Abstract
Purpose
IGF-1Ec is an isoform of Insulin-like growth factor 1 (IGF-1) and has recently been identified to be overexpressed in cancers including prostate and neuroendocrine tumours. The aim of this paper is to investigate the expression of IGF-1Ec in colorectal cancer and polyps compared to normal colon tissues and its association with recurrent disease using semi-quantitative immunohistochemistry.
Methods
Immunohistochemistry for IGF-1Ec expression was performed for colorectal cancer, colorectal polyps and normal colonic tissues. The quantification of IGF-1Ec expression was performed with the use of Image J software and the IHC profiler plugin. Following ethics approval from the National Research Ethics Service (Reference 11/LO/1521), clinical information including recurrent disease on follow-up was collected for patients with colorectal cancer.
Results
Immunohistochemistry was performed in 16 patients with colorectal cancer and 11 patients with colonic polyps and compared to normal colon tissues and prostate adenocarcinoma (positive control) tissues. Significantly increased expression of IGF-1Ec was demonstrated in colorectal cancer (p < 0.001) and colorectal polyps (p < 0.05) compared to normal colonic tissues. Colonic adenomas with high-grade dysplasia had significantly higher expression of IGF-1Ec compared to low-grade dysplastic adenomas (p < 0.001). Colorectal cancers without lymph node metastases at the time of presentation had significantly higher IGF-1Ec expression compared to lymph node-positive disease (p < 0.05). No correlation with recurrent disease was identified with IGF-1Ec expression.
Conclusion
IGF-1Ec is significantly overexpressed in colorectal cancer and polyps compared to normal colon tissues offering a potential target to improve colonoscopic identification of colorectal polyps and cancer and intraoperative identification of colorectal tumours.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer (CRC) is the third most frequently diagnosed cancer in males, and the second in females worldwide with an estimated 1.36 million new cancer cases and 694,000 deaths in 2012 (‘Fact Sheets by Cancer’ 2016). The CONCORD -3 study demonstrated a wide variation in 5-year survival statistics across the different countries in colon and rectal cancer, and the trend has been identified to be either flat or increasing (Allemani et al. 2018). Biomarkers are potentially essential tools in improving outcomes for the management of different cancers. In colorectal cancer, the most frequently used biomarkers include Carcinoembryonic Antigen which has been demonstrated to be the most sensitive investigation for the detection of early recurrent disease compared to radiological and endoscopic investigations and the use of KRAS to predict the effectiveness of anti-EGFR therapies for adjuvant chemotherapy (Tsikitis et al. 2009; Duffy et al. 2014; Allegra et al. 2009; Van Cutsem et al. 2010). The quest for further biomarkers continues to help improve outcomes.
The IGF-1 axis involvement in tumorigenesis, cancer growth and invasion has been demonstrated in vitro and in vivo (Bowers et al. 2015). Numerous studies from as early as the 1980s have demonstrated aberrant IGF-1 signalling in colon cancer, prostatic cancer, pancreatic cancer, osteosarcoma, melanoma and childhood malignancies (Denduluri et al. 2015; Beckwith and Yee 2015; Sachdev and Yee 2007). This led to the development of multiple agents targeting the IGF-1 axis, including receptor targeting agents and drugs reducing ligand bioactivity (Bowers et al. 2015; Beckwith and Yee 2015). However, despite promising preclinical trials with xenograft in vivo models and early phase clinical trials, the results were not replicated in later phase human clinical trials leading to multiple pharmaceutical companies discontinuing these agents from further research (Bowers et al. 2015; Beckwith and Yee 2015). The failure to reflect these results in large scale clinical trials is likely related to the complexity of the IGF-1 signalling axis and its interplay with other growth factor pathways. Clearly, further scrutinization of this pathway is required and this includes the growing research into the different isoforms of IGF-1 and its implications for tumorigenesis and cancer progression. IGF-1Ec is an isoform of the insulin-like growth factor 1 (IGF-1) which is generated from the splicing of exons 4, 5 and 6 of the IGF-1 gene (Matheny et al. 2010). It was initially demonstrated to be upregulated in skeletal muscle after exercise and injury and subsequently identified to have other physiological roles in neuroprotection in the setting of brain ischaemia, promoting cartilage regeneration at sites of damaged cartilage and protection of cardiac tissues in ischaemia (Zanou and Gailly 2013; Dluzniewska et al. 2005; Luo et al. 2015; Stavropoulou et al. 2009). Its role in cancer pathogenesis has been recently investigated and initially confirmed in prostate cancer with prostatic adenocarcinoma tissues demonstrating higher expression of IGF-1Ec compared to normal prostate tissues and intraepithelial neoplasia (Savvani et al. 2013). Subsequently, upregulation of IGF-1Ec expression was demonstrated in osteosarcoma cell lines and in gut neuroendocrine tumours (Shang et al. 2015; Alexandraki et al. 2017). Recently, we demonstrated the over-expression of IGF-1Ec in colorectal cancer cell lines, colorectal cancer and polyp tissues compared to normal tissues (Alagaratnam et al. 2019). To further understand the role of IGF-1Ec in colorectal cancer and potential biomarker roles, we describe the use of quantitative immunohistochemistry to investigate the association between IGF-1Ec expression and the different stages of colorectal cancer and grades of dysplasia of polyps.
Methods
Tissues
Ethics approval was obtained from the National Research Ethics Service committee in West London, UK (Reference 11/LO/1521) for the use of colorectal cancer and polyp tissues from tumours resected from patients who had undergone surgery at our institution. Tissue blocks were identified retrospectively, and the slide cuts were confirmed by a consultant histopathologist. The hospital database was utilised to obtain tissues based on the TNM staging and polyps excised. Basic demographics of the patients, such as age and gender, were collected from the hospital database and follow-up, where available, was reviewed by looking at clinic letters and investigations with an emphasis on recurrent cancer. Six frozen sections of normal colon tissues and prostate cancer tissues fixed in cold acetone were purchased from Ambsio [AMS Biotechnology (Europe) Limited] and Biotechne® with the validity of these specimens confirmed by in-house pathologists in these companies. The morphologies of these tissue slides were further reviewed under light microscopy prior to performing our experiments.
Quantitative immunohistochemistry
Immunohistochemistry staining for IGF-1Ec was performed with an initial incubation with polyclonal anti-IGF-1Ec antiserum (Phoenix Pharmaceuticals INC) at a dilution of 1: 1,000 followed by the application of the secondary biotinylated goat anti-rabbit IgG (ThermoFisher scientific) and visualization of the immunocomplex was obtained by incubating the sections in a solution of 3,3-diaminobenzidine counterstained in haematoxylin. Confirmation of the specificity of the polyclonal anti-IGF-1Ec to the IGF-1Ec peptide was described in our previous work using dot blot experiments (Alagaratnam et al. 2019). Prostate cancer tissues were used as positive control tissues, and negative controls were established by substituting the anti-IGF-1Ec antiserum with PBS. The slides were captured with a Leica SCN400F and scanned at 20× magnification. Semi-quantitative image analysis was performed with the open-source software ImageJ, and the IHC profiler plug-in developed by Varghese et al. (2014). This software performs quantitative antibody staining intensity measurements for DAB/haematoxylin stained tissues with pixel-by-pixel analysis and assigns a score to a four-tier system. The score included high positive (pixel intensity 0–60), positive (pixel intensity 61–120), low positive (pixel intensity 121–180), negative (pixel intensity 181–235), while pixels from 236 to 255 were excluded due to it representing fatty tissues which were not relevant to the score. The final score was calculated by the simple algebraic formula:
The score of the zone was four for high positive, three for positive, two for low positive and 1 for negative. Varghese et al. (2014) validated this software by analysing 1703 DAB-stained IHC images and demonstrated an 88.6% concordance in a comparison study with manual pathological scoring. Examples of recent studies utilising Image J and the IHC profiler are outlined in Table 1. Images were magnified to ×40 and analysed using the IHC profiler plug-in. Three slides for each tumour section were used, and four random areas were captured for analysis. The pixel count for each intensity was utilised and the score calculated using Microsoft Excel using the simple algebraic equation outlined above. Statistical analysis was performed using IBM® SPSS® v22. Comparisons between 2 groups were performed with the Mann–Whitney test and between multiple groups (more than 2) with the Kruskal–Wallis test; p < 0.05 was regarded as statistical significance and denoted with * whilst p < 0.01 was regarded as highly significant and denoted with ** on the graphs. The medians of the samples collected were plotted with 95% confidence interval bars.
Results
Immunohistochemistry
Following ethics approval, cancer tissues were obtained for 16 patients, and polyp tissues obtained for 11 patients (Table 2). This included 7 patients with cancers without lymph node involvement and 9 patients with lymph node involvement with 3 of these patients presenting with distant metastatic disease. The median age for the cancer patient group was 79 (range 67–92) with a 10:6 male: female ratio and a median follow-up of 30 months (Table 2). The polyps examined included adenomas with low-grade dysplasia (5), high-grade dysplasia (3) and serrated adenomas (3).
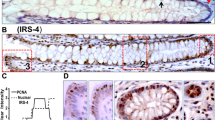
All tissues were stained for IGF-1Ec expression using indirect immunohistochemistry. In cancer tissues, tumour cells demonstrated predominantly cytoplasmic staining. Prostate adenocarcinoma tissues were used as positive controls for methodological confirmation. Figure 1 demonstrates the images captured from the slides, including the positive and negative control tissues, normal colon and colon cancer tissues and colonic polyps. Figure 2 illustrates the difference in IGF-1Ec expression in normal colon tissues compared to colonic polyps and colon cancer tissues. The staining intensity on the IHC profiler was significantly higher (p = 0.03) for the positive control prostate cancer tissues with a median of 1.95 (range 1.4–2.3), while the intensity for the negative control (prostate cancer tissues not incubated with the primary antibody) tissues was a median of 0.4 (range 0.1–0.6). There was a significantly reduced expression of IGF-1Ec in normal colon tissues with a median expression of 1.2 (range 1.1–1.4) compared to colonic polyps (p < 0.05) with a median expression of 1.7 (range 0.7–2.4) and colon cancer tissues (p < 0.001) with a median expression of 1.7 (range 0.7–2.6). There were no differences in the expression of IGF-1Ec between colonic polyps and colonic cancer tissues. There was a significant increase in expression of IGF-1Ec (p = 0.001) in high-grade dysplastic adenomas (median 2, range 0.8–2.4) compared to low-grade dysplastic adenomas (median 1.6, range 1–2.2). Compared to normal colon tissues, a significantly higher expression of IGF-1Ec was identified in low-grade dysplastic adenomas (p = 0.001), high-grade dysplastic adenomas (p < 0.001) and serrated adenomas (p = 0.001) (Fig. 3). Lymph node-negative tumours demonstrated a significantly higher expression of IGF-1Ec (median 1.9, range 1–2.6) compared to lymph node-positive tumours (median 1.6, range 0.7–2.2) as illustrated in Fig. 4 (p = 0.008). Comparison of the scores for the primary colon tumours with different T stages (depth of spread through the layers of the bowel lining) and M stages (metastatic disease at the time of presentation) did not demonstrate any difference in IGF-1Ec expression (Figs. 5 and 6). Similarly, for patients who presented with M0 disease, comparison between recurrent disease and no evidence of recurrence on follow-up did not demonstrate any differences in IGF-1Ec expression (Fig. 7).
Immunohistochemistry for IGF-1Ec expression. a Positive control—prostate cancer. b Negative control—prostate cancer without primary IGF-1Ec antibody. c Normal colon tissue, d COLON cancer tissue, e low-grade dysplastic adenomatous polyp. f high-grade dysplastic adenomatous polyp, g colon cancer tissue with lymph node-negative disease, h colon cancer with lymph node-positive disease
Median immunohistochemistry scores (IHC profiler) with 95% confidence interval bars for different tissues. Positive control—prostate cancer tissues. Negative control—prostate cancer tissues with the primary IGF-1Ec antibody substituted with PBS. Significant differences between the negative and positive control as illustrated. Normal colon tissues expression of IGF-1Ec was significantly lower than colon polyp and colon cancer tissues. p < 0.05 was considered as significant and denoted as * and p < 0.01 was considered as highly significant and denoted as **
Median immunohistochemistry scores (IHC profiler) with 95% confidence interval bars for the expression of IGF-1Ec peptide in the different colonic polyp tissues. Significantly higher expression of IGF-1Ec in high-grade dysplastic tubular adenomas compared to low-grade dysplastic polyps and normal colon tissues. p < 0.01 was considered as highly significant and denoted as **
Discussion
We used an automated immunohistochemistry quantification system to investigate overexpression of an IGF isoform in colorectal cancer and precursor lesions as a potential biomarker of disease. The use of image J IHC profiler plugin has been validated by Varghese et al. (2014) with the growing popularity of this approach due to the objective nature of quantification and the ease and speed of performing this process compared to manual approaches to quantify immunohistochemistry, such as the H score.
The immunohistochemistry findings suggest that IGF-1Ec is overexpressed in colon polyps and cancer tissues at significantly higher levels than normal colon tissues. There appears to be some baseline expression of IGF-1Ec in the normal colon tissues as evidenced by significantly higher levels than the baseline background readings of the negative controls. However, IGF-1Ec expression was unchanged for advancing stages of cancer with similar levels of expression in patients presenting with metastatic disease compared to patients presenting without metastatic disease. Interestingly, there appears to be a higher expression of IGF-1Ec in lymph node-negative disease at presentation compared to lymph node-positive disease. Generally, lymph node involvement is regarded to be associated with an increased risk of recurrent disease and is a rationale for offering postoperative chemotherapy in patients with colorectal cancer to reduce this risk (‘Colorectal Cancer: Diagnosis and Management|Introduction|Guidance and Guidelines|NICE’ n.d. 2016). However, there is an area of uncertainty with stage 2 colorectal cancer (lymph node-negative cancers with T stage > 3) where the incidence of recurrent disease in these patients has been demonstrated to be approximately 20–25% within 5 years with outcomes for these patients similar to those with stage III disease where lymph nodes are involved (Chen and Bilchik 2006; Davies et al. 2008). The use of chemotherapy in all patients with stage II colorectal cancer has not been found to be beneficial, and therefore demonstrates the need to identify the subgroup of patients with stage II cancer who would benefit from chemotherapy, thereby avoiding over- or undertreating patients with adjuvant therapies and their associated side effects and complications. Our study group had insufficient numbers to investigate the relationship between IGF-1Ec expression in stage II colorectal cancer and recurrence but this is an area which would benefit from closer review.
We investigated the expression of IGF-1Ec in colonic polyps which are known precursor lesions for colorectal cancer. The ‘adenoma–carcinoma’ sequence is an accepted concept in the pathogenesis of colorectal cancer, and most cancers are thought to arise from polyps. This hypothesis is supported by the epidemiological evidence that the prevalence of adenomas and carcinomas increases with age, with adenomas peaking 5 years prior to carcinomas and the geographical variations in prevalence of both adenomas and carcinomas are very similar (Leslie et al. 2002). In addition, the distributions of adenomas and carcinomas are very similar in the colon, and removal of the adenomas is known to reduce the long-term incidence of colorectal cancers (Leslie et al. 2002; Atkin et al. 1992; Winawer et al. 1993). We, therefore, looked at the expression of IGF-1Ec in the polyps of different grades of dysplasia (low and high dysplasia) and compared adenomatous polyps to normal colon tissues. The expression of IGF-1Ec was identified to be significantly higher in colonic polyps compared to normal colon tissues with increased expression with a worsening degree of dysplasia. This suggests that IGF-1Ec is involved in the early stages of cancer pathogenesis in colon cancer.
Within the limits of this study, IGF-1Ec expression was identified to occur at a low level in normal colon tissues with significantly higher expression in colonic polyps, particularly with worsening dysplasia. Significantly higher expression of IGF-1Ec was identified in colon cancer compared to normal colon tissues though the former was at similar levels to colonic polyps. The potential involvement of IGF-1Ec in different cancers has been described in the literature. The potential role of IGF-1Ec in prostate cancer has been demonstrated by the Department of Experimental Physiology at the University of Athens (Savvani et al. 2013; Armakolas et al. 2010; 2015; Philippou et al. 2013). Their published work spanning over a few years initially reported higher IGF-1Ec peptide expression, using immunohistochemistry, in prostate cancer tissues compared to normal prostate tissue (Savvani et al. 2013). Subsequent work with semi-quantitative PCR demonstrated increased expression of IGF-1Ec in prostate cancer and intra-epithelial neoplasia (very early stage/in situ cancer) compared to normal prostate tissues and demonstrated a correlation with the level of expression of IGF-1Ec peptide with the stage of prostate cancer. IGF-1Ec expression in osteosarcomas was confirmed in vitro with the cell lines MG-63 and Mhos (Philippou et al. 2011; Shang et al. 2015). Quantitative PCR, western blotting and immunohistochemistry confirmed the over-expression of mRNA IGF-1Ec transcripts in the malignant cell lines (MG-63) compared to ‘less’ malignant cell lines (Mhos), and exogenous administration of a synthetic peptide Ec peptide demonstrated significantly increased the proliferation index compared to controls with increased migration distances and invasion (through an 8 µm pore of polycarbonate membrane) in a dose-dependent manner (Shang et al. 2015). Alexandraki et al. (2017) reported IGF-1Ec peptide expression in neuroendocrine tumours was more prevalent in metastatic tumours with higher proliferation indices compared to primary neuroendocrine tumours with lower proliferation indices.
Further work would include reviewing IGF-1Ec as a prognostic factor in stage II colorectal cancer to identify if this could help stratify patients who would benefit from chemotherapy and overall outcomes. The findings from our study suggest that IGF-1Ec is overexpressed in colonic polyps and cancer tissues compared to normal colon tissue. Further clarification with larger number of tissue samples followed by animal models will be required prior to considering the potential clinical applications including a role as a targeting peptide for drug delivery agents, fluoroscopic agents for intraoperative visualization of colonic tumours and improving colonoscopic visualization of polyps and tumours, thereby reducing missed lesions which have been reported as high as 25% (Zhao et al. 2019).
References
Alagaratnam S, Yang SY, Loizidou M, Fuller B, Ramesh B (2019) Mechano growth factor expression in colorectal cancer investigated with fluorescent gold nanoparticles. Anticancer Res 39(4):1705–1710. https://doi.org/10.21873/anticanres.13276
Alexandraki KI, Philippou A, Boutzios G, Theohari I, Koutsilieris M, Delladetsima IK, Kaltsas GA (2017) IGF-IEc expression is increased in secondary compared to primary foci in neuroendocrine neoplasms. Oncotarget 8(45):79003–79011. https://doi.org/10.18632/oncotarget.20743
Allegra CJ, Milburn Jessup J, Somerfield MR, Hamilton SR, Hammond EH, Hayes DF, McAllister PK, Morton RF, Schilsky RL (2009) American Society of Clinical Oncology Provisional Clinical Opinion: testing for KRAS gene mutations in patients with metastatic colorectal carcinoma to predict response to anti-epidermal growth factor receptor monoclonal antibody therapy. J Clin Oncol 27(12):2091–2096. https://doi.org/10.1200/JCO.2009.21.9170
Allemani C, Matsuda T, Di Carlo V, Harewood R, Matz M, Nikšić M, Bonaventure A et al (2018) Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): analysis of individual records for 37,513,025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. The Lancet 391(10125):1023–1075. https://doi.org/10.1016/S0140-6736(17)33326-3
Alrashdan MS, Angel C, Cirillo N, McCullough M (2016) Smoking habits and clinical patterns can alter the inflammatory infiltrate in oral lichenoid lesions. Oral Surg Oral Med Oral Pathol Oral Radiol 121(1):49–57. https://doi.org/10.1016/j.oooo.2015.08.020
Armakolas A, Philippou A, Panteleakou Z, Nezos A, Sourla A, Petraki C, Koutsilieris M (2010) Preferential expression of IGF-1Ec (MGF) transcript in cancerous tissues of human prostate: evidence for a novel and autonomous growth factor activity of MGF E peptide in human prostate cancer cells. Prostate 70(11):1233–1242. https://doi.org/10.1002/pros.21158
Armakolas A, Kaparelou M, Dimakakos A, Papageorgiou E, Armakolas N, Antonopoulos A, Petraki C et al (2015) Oncogenic role of the Ec peptide of the IGF-1Ec isoform in prostate cancer. Mol Med 21(1):167–179. https://doi.org/10.2119/molmed.2014.00222
Atkin WS, Morson BC, Cuzick J (1992) Long-term risk of colorectal cancer after excision of rectosigmoid adenomas. N Engl J Med 326(10):658–662. https://doi.org/10.1056/NEJM199203053261002
Beckwith H, Yee D (2015) Minireview: were the IGF signaling inhibitors all bad? Mol Endocrinol 29(11):1549–1557. https://doi.org/10.1210/me.2015-1157
Bowers LW, Rossi EL, O’Flanagan CH, deGraffenried LA, Hursting SD (2015) The role of the insulin/IGF system in cancer: lessons learned from clinical trials and the energy balance-cancer link. Front Endocrinol 6:77. https://doi.org/10.3389/fendo.2015.00077
Colorectal Cancer (2016) Diagnosis and Management|Introduction|Guidance and Guidelines|NICE’. n.d. https://www.nice.org.uk/guidance/cg131/chapter/introduction. Accessed 29 Sep 2016
Chen SL, Bilchik AJ (2006) More extensive nodal dissection improves survival for stages I–III of colon cancer: a population-based study. Ann Surg 244(4):602–610. https://doi.org/10.1097/01.sla.0000237655.11717.50
Davies M, Arumugam PJ, Shah VI, Watkins A, Morgan AR, Carr ND, Beynon J (2008) The clinical significance of lymph node micrometastasis in stage I and stage II colorectal cancer. Clin Transl Oncol 10(3):175–179
Denduluri SK, Idowu O, Wang Z, Liao Z, Yan Z, Mohammed MK, Ye J et al (2015) Insulin-like growth factor (IGF) signaling in tumorigenesis and the development of cancer drug resistance. Genes Dis 2(1):13–25. https://doi.org/10.1016/j.gendis.2014.10.004
Dequanter D, Velde MVANDE, Bar I, Nuyens V, Rousseau A, Nagy N, Vanhamme L et al (2016) Nuclear localization of glutamate-cysteine ligase is associated with proliferation in head and neck squamous cell carcinoma. Oncol Lett 11(6):3660–3668. https://doi.org/10.3892/ol.2016.4458
Dluzniewska J, Sarnowska A, Beresewicz M, Johnson I, Srai SKS, Ramesh B, Goldspink G, Górecki DC, Zabłocka B (2005) A strong neuroprotective effect of the autonomous c-terminal peptide of IGF-1 Ec (MGF) in brain ischemia. FASEB J 19(13):1896–1898. https://doi.org/10.1096/fj.05-3786fje
Duffy MJ, Lamerz R, Haglund C, Nicolini A, Kalousová M, Holubec L, Sturgeon C (2014) Tumor markers in colorectal cancer, gastric cancer and gastrointestinal stromal cancers: European Group on Tumor Markers 2014 Guidelines Update. Int J Cancer 134(11):2513–2522. https://doi.org/10.1002/ijc.28384
Fact Sheets by Cancer (2016) 2016. http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx.
Fathollah S, Mirpour S, Mansouri P, Dehpour AR, Ghoranneviss M, Rahimi N, Naraghi ZS, Chalangari R, Chalangari KM (2016) Investigation on the effects of the atmospheric pressure plasma on wound healing in diabetic rats. Sci Rep 6(February):19144. https://doi.org/10.1038/srep19144
Ke C-Y, Yang F-L, Wen-Tien Wu, Chung C-H, Lee R-P, Yang W-T, Subeq Y-M, Liao K-W (2016) Vitamin D3 reduces tissue damage and oxidative stress caused by exhaustive exercise. Int J Med Sci 13(2):147–153. https://doi.org/10.7150/ijms.13746
Kumar G, Tajpara P, Bukhari AB, Ramchandani AG, De A, Maru GB (2014) Dietary curcumin post-treatment enhances the disappearance of B(a)P-derived DNA adducts in mouse liver and lungs. Toxicol Rep 1:1181–1194. https://doi.org/10.1016/j.toxrep.2014.11.008
Leslie A, Carey FA, Pratt NR, Steele RJC (2002) The colorectal adenoma-carcinoma sequence. Br J Surg 89(7):845–860. https://doi.org/10.1046/j.1365-2168.2002.02120.x
Luo Z, Jiang Li, Yan Xu, Li H, Wei Xu, Shuangchi Wu, Wang Y, Tang Z, Lv Y, Yang Li (2015) Mechano growth factor (MGF) and transforming growth factor (TGF)-Β3 functionalized silk scaffolds enhance articular hyaline cartilage regeneration in rabbit model. Biomaterials 52(June):463–475. https://doi.org/10.1016/j.biomaterials.2015.01.001
Matheny RW, Nindl BC, Adamo ML (2010) Minireview: mechano-growth factor: a putative product of igf-i gene expression involved in tissue repair and regeneration. Endocrinology 151(3):865–875. https://doi.org/10.1210/en.2009-1217
Nanda N, Dhawan DK, Bhatia A, Mahmood A, Mahmood S (2016) Doxycycline promotes carcinogenesis & metastasis via chronic inflammatory pathway: an in vivo approach. PLoS ONE 11(3):e0151539. https://doi.org/10.1371/journal.pone.0151539
Philippou A, Armakolas A, Panteleakou Z, Pissimissis N, Nezos A, Theos A, Kaparelou M, Armakolas N, Pneumaticos SG, Koutsilieris M (2011) IGF1Ec expression in MG-63 human osteoblast-like osteosarcoma cells. Anticancer Res 31(12):4259–4265
Philippou A, Armakolas A, Koutsilieris M (2013) Evidence for the possible biological significance of the igf-1 gene alternative splicing in prostate cancer. Front Endocrinol 4:31. https://doi.org/10.3389/fendo.2013.00031
Rotoli D, Morales M, Maeso MDC, Garcia MDP, Morales A, Avila J, Martin-Vasallo P (2016) Expression and localization of the immunophilin FKBP51 in colorectal carcinomas and primary metastases, and alterations following oxaliplatin-based chemotherapy. Oncol Lett. https://doi.org/10.3892/ol.2016.4772
Sachdev D, Yee D (2007) Disrupting insulin-like growth factor signaling as a potential cancer therapy. Mol Cancer Ther 6(1):1–12. https://doi.org/10.1158/1535-7163.MCT-06-0080
Savvani A, Petraki C, Msaouel P, Diamanti E, Xoxakos I, Koutsilieris M (2013) IGF-IEc expression is associated with advanced clinical and pathological stage of prostate cancer. Anticancer Res 33(6):2441–2445
Shang J, Fan X, Liu H (2015) The role of mechano-growth factor E peptide in the regulation of osteosarcoma. Oncol Lett 10(2):697–704. https://doi.org/10.3892/ol.2015.3339
Stavropoulou A, Halapas A, Sourla A, Philippou A, Papageorgiou E, Papalois A, Koutsilieris M (2009) IGF-1 Expression in infarcted myocardium and MGF E peptide actions in rat cardiomyocytes in vitro. Mol Med 15(5–6):127–135. https://doi.org/10.2119/molmed.2009.00012
Tsikitis VL, Malireddy K, Green EA, Christensen B, Whelan R, Hyder J, Marcello P et al (2009) Postoperative surveillance recommendations for early stage colon cancer based on results from the clinical outcomes of surgical therapy trial. J Clin Oncol 27(22):3671–3676. https://doi.org/10.1200/JCO.2008.20.7050
Van Cutsem E, Nordlinger B, Cervantes A, ESMO Guidelines Working Group (2010) Advanced colorectal cancer: ESMO clinical practice guidelines for treatment. Ann Oncol 21(Suppl 5):v93–97. https://doi.org/10.1093/annonc/mdq222
Varghese F, Bukhari AB, Malhotra R, De A (2014) IHC profiler: an open source plugin for the quantitative evaluation and automated scoring of immunohistochemistry images of human tissue samples. PLoS ONE 9(5):e96801. https://doi.org/10.1371/journal.pone.0096801
Winawer SJ, Zauber AG, Ho MN, O’Brien MJ, Gottlieb LS, Sternberg SS, Waye JD, Schapiro M, Bond JH, Panish JF (1993) Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med 329(27):1977–1981. https://doi.org/10.1056/NEJM199312303292701
Zanou N, Gailly P (2013) Skeletal muscle hypertrophy and regeneration: interplay between the myogenic regulatory factors (MRFs) and insulin-like growth factors (IGFs) pathways. Cell Mol Life Sci CMLS 70(21):4117–4130. https://doi.org/10.1007/s00018-013-1330-4
Zhang C, Arentz G, Winderbaum L, Lokman NA, Klingler-Hoffmann M, Mittal P, Carter C, Oehler MK, Hoffmann P (2016) MALDI mass spectrometry imaging reveals decreased CK5 levels in vulvar squamous cell carcinomas compared to the precursor lesion differentiated vulvar intraepithelial neoplasia. Int J Mol Sci 17(7):1088. https://doi.org/10.3390/ijms17071088
Zhao S, Wang S, Pan P, Xia T, Chang X, Yang X, Guo L et al (2019) Magnitude, risk factors, and factors associated with adenoma miss rate of tandem colonoscopy: a systematic review and meta-analysis. Gastroenterology 156(6):1661–1674.e11. https://doi.org/10.1053/j.gastro.2019.01.260
Acknowledgements
We thank Dr Tu Vinh Luong for her expertise as a consultant histopathologist in her help in identifying appropriate colorectal cancer and polyp tissues.
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Ethics approval
National Research Ethics Service (UK) Reference 11/LO/1521.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Alagaratnam, S., Loizidou, M., Yang, S.y. et al. Increased expression of IGF-1Ec with increasing colonic polyp dysplasia and colorectal cancer. J Cancer Res Clin Oncol 146, 2861–2870 (2020). https://doi.org/10.1007/s00432-020-03345-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-020-03345-0