Abstract
Purpose
Non-small cell lung cancer (NSCLC) is the most common type of lung cancer and ranked top in terms of incidence and mortality in men and women. Recently, improvements in treatment approaches for NSCLC have reported, but still, there is a need to devise innovative treatment strategies, especially to manage the advanced and metastatic stage of NSCLC. Aloperine (ALO), an herbal alkaloid, has exerted anti-cancer effects in many cancers. However, the use of any chemotherapeutic agents is dose limited due to possible adverse effects and drug-resistance issues. Therefore, a combination of chemotherapy with viral-based targeted gene therapy may provide a novel treatment strategy for NSCLC.
Methods/results
In this study, the results of the MTT and flow cytometry-based assays showed that Aloperine–Adbic (adenoviral vector expressing p14ARF/p53) combined treatment on NSCLC cells synergistically produced anti-proliferative effects, induced apoptosis, and arrested cell cycle at the G1 phase. Furthermore, the expression analysis suggested that the p53/p21 pathway might contribute to achieving aforesaid cytotoxic effects. The ALO–Adbic combined treatment prolonged the percent survival of NSCLC xenograft models.
Conclusion
In conclusion, ALO–Adbic combination can produce synergistic anti-cancer effects at low doses, and may offer a more effective and less toxic new treatment strategy for NSCLC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lung cancer is recognized as the most common cancer, with a high incidence and mortality rate in the world (Torre et al. 2016). It nearly perpetually has a poor prognosis, and it is the most consistent reason for lung cancer-related deaths (1.6 million annually) (Torre et al. 2015). In Northern America, Northern Europe, and Australia, interestingly, the female percentage of lung cancer patients is on the rise (Ferlay et al. 2012; Jemal et al. 2018). Lung cancer has two primary types, including non-small-cell lung cancer (NSCLC) and small cell lung cancer (SCLC); among these, NSCLC is the most prevalent type of lung cancer (Molina et al. 2008). NSCLC accounts for 90% of all lung cancers (Thomas et al. 2015). Lung adenocarcinoma (LUAD) and lung squamous cell carcinoma (LUSC) are two histological variants of NSCLC (Herbst et al. 2018). In both men and women, it is the most prevalent cancer, with an occurrence rate higher than the combined occurrence of colorectal, breast, and cervical cancers (Maher et al. 2012; Spiro and Porter 2002). At the time of diagnosis, about two-thirds of all NSCLC patients, diagnosed with advanced cancer stage (stage IIIB or IV) (Miller et al. 2016), which left these patients with treatment options of using palliative chemotherapy if targeted anti-cancer agents are of no benefit. Although chemotherapy is nonetheless the most common choice of treatment for operable and inoperable lung cancer patients; however, in patients with advanced NSCLC, the medical advantages of presently used chemotherapeutic agents are modest. At the same time, chemotherapy associated with the drug-resistance issues and the severe side effects like nephrotoxicity and myelosuppression (Andrews and Howell 1990; Gately and Howell 1993; Kelland 2007; Ramalingam and Belani 2008). Therefore, it is urgently needed to devise new treatment strategies using less toxic and more effective anti-cancer agents to substitute the conventional chemotherapeutic agents for a better NSCLC treatment.
Aloperine (ALO) is an alkaloid, extracted from Sophora alopecuroides L., reported having a strong anti-allergic and anti-inflammatory activities (Yuan et al. 2010; Zhou et al. 1989). Quinolizidine alkaloids have recognized as the bioactive components of ALO. Literature states that ALO contains more than 20 types of quinolizidine alkaloids (Wang et al. 2012); of these, sophoridine, matrine, and oxymatrine exhibited anti-tumor activities (Liang et al. 2012; Zhang et al. 1998, 2001, 2012). Multiple studies reported the anti-tumor activities of ALO in many cancers, including multiple myeloma, hepatocellular carcinoma, osteosarcoma, breast, colon, thyroid, and prostate cancers. ALO mainly induces apoptosis and arrests cell cycle advancement to inhibit the growth of tumors (Chen et al. 2018; Lee et al. 2018; Wang et al. 2015; Xu et al. 2017; Zhang et al. 2014). Underlying mechanisms responsible for ALO-induced anti-tumor activities include blocking of Ras signalling pathway in breast cancer, G2/M cell cycle arrest in hepatocellular carcinoma and colon cancer, G1/S cell cycle arrest in prostate cancer, Caspase-Dependent Apoptosis in osteosarcoma, and thyroid cancer (Chen et al. 2018; Lee et al. 2018; Ling et al. 2018; Liu et al. 2019; Tian et al. 2018; Zhang et al. 2014). Therefore, ALO exerted diverse anti-tumor effects on various cancers. However, underlying mechanisms for ALO-induced anti-tumor effects on lung cancer are not well known.
The P53 gene is considered a potent tumor suppressor gene due to its role in cell cycle arrest and triggering of apoptotic pathways. The p53 lead tumor suppression activities initiated in response to various cellular abnormalities associated with cancer, like DNA damage and oncogene expression. Cascade of tumor suppression events started with the p53 protein stabilization, which in turn triggered the expression of its downstream genes responsible for tumor growth suppression (Wu et al. 1993). Literature states that 50–60% of all cancers have either deficiency or mutation in the p53 gene. Lack of p53 protein or its mutated state cannot initiate the p53 pathway, which might be a key factor in the development of cancers (Greenblatt et al. 1994; Hollstein et al. 1991). Several oncogenes (E2F-1, beta-catenin, myc, ras, and adenovirus E1A.) activate p53 through a series of positive feedback events, which also facilitate the transcription activation of p14ARF (Bates et al. 1998; de Stanchina et al. 1998; Palmero et al. 1998). p14ARF then plays its role in stabilizing and maintained the enhanced expression of the p53 by inhibiting the activity of HDM-2 ubiquitin ligase (Honda and Yasuda 1999). The loss of p53 and p14ARF has reciprocally reported in many cancers (Kannan et al. 2000; Pinyol et al. 2000). Mutated p53 and p14ARF tumor suppressor genes most commonly found in NSCLC. It is found that inactivated p53 and p14ARF genes are present in 90% and 70% of NSCLC, respectively (Herbst et al. 2018).
Viral-based gene therapies in humans primarily carried out using adenoviral vectors. These were considered a suitable choice due to exhibiting attractive advantages for gene therapy like improved transduction efficiency, relatively low toxicity, and cost-effective construction of vectors at a large scale (Kallel and Kamen 2015).
In this study for the first time, we have used replication-defective therapeutic adenoviral vectors expressing p14ARF/p53 tumor suppressor genes (Adbic) in combination with ALO chemotherapeutic drug against NSCLC, in vitro, and in vivo. To achieve maximum anti--tumor effects and to make this therapeutic approach a targeted approach, we have administered replication-defective therapeutic adenoviral vectors through E1s’ modified mesenchymal stem cell (MSC-E1s) vehicle system. These MSC-E1s contain type C adenovirus E1A/E1B genes responsible for viral replication. This delivery system supports the propagation and targeted delivery of vectors to tumor site (Muhammad et al. 2019). We have assessed the synergistic anti-cancer effects of combined therapy with ALO and Adbic against NSCLC, in vitro, and in vivo. This combined therapy might provide a synergistic, more effective, and less toxic treatment alternatives for NSCLC.
Materials and methods
Cell lines
Non-small cell lung cancer cells NCI-H1944 (ATCC, CRL-5907) and NCI-H1869 (ATCC, CRL-5900) purchased from ATCC. These were grown and maintained according to the instruction provided by the manufacturer. E1A/E1B-modified mesenchymal stem cell were prepared and cultured as previously described (Muhammad et al. 2019).
Adenoviral vectors and drug
Replication-defective therapeutic adenoviral vectors expressing p14ARF/p53 tumor suppressor gene and replication-defective adenovirus containing GFP (AdGFP), constructed by Huang et al., were kindly provided by Dr. Yinghui Hunag (Huang et al. 2003). Aloperine (98% HPLC-grade pure) procured from Selleck (Houston, TX, USA). ALO was reconstituted in DMSO for experimental usage and kept at − 20 °C for storage.
Xenograft models
Six-to-eight-week old BALB/C nude mice, purchased from the Chinese academy of sciences, were administered 0.1 ml of NCI-H1944 and NCI-H1869 NSCLC cells (3 × 106 cells/ml) subcutaneously into the hind flank. Fifteen days following injection of NSCLC cells, tumors (Vol ~ 50 mm3) appeared in all mice. Tumor mice models randomized into treatment groups, and these treated with PBS, Aloperine, Adbic, or combination of Aloperine and Adbic. Treated mice models kept under surveillance for 90 days. Percent survival of treatment groups was measured and compared using Originpro 9 software. Dead mice in control and treatment groups euthanized, tumors removed, and their volume measured and compared. Tumor volume measured by the formula: tumor volume = 1/2 L × w2. in vivo experimentations on mice models were conducted by strictly obeying the NIH guidelines for the Care and Use of Laboratory Animals.
Preliminary dose–response experiments performed to assess the suitable treatment dose of Aloperine for the study. Experiment models in these experiments were given injections of 50, 40, 30, 20, and 10 mg/kg Aloperine intraperitoneally. Finally, we observed the 30 mg/kg dose suitable for this study (Supplementary Fig. 2B). Adbic dose (5 × 105 MSC-E1s loaded with Adbic 3 × 107 pfu/mouse) for xenograft models was observed efficient in our previous study (Muhammad et al. 2019). We also use the same dose in this study.
Cell viability assay
Non-small cell lung cancer cells NCI-H1944 and NCI-H1869 were cultured in 24-well plates. Cytotoxicity of Aloperine and Adbic, at different concentrations, was assessed against NSCLC cells in DMEM containing 2% FBS for 4 h (37 °C, 5%CO2), and then, treated cells were transferred to 96-well plates containing complete medium (3 wells/ treated and control cells) with 3 × 103 cells/ well. Cells were incubated for 48 h (37 °C, 5%CO2), and then, 20 µl of MTT (5 mg/ml) reagent were added. After 4 h culture medium in wells replaced with 200 µl of DMSO and 96-well plates were left for 10 min at room temperature, and then, cell viability was measured by taking OD of each well 490 nm using microplate reader and expressed as a percentage of control cell viability.
Combination studies
NSCLC cells NCI-H1944 and NCI-H1869 were subjected to different concentrations of Aloperine (0.05, 0.1, 0.25, 0.5, 0.75, and 1.0 mM) and a fixed concentration of Adbic (25 MOI). The CI-isobologram method was applied on obtained data to assess the possible synergistic anti-cancer effects of ALO–Adbic combined therapy using CompuSyn 2.0 program created by Chou and Martin (Chou and Martin 2005). The three outcomes CI < 1, CI = 1, and CI > 1 considered for synergy, additive effect, and antagonism, respectively (Chou 1991).
Apoptosis analysis
NSCLC cells NCI-H1944 and NCI-H1869 were cultured in 6-well plates. After 24 h, cells were given the treatment with ALO, Adbic, or combination of ALO and Adbic. After 48 h, culture media removed, and the monolayer of adherent cells washed with PBS. 1 μg/mL of DAPI stain was applied. Cells left at room temperature for 10 min. After incubation, washing is done. The apoptotic nuclei were detected under 200X magnification using a fluorescent microscope with a 340/400 nm excitation filter and scored according to the percentage of apoptotic nuclei found in samples containing 200–300 cells.
NSCLC cells NCI-H1944 and NCI-H1869 were cultured in 6-well plates, tumor cells (1 × 106 cells/well) treated with ALO, Adbic, or combination of ALO and Adbic. After 24 h and 48 h, cells were collected and double-stained with V-FITC/propidium iodide (Invitrogen, Carlsbad, CA, USA) following the procedure provided by the manufacturer. Stained cells were subjected to flow cytometry for apoptosis. Apoptosis was measured using FACScalibur™ Becton Dickinson) flow cytometer.
Cell cycle analysis
The cells were treated with ALO, Adbic, or combination of ALO and Adbic for 48 h. After incubation, treated cells were collected, mixed with ice-cold 70% ethanol for fixation. Cells were kept at − 20 °C for 48 h. Then, 500 µl of PBS used to wash the cells, and washing repeated three times. Cells were stained with 50 μg/mL of Propidium Iodide (PI) and 25 μg/mL of RNase A (Invitrogen, Carlsbad, CA, USA) and kept in the dark at room temperature for 15 min. These stained cells were subjected to cell cycle analysis using FACScalibur™ (Becton Dickinson) flow cytometer. FlowJo_V10 software used to analyze the data.
Western blotting analysis
NSCLC cells were cultured and subjected to treatment with ALO, Adbic, or combination of ALO and Adbic for 48 h. Then, cells collected and cell lysate obtained by treating the cells with RIPA lysis buffer on ice for 30 min. 30 ug of total protein was subjected to electrophoresis on 15% polyacrylamide gel and transferred onto PVDF membranes (Millipore, Billerica, MA, USA). Membranes were then subjected to treatment with primary antibodies against p53 (1:500), p21 (1:500), Cyclin E (1:500), CDK2 (1:500), pRb (1:500), E2F1(1:500), Bax (1:500), Bcl 2 (1:500), Caspase 3 (1:500), and Caspase 9 and β-actin (BIOSS Antibodies, Boston, USA). After overnight incubation at 4 °C, three times washing of membranes was carried out (15 min each) with TBS. Then, the membranes were subjected to secondary antibodies treatment for 2 h at room temperature. Then, after washing of membranes with TBS, protein bands were quantified using Kodak digital camera and analysis software (Kodak, Rochester, NY, USA). The data normalized to β-actin for analyses and plotting.
Statistical analysis
The data sets from three independent experiments were subjected to statistical analysis (Mean ± Standard deviation, Student’s T Test) to evaluate the significance of experimental results. For statistical analysis, Originpro 9 software was used. Results showed that P value < 0.05 were considered significant.
Results
Tumor suppression by Aloperine, Adbic, or combination of Aloperine and Adbic, Invitro
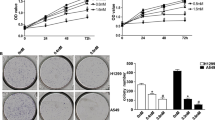
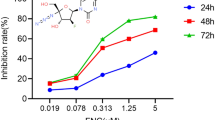
The invitro tumor suppression activity of Aloperine, Adbic, or combination of Aloperine and Adbic against NSCLC cells evaluated. NCI H-1944 and NCI H-1869 cells were treated with Adbic (5.10, 25, 50, 100 MOI) and Aloperine (0.05, 0.1, 0.25, 0.5, 0.75, 1.0 mM) for 48 h, and then, MTT assay was performed to measure the cell viability. Both anti-cancer agents suppressed the tumor cell growth effectively in a dose-dependent manner (Fig. 1a, b). Half, maximal inhibitory concentration (IC50) values of Aloperine and Adbic, were observed 0.25 mM and 50 MOI, respectively.
Effects of Aloperine, Adbic alone, or Aloperine and Adbic combined treatment on NSCLC cells. a NCI-H1944 and b NCI H-1869 cells treated with Aloperine, Adbic, or Aloperine–Adbic combination for 48 h. The cytotoxic effects of the anti-cancer agents assessed through the MTT assay. X-axis (bottom) represents ALO (mM) concentrations, while X-axis (top) represents Adbic (MOI) concentrations. Results shown as the mean ± standard deviation of triplicate samples
We evaluated the maximum tumor suppression activity of these anti-cancer agents in combination treatment. Adbic at 25 MOI (< IC50 Value) concentration combined with various Aloperine concentrations 0.05, 0.1, 0.25 mM (< IC50 Value). Combined treatment with Adbic and Aloperine at concentrations described above remarkably suppressed the tumor cell growth as compared to Adbic or Aloperine alone treatment. Combined treatment significantly reduced the IC50 value compared to Adbic or Aloperine alone treatment (Adbic 25 vs. 50 MOI, Aloperine 0.05 vs. 0.25 mM). The IC50 value of Adbic and Aloperine reduced 50% and 80%, respectively, in combined treatment (Fig. 1). Experimental data showed that anti-tumor cell proliferation activity of combined treatment was significantly (P < 0.05) higher than the alone treatment.
To evaluate the possible synergistic effects of combined therapy, experimental data were subjected to synergy analysis. Combined treatment with Adbic 25 MOI (Fixed concentration) and Aloperine 0.05, 0.1, 0.25 mM (variable concentrations) against NCI H-1944 cells revealed fraction-affected (Fa) values 0.3, 0.18, 0.08 and combination index (CI) values 0.5, 0.3, 0.2. Similar combined treatment against NCI H-1869 cells revealed Fa values 0.38, 0.25, 0.12, and CI values 0.6, 0.37, 0.25 (Fig. 2a). Data indicate that tumor suppression effects of combined treatment against both cell lines are highly synergistic (CI < 1). The most synergistic effects were observed in combined treatment with Adbic 25 MOI and Aloperine 0.25 mM concentration (Fig. 2b).
Synergistic effects of Aloperine and Adbic combined treatment. a Measurements of the Combination Index (CI) of Aloperine and Adbic in NCI-H1944 and NCI H-1869 cells were carried out using the CI-isobologram method. CI < 1 represents the synergistic effects. b Cytotoxic effects of Aloperine (0.25 mM), Adbic (25 MOI), or the combination of Aloperine and Adbic on NSCLC cells growth
Treatment with Adbic, Aloperine, or combination of Adbic and Aloperine-induced apoptosis in NSCLC cells
Rate of apoptosis in NCI H-1944 and NCI H-1869 cells resulted from treatment with Adbic (25 MOI), Aloperine (0.25 mM), or combination of Adbic (25 MOI) and Aloperine (0.25 mM) for 48 h by DAPI staining. The data showed that the nuclei of the control group cells showed no changes, but the condensation of nuclei was observed in ALO, Adbic alone treatment groups, and these changes are more prominent in ALO–Adbic combined treatment groups (Fig. 3a).
Apoptosis induction analysis. a NSCLC cells were treated with ALO, Adbic alone, or ALO–Adbic combination for 48 h, and cells stained with DAPI. Apoptotic cells visualized under the fluorescence microscope. The data presented as the mean of triplicate experiments. b Apoptosis induced by Aloperine, Adbic, or Aloperine and Adbic combined treatment in NSCLC cells after 48 h assessed. NSCLC cells were double-stained with Annexin-V-FITC/PI staining and subjected to flow cytometry analysis by FASCcalibur. c Histogram represents the apoptosis rate in NSCLC cells. Data are representatives of triplicate experiments with mean ± SD. d Expression analysis of genes involved in apoptosis: the expression level of Bax, Bcl2, Caspase 9, and Caspase 3 proteins in pancreatic cancer cells, after 48 h treatment with API, Adbic alone, or API–Adbic combination. Equivalent loading was verified by stripping membranes and re-probing with the actin antibody
Further rate of apoptosis was assessed in treated cells after 24 h and 48 h, by flow cytometry using Annexin-V/Propidium Iodide (PI) staining. Both anti-cancer agents, alone or in combination, caused apoptosis in NSCLC cells compared to control cells after 24 h (Supplementary Fig. 1A) and 48 h (Fig. 3b). Data showed that frequency of apoptosis was significantly (P < 0.05) higher in cells received combined treatment for 2 h h with Adbic and Aloperine (NCI-H 1944 = 57%, NCI-H 1869 = 61%) compared to cells received alone treatment with Adbic (NCI H-1944 = 26%, NCI H-1869 = 23%) or Aloperine (NCI H-1944 = 23%, NCI H-1869 = 20%) (Supplementary Fig. 1B). Results exhibit that rate of apoptosis was significantly (P < 0.05) higher in cells received combined treatment for 48 h with Adbic and Aloperine (NCI H-1944 = 83%, NCI H-1869 = 78%) compared to cells received alone treatment with Adbic (NCI H-1944 = 38%, NCI H-1869 = 33%) or Aloperine (NCI H-1944 = 30%, NCI H-1869 = 28%) (Fig. 3c).
We also evaluated the expression of pro-apoptotic genes Bax, Caspase 9, Caspase 3, and anti-apoptotic gene Bcl2. Upon expression analysis, the level of Bax, Caspase 9, and Caspase 3 proteins found out to be raised, while the expression of Bcl2 protein found out to be reduced in NSCLC cells received treatments compared to control cells. This expression pattern indicates the induction of apoptosis in treated cancer cells (Fig. 3d).
Effects of Adbic, Aloperine alone, or combined treatment on NSCLC cell cycle
NCI H-1944 and NCI H-1869 cells treated with Adbic (25 MOI), Aloperine (0.25 mM), or combination of Adbic (25 MOI) and Aloperine (0.25 mM) for 48 h. The treated cells subjected to cell cycle analysis by flow cytometry using PI staining. Cell cycle analysis revealed that the distribution of treated cells was more in the Sub-G1/G1 phase compared to control cells. Results indicate that treatments with anti-cancer agents either alone or combined arrested the G1-S phase transition of the cell cycle, but the distribution of cells received combined treatment was significantly (P < 0.05) higher in the Sub-G1/G1 phase compared to cells received alone treatment with Adbic or Aloperine (Fig. 4).
Effects of Aloperine, Adbic, or Aloperine and Adbic combined treatment on NSCLC cell cycle. a NCI H-1944 and NCI H-1869 cells subjected to cell cycle analysis after 48 h treatment with both anti-cancer agents. The distribution of treated cells in different phases of the cell cycle was analyzed through flow cytometry by FASCcalibur. b Histogram represents the apoptosis rate in NSCLC cells. Data are representatives of triplicate experiments with mean ± SD shown
Adbic, Aloperine alone, or in combination treatments arrest cell cycle at G1 phase and induce apoptosis through p53/p21 pathway activation.
We evaluated the molecular alterations that lead to cell cycle arrest at the G1 phase and responsible for apoptosis induction. We performed protein expression analysis Adbic, Aloperine alone, and in combination. We assessed the expression of genes involved in p53/p21-triggered cell cycle arrest and apoptosis. NSCLC cells treated with anti-cancer agents alone or in combination revealed elevated expression of p53 and p21 proteins, while the expression of Cyclin E, CDK2, pRb, and E2F1 found to be decreased in both cell lines compared to control cells (Fig. 5). Combination treatment resulted in much higher expression of p53 and p21 and lower expression of Cyclin E, CDK2, Rb, and E2F1 proteins compared to alone treatment, which provides a proof of rationale that combination treatment can efficiently halt cell cycle at G1 phase and lead to tumor cell death.
The Aloperine–Adbic combined treatment prolonged the survival rate in NSCLC xenograft models
To confirm the efficiency of combined treatment, in vivo experimentations performed on male nude mice-bearing NSCLC cell tumors. Twenty-eight NSCLC xenograft models randomized into four groups, each containing 7 mice. The control group received only PBS. Three treatment groups subjected to treatment with anti-cancer agents. Two treatment groups received alone treatment with Aloperine (30 mg/kg) intraperitoneally on days 1 and 9 of treatment and 5 × 105 E1A/B containing mesenchymal stem cells (MSC-E1s) loaded with Adbic 3 × 107 pfu/mouse on days 1, 5, and 9 of treatment via the tail vein. Adbic adenoviral vectors were delivered through MSC-E1s, because this delivery system supports propagation of adenoviral vectors and delivers adenoviral vectors specifically to the tumor site. Prior systemic delivery of MSC-E1s loaded Adbic maximum transfection was achieved. For this purpose, 5 × 105 MSC-E1s transfected with replication-defective adenovirus containing GFP (AdGFP) at the same concentration as Adbic used in this study. MSC-E1s observed under a fluorescent microscope for green fluorescene at different time intervals. A maximum green fluorescene indicative of maximum transfection was observed after 24 h (Supplementary Fig. 3). Before systemic administration, Adbic were allowed to transfect MSC-E1s for 24 h to achieve maximum transfection. The third treatment group received combined therapy with Aloperine 30 mg/kg (days 1 and 9 of treatment) and 5 × 105 MSC-E1s loaded with Adbic 3 × 107 pfu/mouse (days 1, 5, and 9 of treatment). After treatment, control and treatment groups were observed for 90 days, and their survival rate was noted, and survival function data were plotted using originpro 9.0. Results revealed remarkable differences in the survival rate of treated mice models compared to the control group. On day 43 of treatment, 100% of mice models received PBS (control group) died. Percent survival of treatment groups received Aloperine, Adbic, or Aloperine–Adbic combined treatment was 86%, 86%, and 100%, respectively, by day 43 of treatment. On day 90 of the treatment, the percent survival of treatment group received the Aloperine–Adbic combined treatment was extraordinarily high (72%) compared to Aloperine and Adbic alone treatment groups exhibited the percent survival 42% and 42%, respectively (Fig. 6a). Similarly, mice in treatment groups showed a reduction in tumor volume compared to the control group (Supplementary Fig. 2A). The combined treatment group showed a significant (P < 0.05) reduction in tumor volume (Fig. 6b). The reduction in tumor size and prolonged percent survival of the Aloperine–Adbic combined treatment group might be resulted due to the synergistic therapeutic effects of combined treatment. In vivo experimental results are per the in vitro experimental results.
Effects of Aloperine, Adbic, or combination of Aloperine and Adbic on NSCLC mice models. a Kaplan–Meier survival curve for nude mice-bearing NSCLC subcutaneous tumors shows that all control animals (PBS) died by day 43 when 86% of the Aloperine treated, 86% of the Adbic-treated, and 100% of the Aloperine and Adbic combination-treated mice were still alive (*P < 0.05). On day 90, the survival rate of mice models received Aloperine–Adbic combination treatment, Aloperine, and Adbic alone treatment noted 72%, 42%, and 42%, respectively. Combined treatment significantly (P < 0.05) prolonged the survival rate in mice models compared to alone treatments. b Aloperine, Adbic, or ALO–Adbic combined treatments reduced the tumor volume. Tumor volume reduction was significant in ALO–Adbic co-treatment compared to the control group (P < 0.05)
Discussion
NSCLC is the most common type (85%) of lung cancers and ranked top in terms of incidence and cancer-related mortality (1.6 million annually) in both men and women. During the past decade, the management of NSCLC has evolved enormously. Despite such improvements, there is a need to devise innovative therapeutic approaches especially to improve survival rates in patients with advanced NSCLC. Compounds of natural origin have proven to be an attractive option for cancer therapy due to their tumor-suppressive activity, safety, and inexpensiveness. One such natural agent is Aloperine, a natural alkaloid constituent isolated from the herb S. alopecuroides, which has been reported to exhibit anti-proliferative activity against, which has attracted much attention for its anti-cancer effects in many cancers.
Gene therapies based on targeted delivery of therapeutic adenoviral vectors expressing tumor suppressor genes have exerted potent anti-tumor effects in many cancers (Muhammad et al. 2019; Muthana et al. 2011).
In this study, we have demonstrated the anti-NSCLC therapeutic effects of newly devised treatment approach by combining chemotherapy (ALO) and targeted gene therapy (Adbic). The resulted synergistic effects of the combination of low toxicity anti-cancer agents like Adbic with chemotherapeutic agents might overcome drug-resistance and high toxicity issues.
Aloperine has investigated in many studies for its growth inhibition activities against many tumor cell types. The results of such studies proved the anti-tumor-proliferative tendency of Aloperine (Chen et al. 2018; Ling et al. 2018; Liu et al. 2019; Tian et al. 2018; Zhang et al. 2014). Adenoviral vectors also successfully exerted anti-tumor effects against different cancers and contributed in the growth inhibition of tumor cells (Muhammad et al. 2019; Sakhawat et al. 2017). Observations of our study also supported the aforesaid findings that Aloperine and Adbic are potent anti-tumor cell proliferative agents as both have inhibited the growth of NSCLC cells in dose-dependent manner. Combination of both not only reduced the IC50 value of anti-cancer agents, but also exhibited synergistic NSCLC tumor inhibition effects.
Aloperine and Adbic halted the growth of tumor cells by inducing apoptosis and arresting cell cycle progression. Apoptosis induction and cell cycle arrest are considered hallmark cytotoxic effects for many anti-cancer agents (Huang et al. 2017; Kim et al. 2014; Song et al. 2017). Aloperine and adenoviral vectors are associated with inducing apoptosis in many tumor cells (Wang et al. 2015; Xu et al. 2017). In our study, the apoptosis induction ability of both anti-cancer agents was assessed by flow cytometry. The results revealed that Aloperine, Adbic alone, or combined treatment produced effective and synergistic apoptotic effects, respectively, in NSCLC cells.
Previous studies demonstrated that ALO halted the cell cycle progression at the G2/M phase in different cancers (Liu et al. 2019; Zhang et al. 2014). ALO also found out to be associated with the G1 cell cycle arrest in prostate cancer (Ling et al. 2018). We conducted cell cycle analysis through flow cytometry, which revealed that the majority of ALO and Adbic alone treated NSCLC tumor cells population accumulated in the G1 phase. Combined treatment of both anti-cancer agents significantly (P < 0.05) increased the proportion of NSCLC tumor cells in the G1 phase compared to alone treatment.
The mechanism of ALO-induced anti-tumor activities like apoptosis and the G1 phase arrest in NSCLC tumor cells is unclear. Adbic-induced anti-tumor activities could result due to the raised expression of the p53 gene, which in turn triggered the p53 regulated downstream events responsible for cell cycle arrest and apoptosis (Huang et al. 2003; Muhammad et al. 2019). Cell cycle progression is regulated by the cyclins and cyclin-dependent kinases (CDKs), which belong to the serine/threonine kinases family (Williams and Stoeber 2012). Cyclin D-CDK4, cyclin D-CDK6, and cyclin E-CDK2 complexes play a vital role in G1 phase progression through the checkpoints and let the cells follow the cell cycle till its completion (Planas-Silva and Weinberg 1997). The p53 has a role in the upregulation of the p21 tumor suppression gene. The p21 gene has the inhibitory effect on cyclin E-cdk2. This inhibition prevents cyclin E-cdk2 complex to act upon Rb protein. It results in suppression of the E2F1 activity, which is responsible for the expression of genes involved in the progression of the G1-S phase of the cell cycle (Dulić et al. 1994). In our study, we investigated the expression of genes involved in the p53/p21 pathway which leads G1 cell cycle arrest and apoptosis. Western blot analysis on ALO, Adbic, or ALO–Adbic-treated NSCLC cells revealed the upregulation of p53 and p21 genes and downregulation pattern in Cyclin E, CDK2, Rb, and E2F1 genes (Fig. 7a). This upregulations and downregulations of genes mentioned above were much significant in ALO–Adbic combined treated cells. This finding confirmed that both anti-cancer agents employed cell cycle arrest and apoptosis by activating p53/p21-triggered G1 arrest pathway. In combination treatment, anti-cancer activities of ALO and Adbic were augmented, thus produced synergistic effects.
In our study, the results of in vivo experiments on NSCLC mice models second the in vitro findings. The mice models received ALO, Adbic alone treatment survived for a long time and showed reduction in tumor volume compared to the control (PBS) group. Synergistic effects of ALO–Adbic combined treatment contributed to a much-prolonged survival rate in mice models. The activation of the p53/p21 pathway, cell cycle arrest, and apoptosis might be the reasons for the high survival rate in treated mice models.
In this study, we used E1A/E1B-modified MSCs (MSCs-E1s) for the targeted delivery of Adbic to the tumor site. MSCs are easy to acquire, and these can selectively and precisely target the tumor cells due to their tumor tropism ability 46, 47. Recently, a study reported that unmodified MSCs might not be good candidates for cancer therapy in humans due to the imperfect detection of MSCs from the tumor site. The authors of this study confirmed the safety of the systemic delivery of MSCs as none of the participants produced any adverse effect after therapy (Schweizer et al. 2019). Several factors might be responsible for these findings like the insensitivity of techniques used to detect MSCs at the tumor site, lack of sufficient inflammatory signals, required to drive MSCs to the tumor site, and use of genetically unmodified MSCs (Serakinci and Cagsin 2019a, 2019b). However, it is evident that MSCs have systemically delivered therapeutic agents to tumor site, yielded higher concentrations of anti-cancer agents at tumor site with minimal adverse effects to normal tissues in animal models (Brennen et al. 2013, 2017; Myers et al. 2010; Sarkar et al. 2010). In our previous study, we investigated the ability of MSC-E1s as packaging, propagating, and targeted delivery vehicles for Adbic, and we found that MSC-E1s were successfully propagated and delivered the Adbic specifically to the tumor site and reduced the tumor volume in mice models (Muhammad et al. 2019). In this study, we also found out that MSC-E1-mediated delivery of Adbic reduced the tumor volume in mice models.
Conclusion
In our study, the therapeutic effects of ALO, Adbic, or synergistic therapeutic effects of ALO–Adbic against NSCLC investigated. ALO, Adbic, or ALO–Adbic combination produced promising and synergistic anti-tumor effects. Cytotoxic effects of ALO–Adbic combined treatment much enhanced at low doses. This decrease in dosage might contribute to minimizing the side effects of anti-cancer agents. In the current study, the synergistic cytotoxic effects of ALO–Adbic combined treatment suppressed the growth of NSCLC cells in both in vitro and in vivo experimentations. The underlying mechanism that resulted in enhanced cytotoxic effects may be the activation of p53/p21-triggered G1 phase cell cycle arrest and increased apoptosis. Therefore, ALO–Adbic combined treatment has the potential to be used as an alternative therapeutic approach for NSCLC treatment as it offers higher cytotoxic effects at low doses of drugs. However, further investigations need to be done to explore and verify the molecular mechanisms that contribute to producing synergistic anti-tumor effects. The efficacy of this treatment approach needs to be tested in orthotopic immunocompetent models before conducting clinical trials.
Availability of data and material
The data sets used and/or analyzed following standard procedures during the current study and are available from the corresponding author on reasonable request.
References
Andrews P, Howell S (1990) Cellular pharmacology of cisplatin: perspectives on mechanisms of acquired resistance. Cancer Cells (Cold Spring Harbor, NY: 1989) 2:35–43.
Bates S, Phillips AC, Clark PA, Stott F, Peters G, Ludwig RL, Vousden KH (1998) p14 ARF links the tumour suppressors RB and p53. Nature 395:124
Brennen WN, Chen S, Denmeade SR, Isaacs JT (2013) Quantification of mesenchymal stem cells (MSCs) at sites of human prostate cancer. Oncotarget 4:106
Brennen WN et al (2017) Mesenchymal stem cell infiltration during neoplastic transformation of the human prostate. Oncotarget 8:46710
Chen S et al (2018) Aloperine induces apoptosis and inhibits invasion in MG-63 and U2OS human osteosarcoma cells. Biomed Pharmacother 97:45–52
Chou T (1991) The median-effect principle and the combination index for quantitation of synergism and antagonism. Synerg Antagonism Chemother 1:61–102
Chou T, Martin N (2005) A computer program for quantitation of synergism and antagonism in drug combinations, and the determination of IC50 and ED50 and LD50 values CompuSyn for Drug Combinations: PC Software and User’s Guide; ComboSyn: Paramus. NJ, USA
de Stanchina E et al (1998) E1A signaling to p53 involves the p19ARFtumor suppressor. Genes Dev 12:2434–2442
Dulić V et al (1994) p53-dependent inhibition of cyclin-dependent kinase activities in human fibroblasts during radiation-induced G1 arrest. Cell 76:1013–1023
Ferlay J et al (2012) (2015) Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN. Int J Cancer 136:E359–E386
Gately D, Howell S (1993) Cellular accumulation of the anticancer agent cisplatin: a review. Br J Cancer 67:1171
Greenblatt M, Bennett WP, Hollstein M, Harris C (1994) Mutations in the p53 tumor suppressor gene: clues to cancer etiology and molecular pathogenesis. Cancer Res 54:4855–4878
Herbst RS, Morgensztern D, Boshoff C (2018) The biology and management of non-small cell lung cancer. Nature 553:446
Hollstein M, Sidransky D, Vogelstein B, Harris CC (1991) p53 mutations in human cancers. Science 253:49–53
Honda R, Yasuda H (1999) Association of p19ARF with Mdm2 inhibits ubiquitin ligase activity of Mdm2 for tumor suppressor p53. EMBO J 18:22–27
Huang W-S et al (2017) CIL-102-induced cell cycle arrest and apoptosis in colorectal cancer cells via upregulation of p21 and GADD45. PLoS ONE 12:e0168989
Huang Y, Tyler T, Saadatmandi N, Lee C, Borgstrom P, Gjerset RA (2003) Enhanced tumor suppression by a p14ARF/p53 bicistronic adenovirus through increased p53 protein translation and stability. Cancer Res 63:3646–3653
Jemal A et al (2018) Higher lung cancer incidence in young women than young men in the United States. N Engl J Med 378:1999–2009
Kallel H, Kamen AA (2015) Large-scale adenovirus and poxvirus-vectored vaccine manufacturing to enable clinical trials. Biotechnol J 10:741–747
Kannan K et al (2000) The p16INK4alpha/p19ARF gene mutations are infrequent and are mutually exclusive to p53 mutations in Indian oral squamous cell carcinomas. Int J Oncol 16:585–675
Kelland L (2007) The resurgence of platinum-based cancer chemotherapy. Nat Rev Cancer 7:573
Kim MS et al (2014) Naproxen induces cell-cycle arrest and apoptosis in human urinary bladder cancer cell lines and chemically induced cancers by targeting PI3K. Cancer Prev Res 7:236–245
Lee Y-R, Chen S-H, Lin C-Y, Chao W-Y, Lim Y-P, Yu H-I, Lu C-H (2018) Vitro antitumor activity of aloperine on human thyroid cancer cells through caspase-dependent apoptosis. Int J Mol Sci 19:312
Liang L, Wang X-Y, Zhang X-H, Ji B, Yan H-C, Deng H-Z, Wu X-R (2012) Sophoridine exerts an anti-colorectal carcinoma effect through apoptosis induction in vitro and in vivo. Life Sci 91:1295–1303
Ling Z et al (2018) Aloperine executes antitumor effects through the induction of apoptosis and cell cycle arrest in prostate cancer in vitro and in vivo. OncoTargets Ther 11:2735
Liu J-S et al (2019) Aloperine induces apoptosis and G2/M cell cycle arrest in hepatocellular carcinoma cells through the PI3K/Akt signaling pathway. Phytomedicine 61:152843
Maher A, Miake-Lye I, Beroes J, Shekelle P (2012) Treatment of metastatic non-small cell lung cancer: a systematic review of comparative effectiveness and cost-effectiveness
Miller KD et al (2016) Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin 66:271–289
Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. In: Mayo clinic proceedings, 2008, vol 5. Elsevier, pp 584–594
Muhammad T, Sakhawat A, Khan AA, Ma L, Gjerset RA, Huang Y (2019) Mesenchymal stem cell-mediated delivery of therapeutic adenoviral vectors to prostate cancer. Stem Cell Res Ther 10:190
Muthana M et al (2011) Use of macrophages to target therapeutic adenovirus to human prostate tumors. Can Res 71:1805–1815
Myers TJ, Granero-Molto F, Longobardi L, Li T, Yan Y, Spagnoli A (2010) Mesenchymal stem cells at the intersection of cell and gene therapy. Expert Opin Biol Ther 10:1663–1679
Palmero I, Pantoja C, Serrano M (1998) p19 ARF links the tumour suppressor p53 to Ras. Nature 395:125
Pinyol M et al (2000) INK4a/ARFLocus alterations in human non-Hodgkin's lymphomas mainly occur in tumors with wild-type p53 gene. Am J Pathol 156:1987–1996
Planas-Silva MD, Weinberg RA (1997) The restriction point and control of cell proliferation. Curr Opin Cell Biol 9:768–772
Ramalingam S, Belani C (2008) Systemic chemotherapy for advanced non-small cell lung cancer: recent advances and future directions. Oncologist 13:5–13
Sakhawat A, Liu Y, Ling Ma TM, Wang S, Zhang L, Cong X, Huang Y (2017) Upregulation of Coxsackie adenovirus receptor sensitizes cisplatin-resistant lung cancer cells to CRAd-induced inhibition. J Cancer 8:1425
Sarkar D, Vemula PK, Zhao W, Gupta A, Karnik R, Karp JM (2010) Engineered mesenchymal stem cells with self-assembled vesicles for systemic cell targeting. Biomaterials 31:5266–5274
Schweizer MT et al (2019) A phase I study to assess the safety and cancer-homing ability of allogeneic bone marrow-derived mesenchymal stem cells in men with localized prostate cancer. Stem Cells Trans Med 8:441–449
Serakinci N, Cagsin H (2019a) Programming hMSCs into potential genetic therapy in cancer critical reviews™ in eukaryotic gene expression 29
Serakinci N, Cagsin H (2019b) Turning stem cells homing potential into cancer specific drug delivery machines. Ann Transl Med 7
Song X-l et al (2017) Casticin induces apoptosis and G0/G1 cell cycle arrest in gallbladder cancer cells. Cancer Cell Int 17:9
Spiro SG, Porter JC (2002) Lung cancer—where are we today? Current advances in staging and nonsurgical treatment. Am J Respir Crit Care Med 166:1166–1196
Thomas A, Liu SV, Subramaniam DS, Giaccone G (2015) Refining the treatment of NSCLC according to histological and molecular subtypes. Nat Rev Clin Oncol 12:511
Tian D, Li Y, Li X, Tian Z (2018) Aloperine inhibits proliferation, migration and invasion and induces apoptosis by blocking the Ras signaling pathway in human breast cancer cells. Mol Med Rep 18:3699–3710
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A (2015) Global cancer statistics, 2012. CA Cancer J Clin 65:87–108
Torre LA, Sauer AMG, Chen MS Jr, Kagawa-Singer M, Jemal A (2016) Siegel RL (2016) Cancer statistics for Asian Americans, Native Hawaiians, and Pacific Islanders converging incidence in males and females CA. Cancer J Clin 66:182–202
Wang H, Guo S, Qian D, Qian Y, Duan JA (2012) Comparative analysis of quinolizidine alkaloids from different parts of Sophora alopecuroides seeds by UPLC–MS/MS. J Pharmaceut Biomed Anal 67:16–21
Wang H et al (2015) Aloperine executes antitumor effects against multiple myeloma through dual apoptotic mechanisms. J Hematol Oncol 8:26
Williams GH, Stoeber K (2012) The cell cycle and cancer. J Pathol 226:352–364
Wu X, Bayle JH, Olson D, Levine AJ (1993) The p53-mdm-2 autoregulatory feedback loop. Genes Dev 7:1126–1132
Xu Z et al. (2017) Reducing autophagy and inducing G1 phase arrest by aloperine enhances radio-sensitivity in lung cancer cells. Oncol Rep
Yuan X-Y, Liu W, Zhang P, Wang R-Y, Guo J-Y (2010) Effects and mechanisms of aloperine on 2, 4-dinitrofluorobenzene-induced allergic contact dermatitis in BALB/c mice. Eur J Pharmacol 629:147–152
Zhang L et al (2001) Effects of matrine on proliferation and differentiation in K-562 cells. Leukemia Res 25:793–800
Zhang Z et al (2012) Effects of matrine on proliferation and apoptosis in gallbladder carcinoma cells (GBC-SD). Phytother Res 26:932–937
Zhang L, Jiang J, Tan R (1998) Effects of matrine on telomerase activity and cell cycle in K562 cell. Chin J Oncol 20:328–329
Zhang L, Zheng Y, Deng H, Liang L, Peng J (2014) Aloperine induces G2/M phase cell cycle arrest and apoptosis in HCT116 human colon cancer cells. Int J Mol Med 33:1613–1620
Zhou C, Gao H, Sun X, Shi H, Liu W, Yuan H, Wang Z (1989) Anti-inflammatory and anti-allergic action of aloperine. Acta Pharmacol Sin 10:360–365
Acknowledgements
We are very thankful to Ming Zhao in Torrey Pines Institute for Molecular Studies for her technical assistance, and Beijing Inmay Future Biopharma Inc. to provide experimental materials.
Funding
This research was funded by National Natural Science Foundation OF China (Grant # 81472209) and Key Programs of Beijing Municipal Science and Technology Commission (Grant # K2015311201501).
Author information
Authors and Affiliations
Contributions
TM and AS contributed in designing and conducting experiments in this study. KAA also performed experiments. HH and HRK assisted in data analysis and manuscript writing. WJ and YH overall supervised the research work.
Corresponding author
Ethics declarations
Conflict of interest
Authors declared no conflict of interest.
Ethical approval
Study was approved by ethical committee of Beijing University of Technology (approval number IRB-1507).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Muhammad, T., Sakhawat, A., Khan, A.A. et al. Aloperine in combination with therapeutic adenoviral vector synergistically suppressed the growth of non-small cell lung cancer. J Cancer Res Clin Oncol 146, 861–874 (2020). https://doi.org/10.1007/s00432-020-03157-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-020-03157-2