertise. Consequently, radiosurgery may require specialized center and this could impact on health equity. The implementation of radiosurgery and observation was considered acceptable, and both treatment modalities were judged as feasible and effective. Finally, the panel voted for a weak recommendation in favor of adjuvant radiosurgery (5 votes/5 participants, 100% attendance).
The quality of evidence for adjuvant WBRT vs. radiosurgery was graded as very low as most of the selected studies are retrospective, with a small sample size and included heterogeneous treatment modalities. Moreover, two works published by Kepka et al. on the same population (Kępka et al. 2016; Kepka et al. 2018) were not considered, despite PICO B was fully addressed and their prospective and randomized design. Our choice was motivated by the fact that, as declared by the Authors themselves, the study was underpowered and did not reach the sample size to demonstrate the non-inferiority of SRS vs. WBRT. Additionally, the interpretation of the results- and the subsequent recommendation from the panel- could have been at least partially hampered by the fact that only 72% of those enrolled in the intervention arm actually received SRS. Finally, another concern was that a comparable relevance was given to cognitive/neurological performance and neurological death from any cause. As explained by the Authors (Kępka et al. 2016), this choice is disputable, because the two outcomes are not equally meaningful from a clinical standpoint, and a discrimination would have been critical to properly assess the risk/benefit ratio of SRS as compared to WBRT in this subset of patients.
Overall, the included studies suggest better cognitive outcomes and QoL, with contrasting evidence regarding oncological outcomes. It is uncertain which treatment mays guarantee better toxicity profile and lower adverse events as radiosurgery has a higher risk of symptomatic radionecrosis and WBRT correlates with worse cognitive deterioration and morbidity. Overall, the stakeholders judged the cost/benefit ratio as favoring radiosurgery. Radiosurgery is expected to require advanced technologies and qualified personnel, thus leading to potential inequalities in patients’ access to the healthcare across the country. The risk/benefit comparison confirmed that radiosurgery is acceptable for a well selected subset of patients. To conclude, the panel expressed a weak recommendation favoring radiosurgery over WBRT as adjuvant treatment to the surgical bed (5 votes/5 participants, 100% attendance).
Considering the results of our systematic literature review as a whole, we can conclude that for patients with surgically resected BM, adjuvant radiosurgery to the tumor bed is an option that referring Radiation Oncologists should consider for suitable patients over either observation and WBRT. Further clinical trials are requested to better understand prognostic factors and criteria for patients’ selection.
References
Andrews J, Guyatt G, Oxman AD, Alderson P, Dahm P, Falck-Ytter Y et al (2013) GRADE guidelines: 14. Going from evidence to recommendations: the significance and presentation of recommendations. J Clin Epidemiol 66:719–725. https://doi.org/10.1016/j.jclinepi.2012.03.013
Brown PD, Jaeckle K, Ballman KV, Farace E, Cerhan JH, Anderson SK et al (2016) Effect of radiosurgery alone vs radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases: a randomized clinical trial. JAMA 316:401. https://doi.org/10.1001/jama.2016.9839
Chang EL, Wefel JS, Hess KR, Allen PK, Lang FF, Kornguth DG et al (2009) Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol 10:1037–1044. https://doi.org/10.1016/S1470-2045(09)70263-3
Chung SY, Chang JH, Kim HR, Cho BC, Lee CG, Suh C-O (2017) Optimal dose and volume for postoperative radiotherapy in brain oligometastases from lung cancer: a retrospective study. Radiat Oncol J 35:153–162. https://doi.org/10.3857/roj.2017.00094
Gaspar L, Scott C, Rotman M, Asbell S, Phillips T, Wasserman T et al (1997) Recursive partitioning analysis (RPA) of prognostic factors in three radiation therapy oncology group (RTOG) brain metastases trials. Int J Radiat Oncol 37:745–751. https://doi.org/10.1016/S0360-3016(96)00619-0
Hwang SW, Abozed MM, Hale A, Eisenberg RL, Dvorak T, Yao K et al (2010) Adjuvant Gamma Knife radiosurgery following surgical resection of brain metastases: a 9-year retrospective cohort study. J Neurooncol 98:77–82. https://doi.org/10.1007/s11060-009-0051-x
Kępka L, Tyc-Szczepaniak D, Bujko K, Olszyna-Serementa M, Michalski W, Sprawka A et al (2016) Stereotactic radiotherapy of the tumor bed compared to whole brain radiotherapy after surgery of single brain metastasis: results from a randomized trial. Radiother Oncol 121:217–224. https://doi.org/10.1016/j.radonc.2016.10.005
Kepka L, Tyc-Szczepaniak D, Osowiecka K, Sprawka A, Trąbska-Kluch B, Czeremszynska B (2018) Quality of life after whole brain radiotherapy compared with radiosurgery of the tumor bed: results from a randomized trial. Clin Transl Oncol 20:150–159. https://doi.org/10.1007/s12094-017-1703-5
Lee C-H, Kim DG, Kim JW, Han JH, Kim YH, Park C-K et al (2013) The role of surgical resection in the management of brain metastasis: a 17-years longitudinal study. Acta Neurochir (Wien) 155:389–397. https://doi.org/10.1007/s00701-013-1619-y
Ling DC, Vargo JA, Wegner RE, Flickinger JC, Burton SA, Engh J et al (2015) Postoperative stereotactic radiosurgery to the resection cavity for large brain metastases. Neurosurgery 76:150–157. https://doi.org/10.1227/NEU.0000000000000584
Mahajan A, Ahmed S, McAleer MF, Weinberg JS, Li J, Brown P et al (2017) Post-operative stereotactic radiosurgery versus observation for completely resected brain metastases: a single-centre, randomised, controlled, phase 3 trial. Lancet Oncol 18:1040–1048. https://doi.org/10.1016/S1470-2045(17)30414-X
McPherson CM, Suki D, Feiz-Erfan I, Mahajan A, Chang E, Sawaya R et al (2010) Adjuvant whole-brain radiation therapy after surgical resection of single brain metastases. Neuro-Oncol 12:711–719. https://doi.org/10.1093/neuonc/noq005
Minniti G, Esposito V, Clarke E, Scaringi C, Lanzetta G, Salvati M et al (2013) Multidose stereotactic radiosurgery (9 Gy × 3) of the postoperative resection cavity for treatment of large brain metastases. Int J Radiat Oncol 86:623–629. https://doi.org/10.1016/j.ijrobp.2013.03.037
Owonikoko TK, Arbiser J, Zelnak A, Shu H-KG, Shim H, Robin AM et al (2014) Current approaches to the treatment of metastatic brain tumours. Nat Rev Clin Oncol 11:203–222
Patchell RA, Tibbs PA, Walsh JW, Dempsey RJ, Maruyama Y, Kryscio RJ et al (1990) A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med 322:494–500. https://doi.org/10.1056/NEJM199002223220802
Patel KR, Prabhu RS, Kandula S, Oliver DE, Kim S, Hadjipanayis C et al (2014) Intracranial control and radiographic changes with adjuvant radiation therapy for resected brain metastases: whole brain radiotherapy versus stereotactic radiosurgery alone. J Neurooncol 120:657–663. https://doi.org/10.1007/s11060-014-1601-4
Santos CMC, Pimenta CAM, Nobre MRC (2007) The PICO strategy for the research question construction and evidence search. Rev Lat Am Enfermagem 15:508–511. https://doi.org/10.1590/S0104-11692007000300023
Scheitler-Ring K, Ge B, Petroski G, Biedermann G, Litofsky NS (2016) Radiosurgery to the postoperative tumor bed for metastatic carcinoma vs. whole brain radiation after surgery. Cureus. https://doi.org/10.7759/cureus.885
Soffietti R, Abacioglu U, Baumert B, Combs SE, Kinhult S, Kros JM et al (2017) Diagnosis and treatment of brain metastases from solid tumors: guidelines from the European Association of Neuro-Oncology (EANO). Neuro-Oncol 19:162–174. https://doi.org/10.1093/neuonc/now241
Sperduto PW, Chao ST, Sneed PK, Luo X, Suh J, Roberge D et al (2010) Diagnosis-specific prognostic factors, indexes, and treatment outcomes for patients with newly diagnosed brain metastases: a multi-institutional analysis of 4259 patients. Int J Radiat Oncol 77:655–661. https://doi.org/10.1016/j.ijrobp.2009.08.025
Vecht CJ, Haaxma-Reiche H, Noordijk EM, Padberg GW, Voormolen JHC, Hoekstra FH et al (1993) Treatment of single brain metastasis: radiotherapy alone or combined with neurosurgery. Ann Neurol 33:583–590. https://doi.org/10.1002/ana.410330605
Vuong DA, Rades D, van Eck ATC, Horstmann GA, Busse R (2013) Comparing the cost-effectiveness of two brain metastasis treatment modalities from a payer’s perspective: Stereotactic radiosurgery versus surgical resection. Clin Neurol Neurosurg 115:276–284. https://doi.org/10.1016/j.clineuro.2012.05.005
Acknowledgements
The authors would like to jointly thank the Scientific Committee and the Board of AIRO for the critical revision of the paper. Stefania Volpe was partially supported by the Italian Ministry of Health with “Progetto di Eccellenza”. Stefania Volpe MD is a Ph.D. student within the European School of Molecular Medicine (SEMM).
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author have no conflict/competing interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Reverberi, C., Volpe, S., Balestrini, D. et al. Post-operative management of brain metastases: GRADE-based clinical practice recommendations on behalf of the Italian Association of Radiotherapy and Clinical Oncology (AIRO). J Cancer Res Clin Oncol 147, 793–802 (2021). https://doi.org/10.1007/s00432-021-03515-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-021-03515-8