Abstract
Background
The CD-TK double suicide gene has become an effective therapy for bladder cancer. A novel molecular-targeted ultrasound (US) method has been developed to precisely guide nanobubbles loaded with this gene to regions within bladder tumor cells and is widely used due to its efficiency in delivering drugs to the target tumor.
Methods
Uniform nanoscaled nanobubbles loaded with CD-TK double suicide gene were developed using a thin-film hydration sonication, carbodiimide chemistry approaches, and electrostatic adsorption methods.
Results
In the present study, we synthesized CD-TK double suicide gene-loaded cationic nanobubbles conjugated with anti-VEGFR2 that can bind with VEGFR2-positive cells. Fluorescence and flow cytometry evidence show that CD-TK double suicide gene-loaded nanobubbles were successfully developed. CD-TK-CNBs delivered via US-mediated nanobubble destruction (UMND) enhanced transfection efficiency, overexpression of CD-TK double suicide gene, and tumor cell apoptosis, and inhibited tumor cell growth in vitro.
Conclusions
These CD-TK-CNBs may become a novel treatment for bladder cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Bladder cancer is one of the most common types of cancer in China. Approximately 70% of patients with early stage non-muscle invasive bladder cancer suffer disease recurrence after the initial surgical treatment (Siegel et al. 2013). The development of a novel treatment approach for bladder cancer is paramount, since currently approved treatments show several side effects and a high recurrence rate. The occurrence of bladder cancer is related to many gene defects. In recent years, with the application of gene-targeted therapy and immunotherapy, the survival rate and health of patients with bladder cancer have improved. However, the overall therapeutic effect has not been overly beneficial, and thus, novel strategies and methods should be explored to improve the therapeutic effect of currently available drugs.
Gene therapy can change the intensity of defective gene expression or express “suicide genes” with a chemotherapeutic prodrug that ceases cell proliferation, leads to tumor decline, and eventually results in tumor necrosis (Amer 2014; Wirth and Ylä-Herttuala 2014; Hu et al. 2019). While chemotherapeutic drugs harm both healthy and cancerous cells, suicide gene therapy utilizes genes that transform initially harmless prodrugs into those that are highly toxic, but specifically inside only tumor cells (Zarogoulidis et al. 2013; Vago et al. 2016). Cytosine deaminase/5-fluorine cytosine (CD/5-FC) and thymine kinase gene/Ganciclovir (TK/GCV) are currently used as a mature suicide gene/prodrug system.
Gene therapy requires high gene transfection efficiency and gene expression. Viral vectors delivered by adenoviruses and lentiviruses possess a high transduction efficiency; nevertheless, their latent infection and immunogenicity risks remain unresolved. To date, even though liposome-mediated DNA transfection performs an ideal function in vitro, its transfection efficiency is low and the procedure relatively expensive. With the continuous progress of biomedical engineering fields, the application of nanobubbles to treat certain diseases such as liver fibrosis is becoming increasingly popular, and multifunctional imaging and diagnostic therapy have now been achieved. There exists an alternative technique that can increase the local gene concentration via the production of nanobubbles targeting aggregations. VEGFR-2 mRNA and protein expression was specifically elevated in highly invasive bladder cancer cell lines T-24 (Obrien et al. 1995; Verma et al. 2011; Chen et al. 2013, 2017). VEGFR2-targeted therapy may be effective in treating invasive bladder cancers. Thus, we produced a selection of anti-VEGFR2 antibodies as the targeting agent toward cells specifically expressing VEGFR2. Following injection of blood containing nanobubbles, we observed via monitoring US that the nanobubbles rapidly accumulated in tumor tissue via the bloodstream. Simultaneously, using US irradiation on the body surface in the tumor target area, the US-mediated nanobubble fragments rapidly release the CD-TK double suicide gene at the tumor site. The cavitation effect of the nanobubble fragments and the acoustic pore effect not only directly damage the tumor cells, but also improve the efficacy of the chemotherapeutic drug (Wood and Sehgal 2015; Li et al. 2016). This method is expected to be efficient in the treatment of bladder cancer using US irradiation in combination with nanobubbles loaded with the CD-TK double suicide gene.
Materials and methods
Materials and instruments
All experiments involving animals were approved by the Institutional Administrative Panel on Laboratory Animal Care. The bladder cancer cell line, T-24, was obtained from American Type Culture Collection (ATCC, Manassas, VA) and the cell line was approved by The Ethics Committee of Chongqing Medical University. RPMI-1640 medium and fetal bovine serum were obtained from Gibco. 0.25% trypsin,EDC and NHS was bought from Beyotime. The CMV-GFPa1-IRES-CDglyTK double gene plasmid was sourced from Shanghai Nobel, 5-fluorocytosine (5-FC) and ganciclovir (GCV) from Chongqing Saimike, and 1,2-Dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) and carboxyl-modified 1,2-distearoyl-sn-glycero-3-phosphoetha-nolamine (DSPE-PEG 2000)-COOH from Avanti, US, Cationic cholesterol was purchased from Avanti, US, and the TUNEL kit, PCNA kit, and propidium iodide from Roche. The diagnostic instrument was bought from Baisheng, Italy.
Synthesis and characterization of control and VEGFR2-targeted CD-TK-loaded nanobubbles
A certain proportion (4:1:5) of DPPC, DSPE-PEG2000-COOH, and DC cholesterol was dissolved in 10 mL chloroform in a round-bottom flask and placed in a rotary vacuum evaporator at 50 °C to remove organic solvents and form a uniform lipid film. A volume of 0.5 mL hydration solution consisting of phosphate-buffered saline (V/V: 1:9) was added to a 1.5-mL microfuge tube followed by the lipid suspension and sealed with a rubber cap. Subsequently, the air in the tube was replaced with perfluoropropane and the tube was incubated for 30 min in a water bath at 42 °C. The suspension was then vibrated using a mechanical vibrator for 90 s to obtain nanobubbles and sealed at 4 °C until further use.
After the synthesis of the nanobubbles containing the DSPE-PEG2000-COOH lipid molecule, 0.4 M EDC and 0.1 M NHS were inserted at the ratio of 1:1 (v/v), and the nanobubbles were incubated for 30 min on a shaker. Thereafter, an amine-modified VEGFR2 at a certain concentration was inserted, followed by performance of the covalently coupling reaction at room temperature for a time period of 30 min. Subsequent to the accomplishment of the covalently coupling reaction, targeted nanobubbles were attained with the use of the centrifugal flotation methodology.
CD-TK suicide gene was divided into 10 μg, 20 μg, 30 μg, and 40 μg groups. The CD-TK suicide gene was added to 5 × 108/mL nanobubbles and fully mixed. The nanobubbles were incubated with CD-TK suicide gene at 4 °C for 30 min followed by 400 rpm/min centrifugation for 3 min at 4 °C and standing for 30 min to obtain the gene-loaded nanobubbles. Subsequently, the nanobubbles were diluted in PBS and labeled with PI and FITC for observation by fluorescence microscopy. The upper suspension comprised the nanobubbles that were combined with the CD-TK suicide gene, and the lower layer comprised the free CD-TK suicide gene. The concentration of the free gene in the lower suspension was measured using a nucleic acid detector (260 nm measurement for nucleic acid). Gene-binding rate of nanobubbles = (total gene − total unbound gene)/total gene × 100%. The average size, zeta potential, and polydispersity index (PDI) of nanobubbles were assessed using a dynamic light scattering apparatus (Malvern Instruments, Malvern, UK), and the concentrations of nanobubbles were measured using a hemocytometer and optical microscope. A concentration of 1 × 105 bladder cancer cells/well were seeded in 6-well plate and cultured for 24 h. The treatment groups were transfected with the nanobubbles containing the carrier plasmid for 48 h, and the control group was cultured in RPMI-1640 medium. Following the incubation period, centrifugation was carried out and GFP were analyzed by flow cytometry. The experiment was repeated at least three times.
Cell culture
The bladder cancer cell line, T-24, was cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum and 100 U/mL penicillin and streptomycin at 37 °C, 5% CO2 for 2 days to 90% confluence.
Detection of low-frequency US sensitivity in vitro and in vivo
A 2% (w/v) agarose gel was prepared to be utilized in the in vitro ultrasonography gel model, and 200 μL of the nanobubbles was diluted fourfold with PBS and added to the gel model; following injection of 200 μL nanobubbles via the tail vein into nude mice. The nanobubble contrast-enhanced US imaging effects were observed in vitro and in vivo (the Esaote MyLab 90 ultrasonic diagnostic system, an L5-12 probe, MI 0.10, 88% gain, CnTI contrast mode, and 1.5-cm depth).
Additionally, the procedure involved custom-developed low-frequency US with destruction to nanobubbles for 1 min (1 MHz, 1.0 W/cm2, duty cycle 50%) (Shang et al. 2019; Du et al. 2011; Bruix et al. 2014). Contrast-enhanced US images were recorded before and after destruction with MyLab 90. This process was repeated at least three times.
Animal experiments
60 nude mice aged 4–6 weeks were purchased from the Experimental Animal Center of Chongqing Medical University. Cultured bladder cancer cells were removed from the flask using 0.25% trypsin at 37 °C for 5 min, resuspended at 1 × 107 cells per 0.2 mL PBS, and injected subcutaneously into the right hip joint of the mice. One week after inoculation of the tumor cells, successful xenotransplantation (100 mm3) was verified and the experiment started. All mice were approved by the Animal Ethics Committee of the Medical University of Chongqing. Mice were randomly divided into five groups, with 12 mice in each: control; US + CD-TK gene nanobubbles; US + CD-TK gene nanobubbles + 5-FC; US + CD-TK gene nanobubbles + GCV; and US + CD-TK gene nanobubbles + 5-FC + GCV. The control group did not undergo any treatment. The treatment groups were IV injected with 200 μL nanobubbles once a day for 1 week and irradiated with US(1 MHz, 1.0 W/cm 2, duty cycle 50%). A concentration of 400 mg/kg 5-FC and/or 40 mg/kg GCV was IP injected once a day for 1 week in those groups. The length and diameter of the tumors were measured weekly, and after weeks, the mice were sacrificed and the tumors were removed, measured, weighed, and sliced. The volume of the tumor = long diameter (a) × short diameter2 (b2)/2.
Cell apoptosis and cell cycle analysis
A concentration of 1 × 105 bladder cancer cells/well were seeded in 6-well plate and cultured for 24 h. The treatment groups, U + M + CD-TK, U + M + CD-TK + 5-FC, U + M + CD-TK + GCV, and U + M + CD-TK + GCV + 5-FC, were transfected with the nanobubbles containing the carrier plasmid for 24 and 48 h, and the control group was cultured in RPMI-1640 medium. The latter three experimental groups were also incubated with 2 mg/mL 5-FC and/or 0.5 mg/mL GCV, according to the experimental design. Following the incubation period, centrifugation was carried out at a speed of RCF 560g to remove the supernatant, and the cells were resuspended. A volume of 5 mL annexin V-FITC and 5 mL PI was added to each tube, and the samples were mixed and incubated for 15 min in the dark. Cells were removed using 0.25% trypsin and resuspended in 0.4 mL PBS, fixed, and cell apoptosis and cell cycle were analyzed by flow cytometry in at least triplicate.
Immunofluorescence determination of TUNEL and PCNA
Paraffin sections of tumors were created using a kit, which included paraffin sectioning, dewaxing antigen repair, and incubation with a single antibody (TdT or PCNA) overnight at 4 °C. Two anti-covering tissues marked with FITC fluorescein were added to the homologous genus and incubated for 50 min at 23 °C. Anti-fluorescence quenching was performed using sealing agents. The sections were observed by fluorescence microscopy.
Statistical analysis
All data are expressed as the mean ± standard deviation (SD), and a Student’s t test and analysis of variance were carried out. Variation among groups was analyzed using a one-way ANOVA and LSD using the SPSS software (version 19.0 for Windows, SPSS, Chicago, Illinois, USA). p < 0.05 is considered statistically significant.
Results
Basic characteristics and transfection efficiency of double suicide gene-loaded nanobubbles
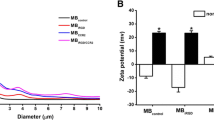
The average size of the nanobubbles was 698.23 ± 78.25 nm, with a PDI of 0.218 ± 0.045 (n = 3), as shown by a Malvern particle-size analyzer. The low PDI indicated a high homogeneity in the size distribution of the nanobubbles (Fig. 1a). The average concentration of the nanobubbles was 5.15 ± 0.32 (× 108/mL) (n = 3), and the zeta potential of the nanobubbles was (36.32 ± 5.44 mV) (Fig. 1b). Nanobubbles were observed by light microscopy (Fig. 1c). The higher zeta potential of the nanobubbles could combine with the negative charges of the DNA. Furthermore, the nanobubbles loaded with the suicide gene were stained with PI, PI was then combined with the plasmid, and the nanobubbles were stained with red fluorescence. As the nanobubbles shell was labeled using a PI to form PI-labeled nanobubbles, they could be more easily observed by light microscopy, which showed that the distribution was uniform and that there was no obvious aggregation (Fig. 1d). VEGFR2-targeted nanobubbles were stained with green fluorescence (Fig. 1e). Additionally, the efficiency with which VEGFR2 bound to nanobubbles was 92.75% ± 4.45%, as recorded using FCM (Fig. 1f). The nanobubble complexes were developed with the use of an electrostatic adsorption strategy based on the negative charge of the DNA and positive charge of the nanobubbles. The concentration of the nanobubbles was fixed (5 × 10 8/mL) and the amount of DNA was incrementally increased. With the addition of DNA, the gene-loading capacity of the nanobubbles increased gradually. When 30 μg of DNA was added, the nanobubbles gene-loading capacity no longer increased and approached saturation (Fig. 1g). Fluorescence imaging was put to use for evaluating the targeting efficacy of VEGFR2-nanobubbles to T24 cells (Fig. 1h).
Preparation of VEGFR2-targeted CD-TK-loaded cationic nanobubbles. Black dots mean the prepared PI-stained nanobubbles. a, b Size distribution and zeta potential of the nanobubbles were determined by Malvern particle-size analyzer. c Light microscopy (× 400). d PI-stained red fluorescence (48 h × 400). e VEGFR2-targeted nanobubbles stained with green fluorescence. f The efficiency with which VEGFR2 bound to nanobubbles was recorded using FCM. g The dose-dependent relationship between the DNA and nanobubbles. h Blue (DAPI) is a representation of cell nuclei, whereas the red (PI) dots imply PI-labeled nanobubbles. Light microscopy Scale bars = 5 μm
The loading capacity of the nanobubbles with DNA was 20.15 ± 1.65 μg/5 × 108. This indicates that the nanobubbles successfully carried the CD-TK double suicide gene, and the binding rate of DNA to nanobubbles was 67.17% ± 0.06%. Green fluorescence could be observed by microscopy 24 h and 48 h after transfection of bladder cancer cells with the double suicide gene-loaded nanobubbles. The ultrasound group showed the highest intensity of green fluorescence, and the empty GFP plasmid was clearly seen in the cytoplasm of the control group (Fig. 2a). Flow cytometry showed that the transfection rate of bladder cancer cells with the nanobubbles was 32.64% in the ultrasound group (Fig. 2b).
Assessment of the double suicide gene-loaded nanobubbles by ultrasound in vivo and in vitro
The prepared double suicide gene-loaded nanobubbles were diluted four times and placed in 1 mL agarose gel. The nanobubbles were observed under ultrasound and contrast-enhanced ultrasound (Fig. 3a), which were significantly decreased after 1 min of destruction to low-frequency US (Fig. 3b). Following injection of 200 μL nanobubbles into nude mice, their development was observed under ultrasound and contrast-enhanced ultrasound (Fig. 3c), which were significantly decreased after 1 min of destruction to low-frequency US (Fig. 3d). The in vitro gel tests showed that the nanobubbles exhibited notably enhanced the contrast. After 1 min of destruction to low-frequency US, the contrast intensity of the nanobubbles decreased significantly, indicating that low-frequency US can effectively destruct the nanobubbles and that the nanobubbles are very sensitive to low-frequency US.
Cytotoxic efficacy of the prodrugs combined with double suicide gene-loaded nanobubbles in bladder cancer cells
The prodrug 5-FC and GCV nanobubbles carrying the CD-TK gene induced apoptosis of the bladder cancer cells as compared with the control group (p < 0.01), which was more efficient than the monotherapy (the 5-FC group along with the GCV group, p < 0.05). Flow cytometry results showed that as compared with the untreated control group, TK/GCV arrested the bladder cancer cells in S phase (p < 0.05), and the ultrasound irradiation + nanobubbles + plasmid S phase cells were different as compared with the untreated group (p < 0.01) along with the GCV group (p < 0.05) (Fig. 4). As demonstrated on the observations in flow cytometry data, cell cycle arrest plots, and comparison of 24 and 48 h early late and total apoptosis, double suicide gene therapy is important in controlling tumor growth, reducing cell survival, and eradicating tumors. We transferred CD-TK genes into human bladder cancer cells and investigated the efficacy of single prodrug and combined prodrugs in vitro. Prodrugs in combination have a more powerful killing effect and a higher apoptotic rate compared with using single prodrug.
Cytotoxic efficacy of the prodrugs combined with suicide gene-loaded nanobubbles on bladder tumors in nude mice
As can be seen from the mouse tumor growth curve, the tumor growth was fastest in the control group followed by the CD-TK double suicide gene-loaded nanobubble group. The tumor growth was significantly slower in the presence of 5-FC or GCV, but the slowest when both the prodrugs were combined. Before the prodrugs were combined with the CD-TK gene-loaded nanobubbles, they showed a marked anti-tumor effect, and the tumor volume was significantly lower than those in the control group (Fig. 5a). TUNEL fluorescence staining was used to detect apoptosis in tumor tissues; fluorescence microscopy showed that 5-FC and GCV prodrugs combined with CD-TK double suicide gene-loaded nanobubbles substantially increased apoptosis (green fluorescence shown in Fig. 5b) as compared with the control group. Apoptosis was also significantly increased in the dual prodrug group as compared with the single prodrug group. PCNA staining was used to detect cell proliferation in tumor tissue. As shown by fluorescence microscopy, proliferative cells in the group containing 5-FC and GCV combined with CD-TK-loaded nanobubbles were significantly reduced as compared with those in control group. Proliferation was also significantly reduced in the dual prodrug group as compared with the single prodrug group. Compared with the other groups, both the prodrugs were combined group showed a better therapeutic effect; 50% of the mice survived for more than 70 days, and the longest survival time was 78 days.
Control group, ultrasound + CD-TK gene-loaded nanobubbles, ultrasound + CD-TK gene-loaded nanobubbles +5-FC, ultrasound + CD-TK gene-loaded nanobubbles + GCV, ultrasound + CD-TK gene-loaded nanobubbles + 5-FC + GCV. a Tumor size, volume change 5 W (mm3). b TUNEL immunofluorescence (green) and PCNA immunofluorescence (red). *p < 0.05, **p < 0.01, **p < 0.001. c Cumulative survival outcome of the T-24 tumor-bearing nude mice in the various treatment groups, scale bars 20 μm
Discussion
Tumorigenesis is a complex pathophysiological process caused by multiple factors involving several genes related to cell proliferation, differentiation, and apoptosis (Wu et al. 2017, 2018). Surgery, radiotherapy, and chemotherapy have many limitations and side effects (Wilhelm et al. 2016; You and Park 2011; Cheng et al. 2018; Wang et al. 2014; Heath and Davis 2008; Celli et al. 2010; Park et al. 2009); therefore, it is of paramount importance to continue to explore novel ways in which to circumvent the limitations of traditional treatment methods. Correcting defective genes or introducing foreign genes can provide a novel way to treat cancer. Gene therapy mainly involves the use of viral and non-viral vectors (Pahle and Walther 2016; Chira et al. 2015; Hayashi et al. 2009). It has been reported that microbubbles can be transfected using ultrasound, and due to the advantages of their non-invasive and non-viral nature and targeted transfection ability, microbubbles have attracted much attention, despite the transfection efficiency being low. If the transfection efficiency can be improved, their application in medical treatment will be of great significance; therefore, the enhancement of transfection efficiency is becoming the focus of related research. In comparison with microbubbles, nanobubbles are smaller in size and more easily penetrate the endothelial space to reach tumor cells for subsequent targeted release. The nanobubbles demonstrated stability in suspension for a period of 1 week at a temperature of 4 °C. Nanobubbles have improved stability and increased gene-loading capacity. Using ultrasound-mediated nanobubbles as the carrier of an anti-tumor suicide gene has the advantages of high gene-loading capacity, good stability, good targeting, no immunogenicity, and easily modified and relatively non-invasive. This method plays a major role in the diagnosis and treatment of tumors (Zhou et al. 2010, 2012; Chen et al. 2012; Xu et al. 2015; Tang et al. 2012; Fischer et al. 2005; Cao et al. 2004).
We successfully prepared nanobubbles loaded with the pGFP-CD-TK double suicide gene, which were detected by red PI fluorescence. The binding rate of nanobubbles and DNA was calculated to be 67.17% ± 0.06%, indicating that the double suicide gene was successfully carried by the nanobubbles and the combination efficiency was high. It was observed that the T-24 cells were successfully transfected with the nanobubbles loaded with the pGFP-CD-TK by ultrasound irradiation. The green fluorescence intensity was the strongest at 48 h and the transfection rate was approximately 32.64%. Sonoporation (transient hole), induced by acoustic cavitation near the cell surface, has been shown to enhance the intracellular delivery of both gene and drug (Brujan 2004; Karshafian et al. 2009; Horie et al. 2009). Our experiments showed that the ultrasound irradiation may produce an instant opening effect of the T-24 cells, indicating the sound hole effect. In the agarose gel model, in comparison with the PBS control group, the ultrasound-mediated nanobubbles showed obvious enhancement in vitro. After low-frequency US irradiation, the nanobubbles were destructed. Our study indicates that nanobubbles are very sensitive to low-frequency US.
At the same time, the T-24 cells were still growing. The pEGFP-CD-TK double suicide gene-loaded nanobubbles were injected into the tail vein of nude mice to reach the tumor tissue. The nanobubbles were observed to be concentrated in a mass under the contrast mode, and the enhanced signal was not observed following low-frequency US irradiation. The nanobubbles carrying the CD-TK double suicide gene were irradiated by ultrasound. The cavitation effect and the sound hole effect were produced to achieve the release of the suicide gene at the tumor site. The present results show that nanobubbles loaded with the CD-TK double suicide gene were successfully created and can target the tumor. Ultrasound-facilitated gene delivery has huge translational potential in the clinic; thus, the present study may provide useful information for researchers in the field of gene delivery.
In the present study, the human bladder cancer cell line, T-24, was transfected with CD-TK double suicide gene-loaded nanobubbles. In the presence of the prodrug, 5-FC, the rate of cytotoxicity was 25.8%; in the presence of the prodrug, GCV, the rate of cytotoxicity was 35.4%; and in the presence of both prodrugs, the rate of cytotoxicity was 58.6% (Fig. 4). In flow cytometry, the percentage of cells arrested in S phase increased massively in the prodrug combination group as compared with the control and single prodrug groups. In the in vivo experiments, the pEGFP-CD-TK double suicide gene-loaded nanobubbles were injected into the tail vein of nude mice, and IP injection of 5-FC or GCV caused tumor growth suppression. The combined effect was even more significant, indicating the synergistic effect of the CD-TK double suicide gene. Moreover, TUNEL and PCNA immunofluorescence staining of the five groups of tumors were compared to observe apoptosis and cell cycle arrest. Initially, the two stages of the cell cycle G1-to-S phase and G2-to-M phase were active and easily manipulated by the environment. Thymidine kinase encoded by the CD-TK double suicide gene could phosphorylate GCV to produce GCV-triphosphate—a nucleotide analog—which combined with the S phase of the cell and replaced the normal responding nucleotides. This resulted in interference with the biosynthesis of DNA, causing the cells entering the S phase to be mutated; therefore, the CD-TK gene suppressed the growth of the tumor cells at the gene level. Cytosine deaminase encoded by the CD-TK gene transformed the non-toxic prodrug, 5-FC, to 5-FU, allowing conversion to the toxic 5-FUTP or 5-FdUMP, which improved the response of the cells to the GCV prodrug and strengthened the cytotoxic effect of GCV. The two enzymes encoded by the CD-TK double suicide gene complement each other to enhance their lethality; moreover, 5-FdUMP can permeate cells, while the three phosphoric acid GCV is unable to permeate cells. The combination of the two can simultaneously destroy tumor cells in vivo and in vitro (Wirth et al. 2013; Li et al. 2012; Yang et al. 2018; Lv et al. 2017; Yoshimura et al. 2001; Carrio et al. 2002). The 5-FC and GCV prodrugs combined with CD-TK double suicide gene-loaded nanobubbles were able to inhibit the proliferation of bladder tumor cells in vitro and bladder cancer in nude mice, and the dual prodrugs had a more significant effect than the single prodrugs.
Conclusions
The nanobubbles synthesized in the present study are of great value to the molecular imaging field and an important tool for the follow-up study of bladder cancer. The paper describes the development of cationic nanobubbles that are covalently modified with a ligand to specifically target VEGFR2. These modified bubbles are loaded with a plasmid designed to be overexpressed by cancer cells and induce cell death. In conclusion, here, we report a strategy using ultrasound to trigger the destruction of nanobubbles, which in turn enhanced the transfection efficiency and in vitro therapeutic efficacy.
Availability of data and materials
Materials are available upon request.
Abbreviations
- UMND:
-
Ultrasound-mediated nanobubble destruction
- CD:
-
Cytosine deaminase
- US:
-
Ultrasound
- 5-FC:
-
5-Fluorine cytosine
- TK:
-
Thymine kinase gene
- GCV:
-
Ganciclovir
- DPPC:
-
1,2-Dipalmitoyl-sn-glycero-3-phosphocholine
- (DSPE-PEG 2000)-COOH:
-
Carboxyl-modified1,2-distearoyl-sn-glycero-3-phosphoetha-nolamine
References
Amer MH (2014) Gene therapy for cancer: present status and future perspective[J]. Mol Cell Ther 2:27
Bruix J, Gores GJ, Mazzaferro V (2014) Hepatocellular carcinoma: clinical frontiers and perspectives. Gut 63:844–855
Brujan EA (2004) The role of cavitation microjets in the therapeutic applications of ultrasound. Ultrasound Med Biol 30(3):381–387
Cao HQ, Meng XM, Liu DQ et al (2004) Killing effect of coexpressing cytosine deaminase and thymidine kinase on rat vascular smooth muscle cells [J]. Chin Med J (Engl) 117(10):1464–1470
Carrio M, Visa J, Cascante A et al (2002) Intratumoral activation of cyclophosphamide by retroviral transfer of the cytochrome P450 2B1 in a pancreatic tumor model. Combination with the HSV-tk/GCV system. J Gene Med 4:141–149
Celli JP, Spring BQ, Rizvi I et al (2010) Imaging and photodynamic therapy: mechanisms, monitoring, and optimization. Chem Rev 110(5):2795–2838
Chen ZY, Sun XF, Liu JQ et al (2012) Augmentation of transgenic expression by ultrasound mediated liposome nanobubbles destruction [J]. Mol Med Rep 5:964–970
Chen MC, Lee CF, Huang WH et al (2013) Magnolol suppresses hypoxia-induced angiogenesis via inhibition of HIF-1α/VEGF signaling pathway in human bladder cancer cells. Biochem Pharmacol 85(9):1278–1287
Chen MC, Hsu WL, Chang WL et al (2017) Antiangiogenic activity of phthalides-enriched Angelica Sinensis extract by suppressing WSB-1/pVHL/HIF-1α/VEGF signaling in bladder cancer. Sci Rep. 7(1):5376
Cheng K, Sano M, Jenkins C et al (2018) Synergistically enhancing therapeutic effect of radiation therapy with radiation activatable and reactive oxygen species-releasing nanostructures. ACS Nano 12(5):4946–4958
Chira S, Jackson CS, Oprea I et al (2015) Progresses towards safe and efficient gene therapy vectors [J]. Oncotarget 6:30675–30703
Du L, Jin Y, Zhou W, Zhao J (2011) Ultrasound-triggered drug release and enhanced anticancer effect of doxorubicin-loaded poly (d, l-lactide-co-glycolide)-methoxy-poly(ethylene glycol) nanodroplets. Ultrasound Med Biol 37:1252–1258
Fischer U, Steffens S, Frank S et al (2005) Mechanisms of thymidine kinase/ganciclovir and cytosine deaminase/5-fluorocytosine suicide gene therapy-induced cell death in glioma cells [J]. Oncogene 24:1231–1243
Hayashi S, Mizuno M, Yoshida J et al (2009) Effect of sonoporation on cationic liposome mediated IFN beta gene therapy for metastatic hepatic tumors of murine colon cancer [J]. Cancer Gene Ther 16:638–643
Heath JR, Davis ME (2008) Nanotechnology and cancer. Annu Rev Med 59:251–265
Horie T, Nishino T, Baba O et al (2009) MicroRNA-33 regulates sterol regulatory element-binding protein 1 expression in mice. Ultrasound Med Biol 35(5):847–860
Hu C, Zhang R, Jiang D (2019) TMEM16A as a potential biomarker in the diagnosis and prognosis of lung cancer. Arch Iran Med. 22(1):32–38
Karshafian R, Bevan PD, Williams R et al (2009) Sonoporation by ultrasound-activated microbubble contrast agents: effect of acoustic exposure parameters on cell membrane permeability and cell viability. Ultrasound Med Biol 35(5):847–860
Li XH, Zhou P, Wang LH et al (2012) The targeted gene (KDRP-CD/TK) therapy of breast cancer mediated by SonoVue and ultrasound irradiation in vitro [J]. Ultrasonics 52:186–189
Li J, Zhou P, Li L, Zhang Y et al (2016) Effects of cationic nanobubbles carrying CD/TK double suicide gene and αVβ3 integrin antibody in human hepatocellular carcinoma HepG2 cells [J]. PLoS One 11:e0158592
Lv Y, Cao Y, Li P et al (2017) Ultrasound-triggered destruction of folate-functionalized mesoporous silica nanoparticle-loaded microbubble for targeted tumor therapy. Adv Healthc Mater 6(18):1700354
Obrien T, Cranston D, Fuggle S et al (1995) Different angiogenic pathways characterize superficial and invasive bladder cancer. Cancer Res 55:510–513
Pahle J, Walther W (2016) Vectors and strategies for nonviral cancer gene therapy [J]. Expert Opin Biol Ther 16:443–461
Park H, Yang J, Lee J, Haam S et al (2009) Multifunctional nanoparticles for combined doxorubicin and photothermal treatments. ACS Nano 3(10):2919–2926
Shang H, Wu B, Liang X et al (2019) Evaluation of therapeutic effect of targeting nanobubbles conjugated with NET-1 siRNA by shear wave elastography: an in vivo study of hepatocellular carcinoma bearing mice model. Drug Deliv 26(1):944–951
Siegel R, Naishadham D, Jemal A (2013) Cancer statistics, 2013. CA Cancer J Clin 63:11–30
Tang Q, He X, Liao H et al (2012) Ultrasound nanobubbles contrast agent-mediated suicide gene transfection in the treatment of hepatic cancer [J]. Oncol Lett 4:970–972
Vago R, Collico V, Zuppone S et al (2016) Nanoparticle-mediated delivery ofsuicidegenesin cancertherapy. Pharmacol Res. 111:619–641
Verma A, Degrado J, Hittelman AB et al (2011) Effect of mitomycin C on concentrations of vascular endothelial growth factor and its receptors in bladder cancer cells and in bladders of rats intravesically instilled with mitomycin C. BJU Int 107(7):1154–1161
Wang Y, Zhou K, Huang G et al (2014) A nanoparticle-based strategy for the imaging of a broad range of tumours by nonlinear amplification of microenvironment signals. Nat Mater 13:204–212
Wilhelm S, Tavares AJ, Dai Q et al (2016) Analysis of nanoparticle delivery to tumours. Nat Rev Mater. 1:16014
Wirth T, Ylä-Herttuala S (2014) Gene therapy used in cancer treatment. Biomedicines 2(2):149–162
Wirth T, Parker N, Ylä-Herttuala S (2013) History of gene therapy [J]. Gene 25:162–169
Wood AK, Sehgal CM (2015) A review of low-intensity ultrasound for cancer therapy [J]. Ultrasound Med Biol 41:905–928
Wu M, Wang Y, Wang Y et al (2017) Paclitaxel-loaded and A10-3.2 aptamer-targeted poly(lactide-co glycolic acid) nanobubbles for ultrasound imaging and therapy of prostate cancer [J]. Int J Nanomedicine 12:5313–5330
Wu M, Zhao H, Guo L et al (2018) Ultrasound mediated nanobubble destruction (UMND) facilitates the delivery of A10-3.2 aptamertargeted and siRNA-loaded cationic nanobubbles for therapy of prostate cancer. Drug Deliv 25(1):226–240
Xu Y, Xie Z, Zhou Y et al (2015) Experimental endostatin-GFP gene transfection into human retinal vascular endothelial cells using ultrasound-targeted cationic nanobubbles destruction [J]. Mol Vis 21:930–938
Yang J, Su H, Sun W et al (2018) Dual chemodrug-loaded single-walled carbon nanohorns for multimodal imaging-guided chemo-photothermal therapy of tumors and lung metastases. Theranostics 8(7):1966–1984
Yoshimura I, Suzuki S, Tadakuma T et al (2001) Suicide gene therapy on LNCaP human prostate cancer cells. Int J Urol 8:S5–S8
You HB, Park K (2011) Targeted drug delivery to tumors: myths, reality and possibility. J Control Release 153:198–205
Zarogoulidis P, Darwiche K, Sakkas A et al (2013) Suicide gene therapy for cancer–current strategies [J]. J Genet Syndr Gene Ther 4:16849
Zhou S, Li S, Liu Z et al (2010) Ultrasound-targeted nanobubbles destruction mediated herpes simplex virus-thymidine kinase gene treats hepatoma in mice [J]. J Exp Clin Cancer Res 29:170
Zhou SJ, Li SW, Wang JJ et al (2012) High-intensity focused ultrasound combined with herpes simplex virus thymidine kinase gene-loaded ultrasound-targeted nanobubbles improved the survival of rabbits with VX2 liver tumor [J]. J Gene Med 14:570–579
Acknowledgements
This study was approved by The Animal Ethics Committee of Chongqing Medical University and the ARRIVE guidelines followed for the welfare of the animals.
Funding
The present work was sponsored by the National Science Foundation of China No. 81650003, Research on the Frontiers and Application of Chongqing Science and Technology Commission No. cstc2015jcyjA10030, Key Medical Scientific Research Project of Chongqing Municipal Health department No. 2012-1-037, and the Science and Technology Project of Chongqing Yuzhong District No. 20130117, Project of Science and Technology Innovation Foundation of Zhongnan Hospital of Wuhan University No. znpy2018021.
Author information
Authors and Affiliations
Contributions
CH and RG Z conceived of and designed the study. CH, MW, JW, and DP J performed the experiments. CH, JW, and RG Z analyzed and interpreted the data. CH wrote the manuscript. RG Z and JW revised the paper. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hu, C., Jiang, D., Wu, M. et al. Ultrasound-mediated nanobubble destruction (UMND) facilitates the delivery of VEGFR2-targeted CD-TK-loaded cationic nanobubbles in the treatment of bladder cancer. J Cancer Res Clin Oncol 146, 1415–1426 (2020). https://doi.org/10.1007/s00432-020-03160-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-020-03160-7