Abstract
Purpose
Previously, the combination of epidermal growth factor receptor-tyrosine kinase inhibitors (EGFR-TKIs) and bevacizumab (BEV) was investigated. A subgroup analysis of the IMpower150 trial, which investigated the combination of atezolizumab, carboplatin, paclitaxel, and bevacizumab (ABCP), demonstrated the benefit of ABCP in patients harboring EGFR mutations. This study aims to assess the prognostic significance of the qualification for BEV use and the proportion of patients who potentially benefit from BEV-containing combination therapy before and after initial EGFR-TKI treatment.
Methods
We retrospectively analyzed the data of 297 patients with advanced or recurrent non-squamous non-small cell lung cancer (NSCLC) harboring EGFR mutations who had received EGFR-TKIs. We performed statistical analyses using the Kaplan–Meier method and the Cox regression adjusted for risk factors.
Results
Of the 297 patients, 203 (68%) were eligible to receive BEV (“BEV fit”) at the time of EGFR-TKI initiation. Among the “BEV unfit” patients at baseline (n = 70), 14 (20%) became eligible to receive ABCP (“ABCP fit”) at the time of EGFR-TKI failure. The median overall survival (OS) of the “BEV fit” and “BEV unfit” patients was 26.2 [95% confidence interval (CI) 23.7–31.2] and 19.1 (95% CI 15.0–25.1) months, respectively (P < 0.001). The multivariate analysis revealed a marked correlation between survival and the qualification for BEV use.
Conclusions
The qualification for BEV use at baseline is independently related to the OS. Some patients harboring EGFR mutations, including those who were “BEV unfit” at baseline, could be eligible for the ABCP regimen after EGFR-TKI treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Globally, lung cancer is one of the leading causes of cancer-related mortality with a poor prognosis (Goldstraw et al. 2016). Recent developments in molecular targeted therapies for oncogenic driver mutations of non-small cell lung cancer (NSCLC) have improved the prognosis in patients with tumors that express the appropriate molecular target for inhibitory agents. Reportedly, epidermal growth factor receptor (EGFR) mutations are the leading driver genetic alterations; they also serve as prognostic factors and predictive factors in targeted therapy with EGFR-tyrosine kinase inhibitors (TKIs) (Paez et al. 2004; Sannomiya et al. 2004; Takano et al. 2005). To date, five EGFR-TKIs, including gefitinib, erlotinib, afatinib, dacomitinib, and osimertinib, have been recognized as the standard of care for patients with advanced NSCLC harboring EGFR mutations. Nevertheless, acquired resistance to EGFR-TKIs and relapse are inevitable within 1–2 years.
Combining EGFR-TKIs and cytotoxic agents or angiogenesis inhibitors has been attempted to improve outcomes. Bevacizumab (BEV), a chimeric monoclonal antibody for vascular endothelial growth factor (VEGF), is a promising agent that could exert a synergetic antitumor effect when combined with EGFR-TKIs. Preclinical studies have suggested a correlation between VEGF and EGFR pathways; furthermore, studies suggest that elevated VEGF levels and tumor angiogenesis contribute to the primary or acquired resistance to EGFR inhibition or the suppression of immunity in the tumor microenvironment through tumor-associated macrophages (Ciardiello et al. 2000, 2001; Bruns et al. 2000; Solorzano et al. 2001; Viloria-Petit et al. 2001; Swinson et al. 2004; Luwor et al. 2005; Swinson and O’Byrne 2006; Amin et al. 2006; Pore et al. 2006; Byers and Heymach 2007). Clinically, several trials have reported the superior efficacy and acceptable toxicity of the EGFR-TKI and BEV combination (Rosell et al. 2017; Seto et al. 2014; Dingemans et al. 2011; Zhao et al. 2018; Ichihara et al. 2015; Ninomiya et al. 2018; Hattori et al. 2015; Kurata et al. 2017; Tamiya et al. 2018). At the 2018 American Society of Clinical Oncology (ASCO) annual meeting, a phase III trial (NEJ026) testing the first-line combination therapy of erlotinib and bevacizumab (EB) illustrated its superiority in an interim analysis (Saito et al. 2018). In addition, the median progression-free survival (PFS) as the primary endpoint was significantly prolonged in the EB arm compared to the erlotinib monotherapy arm [16.9 vs. 13.3 months; hazard ratio (HR), 0.61; 95% confidence interval (CI) 0.42–0.88; P < 0.016] (Saito et al. 2018).
Currently, immune checkpoint inhibitors (ICIs) have been approved for treating advanced-stage NSCLC as monotherapies or in combination with platinum-doublet cytotoxic chemotherapy. Nevertheless, some clinical trials investigating ICIs as monotherapies after the failure of EGFR-TKIs have not reported a survival benefit of ICIs compared to cytotoxic chemotherapy for patients harboring EGFR mutations (Lee et al. 2017). In addition, some of the subsequent phase III trials validating the combination regimens of ICIs and platinum-doublet chemotherapy excluded this patient population. Amid such scenarios, a subgroup analysis of a phase III trial (IMpower150) testing the combination of atezolizumab, carboplatin, paclitaxel, and bevacizumab (ABCP) demonstrated the benefit of ABCP in improving the PFS in patients with EGFR or anaplastic lymphoma kinase (ALK) genetic alterations (Socinski et al. 2018), which possibly implicates the potential clinical benefit of ICI use in the form of the ABCP regimen for patients with NSCLC harboring EGFR mutations who failed to respond to EGFR-TKIs.
Despite such promising results, not all patients with advanced NSCLC harboring EGFR mutations can benefit from BEV-containing combination therapy because of concerns for its adverse events. In addition, the findings warrant cautious interpretation because the risk factors for BEV use could negatively affect patient survival. However, the following unanswered clinical questions persist concerning BEV use for patients with NSCLC harboring EGFR mutations: (1) whether the qualification for BEV use itself affects the survival of patients, (2) what proportion of patients would potentially benefit from the combination therapy of EGFR-TKIs and BEV as the initial EGFR-TKIs, and (3) what proportion of patients (including those who were initially unable to use BEV) could benefit from the ABCP regimen after the initial EGFR-TKIs.
Hence, this study aims to investigate the impact of the qualification for BEV use on the clinical outcomes of patients treated with EGFR-TKI therapy for advanced NSCLC. In addition, this study aims to estimate the proportions of patients who were qualified to use BEV-containing combination therapy before and after the initial EGFR-TKIs.
Methods
Patients
In this study, we retrospectively reviewed the medical records of patients with advanced or recurrent NSCLC harboring EGFR mutations who had received EGFR-TKIs between December 2013 and August 2018 at our institution. Patients who fulfilled the following criteria were enrolled: (1) histologically or cytologically confirmed advanced unresectable (stage III or IV) or recurrent non-squamous (non-SQ) NSCLC harboring EGFR mutations; (2) received EGFR-TKIs (gefitinib, erlotinib, or afatinib) as either the first-line or later lines of therapy and were never treated with any other EGFR-TKIs; (3) the qualification for BEV use (detailed later) could be evaluated by either computed tomography (CT) with a thickness of < 5.0 mm or via a bronchoscopy. In addition, we examined the following clinical factors of patients as the baseline characteristics: sex, age, Eastern Cooperative Oncology Group-Performance Status (ECOG-PS), EGFR mutation status (e.g., exon 19 deletion, exon 21 L858R mutation, or other “uncommon” mutations), staging (UICC classification, eighth edition), presence of brain and liver metastases, history of thoracic radiotherapy, and smoking status.
Qualification for BEV use before first-line EGFR-TKI therapy
We assessed the qualification for BEV use (“BEV fit” or “BEV unfit”) based on the findings from the CT scans and bronchoscopies together with the patients’ medical history. Based on the major exclusion criteria in the preceding clinical trials (Rosell et al. 2017; Seto et al. 2014; Sandler et al. 2006; Reck et al. 2009; Zhou et al. 2015; Zinner et al. 2015), we classified patients as “BEV unfit” if they had any of the following conditions: evidence of a tumor invading major blood vessels, exposure of a tumor to airways (from the trachea, main bronchus to the subsegmental bronchi), presence of cavitating lesions, history of ≥ grade 2 hemoptysis, major surgical procedure or prominent injury within 28 days, active peptic ulcer disease, evidence of bleeding diathesis or coagulopathy, presence of thrombosis or ongoing treatment with anticoagulant or antiplatelet drugs, persistent proteinuria ≥ 2+, and uncontrolled hypertension.
Qualification for ABCP use after first-line EGFR-TKI therapy
We assessed the qualification for ABCP use (“ABCP fit” or “ABCP unfit”) based on the findings from the CT scans or bronchoscopies together with the patients’ medical history. In addition to the eligibility criteria for BEV use (described in “Qualification for BEV use before first-line EGFR-TKI therapy”), we classified patients as “ABCP unfit” if they had any of the following conditions: ECOG-PS ≥ 2, presence of an autoimmune disease, and systemic immunosuppressive medications within 2 weeks.
Statistical analysis
In this study, we used descriptive statistics to summarize the patients’ baseline characteristics. Using Fisher’s exact test for the categorical data and the Mann–Whitney U test for all continuous variables, we assessed the between-group differences at baseline. We defined PFS as the time from the start of EGFR-TKI use to the first-documented disease progression or the date of death. The overall survival (OS) was determined from the date of beginning EGFR-TKI use to the date of death, irrespective of the cause of death. Of note, patients who had not progressed or died at the time of analysis were censored at the date of the last contact. We estimated the survival distributions (PFS and OS) using the Kaplan–Meier method and compared differences between groups using the log-rank test. Moreover, the potential predictors for survival were explored using the Cox regression. All P values in this study are two sided, and P < 0.05 was considered statistically significant. We included the variables with P < 0.1 based on the univariate analysis in the multivariate analysis. All statistical analyses were performed using the JMP 11 software (SAS Institute, Cary, NC).
Results
Baseline characteristics
Overall, 297 patients with non-SQ NSCLC (215 females and 82 males) received EGFR-TKIs. As the initial EGFR-TKI, 228 patients (77%) were treated with gefitinib, 54 (18%) with erlotinib, and 15 (5%) with afatinib; the median patient age was 69 (range 39–89 years). At the time of the EGFR-TKI initiation, based on the 8th edition of the TNM Classification for Lung Cancer, 9 (3%), 47 (16%), 168 (57%), and 73 (24%) patients presented with stage III, stage IVA, stage IVB, and recurrent disease, respectively. Overall, 282 patients (95%) had adenocarcinoma, and 10 (3%) had NSCLC, not other specified (NOS). Table 1 summarizes the patients’ characteristics.
Qualification for bevacizumab use at baseline
Based on the evaluation criteria for the qualification of BEV use, 203 of the 297 patients (68%) were eligible for BEV administration (“BEV fit”), and 94 (32%) were classified as “BEV unfit” at the time of EGFR-TKI initiation (Fig. 1). Table 1 presents the patient characteristics of each subgroup. Among the “BEV unfit” patients (n = 94), 48 (47%) exhibited exposure of the tumor to airways, 36 (38%) were receiving treatment with anticoagulant or antiplatelet drugs, and 13 (14%) presented evidence of the tumor invading major blood vessels. Table 2 presents other reasons for the disqualification of BEV use.
Qualification for bevacizumab use with the development of progressive disease after initial EGFR-TKI therapy
In this study, 157 of the 203 “BEV fit” patients (77%) and 70 of the 94 “BEV unfit” (75%) patients were eligible to be assessed for being qualified for ABCP use with the development of progressive disease (PD) after initial EGFR-TKI therapy. Of note, those who either died or did not experience PD were excluded from this analysis (Fig. 1). Among the “BEV fit” patients at baseline (n = 157), 114 (73%) became “ABCP fit,” whereas 43 (27%) became “ABCP unfit” at the time of PD, based on the evaluation criteria. Conversely, among the “BEV unfit” patients at baseline (n = 70), 56 (80%) became “ABCP unfit,” whereas 14 (20%) became “ABCP fit” (Table 3).
Clinical outcomes of EGFR-TKI therapy
The median PFS time for all patients treated with EGFR-TKIs was 11.5 months (95% CI 9.9–13.5), and the median OS time was 24.3 months (95% CI 21.1–27.2) (Supplemental Fig. 1).
Clinical outcomes of EGFR-TKI therapy in the “fit” or “unfit” for bevacizumab use subgroups
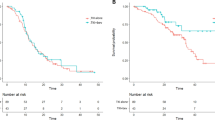
In this study, the median PFS time of the “BEV fit” and “BEV unfit” patients was 12.6 (95% CI 9.8–15.3) and 10.1 (95% CI 7.9–12.7) months, respectively. The median OS time of the “BEV fit” and “BEV unfit” patients was 26.2 (95% CI 23.7–31.2) and 19.1 (95% CI 15.0–25.1) months, respectively. Notably, the differences between the survival curves regarding the PFS and OS were statistically significant (P = 0.02 and < 0.001, respectively; Fig. 2). Table 2 summarizes the reasons for the qualification of ABCP use at the time of PD in patients initially classified as “BEV unfit”.
Univariate and multivariate analyses
The univariate analysis revealed that age, smoking status, ECOG-PS, the presence of brain metastasis, the presence of liver metastasis, the presence of pleural effusions, EGFR mutation status, and qualification for BEV use were markedly correlated with OS (Supplemental Table 1). The multivariate analysis suggested that age, smoking status, ECOG-PS, the presence of brain metastasis, EGFR mutation status, and qualification for BEV use were markedly correlated with OS. Furthermore, the patients qualified to use BEV (i.e., “BEV fit”) exhibited a significantly lower risk of death than the “BEV unfit” patients (HR, 0.72; 95% CI 0.53–0.98; P = 0.038; Table 4).
Discussion
This study assessed the prognostic significance of the qualification for BEV use at baseline in patients with advanced non-SQ NSCLC harboring EGFR mutations; the findings also determined the proportion of patients potentially eligible for BEV-containing combination therapy in a real-world setting. Our findings revealed significant differences in OS between patients who were eligible and ineligible for BEV use. The multivariate analysis revealed that the qualification for BEV use was independently correlated with the OS of patients treated with EGFR-TKIs. In addition, we illustrated that approximately one-third of patients with advanced non-SQ NSCLC harboring EGFR mutations were ineligible for BEV use at baseline. Furthermore, some “BEV unfit” patients became potentially eligible to use ABCP after the initial EGFR-TKI therapy.
Some preceding clinical trials have noted the effect of BEV in combination with EGFR-TKIs. In a subgroup analysis of EGFR mutation-positive participants in the BeTa Lung phase III study, the median PFS duration as the primary endpoint was 17.1 months for the EB arm (n = 12) and 9.7 months for the erlotinib alone arm (n = 18) (Herbst et al. 2011). In the following JO25567 randomized phase II trial, the median PFS duration as the primary endpoint was 16.0 (95% CI 13.9–18.1) months for the EB arm and 9.7 (95% CI 5.7–11.1) months for the erlotinib alone arm (HR, 0.54; 95% CI 0.36–0.79; P = 0.0015) (Seto et al. 2014). As the secondary endpoint of the update analysis presented at the 2018 ASCO Annual Meeting, the median OS duration for those two arms was 47.0 and 47.4 months, respectively (HR, 0.81; 95% CI 0.53–1.23; P = 0.33) (Yamamoto et al. 2018). In the following NEJ026 randomized phase III trial, the median PFS duration as the primary endpoint was 16.9 and 13.3 months for the EB and erlotinib alone arms, respectively (HR, 0.61; 95% CI 0.42–0.88; P = 0.016). At present, another phase III trial comparing EB therapy and erlotinib alone as the first-line therapy is ongoing under the encouraging proof-of-concept results from preceding clinical trials (Gridelli et al. 2016). At the 2019 ASCO annual meeting, another phase III trial testing the combination of erlotinib and ramucirumab (RAM), another angiogenesis inhibitor, illustrated its superior PFS over erlotinib monotherapy (median PFS: 19.4 vs. 12.4 months, HR, 0.591 (95% CI 0.461–0.760), P < 0.0001). This provides more evidence of the beneficial potential of the combination regimens of EGFR-TKIs and angiogenesis inhibitors (Nakagawa et al. 2019). Likewise, several other phase I–III trials have reported favorable outcomes of EGFR-TKIs, including gefitinib and afatinib, and of erlotinib, in combination with BEV or platinum-doublet regimens and both (Rosell et al. 2017; Seto et al. 2014; Sandler et al. 2006; Reck et al. 2009; Zhou et al. 2015; Zinner et al. 2015). Furthermore, trials validating other regimens, including osimertinib in combination with angiogenesis inhibitors (BEV or RAM), are ongoing (Akamatsu et al. 2018, 2019; Hiranuma et al. 2019).
The addition of BEV raises concerns for its adverse events, including the incidence of life-threatening hemorrhage or thromboembolic events, hypertension, and proteinuria. Prior research has identified several risk factors, such as squamous cell carcinoma, centrally located tumors, cavitation of tumors, and others (Johnson et al. 2004), and patients with such risk factors were excluded from some clinical trials (Rosell et al. 2017; Seto et al. 2014; Sandler et al. 2006; Reck et al. 2009; Zhou et al. 2015; Zinner et al. 2015). An assessment of the prognostic significance of the qualification for BEV use may be essential in the upcoming clinical application of the combination therapy of EGFR-TKIs and BEV. In a previous study (n = 154), the qualification for BEV itself represented a powerful prognostic factor for patients with advanced non-SQ NSCLC (Takagi et al. 2013). However, more than 90% of the participants exhibited negative or unknown EGFR mutation status. To date, the prognostic significance of the qualification for BEV use in EGFR mutation-positive patients has not been well investigated.
In this study, we observed marked correlations between survival and the qualification for BEV use in patients with advanced non-SQ NSCLC harboring EGFR mutations irrespective of actual BEV use. We did not observe significant differences in OS between “BEV fit/angiogenesis inhibitors-not-use” (n = 191) and “BEV unfit/angiogenesis inhibitors-not-use” (n = 92). The median OS time was 26.2 (95% CI 23.7–31.2) and 18.8 (95% CI 14.4–22.1) months, respectively (P < 0.001). It might be inappropriate to conclude that patients with poor survival experienced this exclusively due to their BEV-unfitness. Furthermore, BEV-unfitness substantially reflects intrinsic poor prognosis factors (e.g., PS, staging, comorbidities, etc.). However, the qualification for BEV fit or BEV-unfitness could be a surrogate indicator or approximate sum of the properties of the tumor in each patient. In addition, we examined the prognostic impact of the qualification for BEV in real-world settings, which is one of the strengths of the current study. In the preceding major trials including the NEJ026 trial, patients with brain metastases were excluded. Practically speaking, it may be difficult to define poor prognostic factors as a whole. That is one of the reasons we believe the qualification for BEV use is another viewpoint worthy of attention. Specifically, in this study, we intended to evaluate the clinical significance of the qualification for BEV use along with known prognostic factors.
Moreover, one-third of patients with non-SQ NSCLC harboring EGFR mutations may potentially not benefit from EGFR-TKI and BEV combination therapy at baseline in real-world settings. Considering the clinical significance of the qualification for BEV itself, as illustrated in this study, upcoming outcomes of trials on EGFR-TKI and BEV combination therapy should be interpreted cautiously because patients enrolled in these studies do not represent overall patients with non-SQ NSCLC harboring EGFR mutations. In other words, the targeted population (“BEV fit” patients) signifies a selected cohort with a relatively better prognosis, which is often discussed in terms of the presence of brain metastases; this could be clinically plausible because the disqualification for BEV use might denote the extensiveness of tumors and underlying comorbidities.
At present, a paucity of prospective data exists regarding the survival benefit from ICIs or BEV-containing regimens in the later line settings after the failure of the initial EGFR-TKI therapy. Moreover, osimertinib for individuals with acquired T790M mutations or cytotoxic chemotherapy is an accepted standard of care after treatment failure with the initial EGFR-TKIs (Mok et al. 2017). Notably, a subgroup analysis of the IMpower 150 trial reported the superior therapeutic benefit of the ABCP combination regimen over that of the combination of BEV, carboplatin, and paclitaxel, even in patients with EGFR mutations (Socinski et al. 2018); these findings allude to the potential value of ICIs even for patients harboring EGFR mutations. Such a combination therapy could effectively prevent early death after treatment failure with EGFR-TKIs. Based on these study outcomes, we could estimate the proportion of patients potentially eligible for ABCP regimens as a sequential therapy. In this study, 72% (114/157) of the “BEV fit” patients maintained the status quo, whereas 20% (14/70) of the “BEV unfit” patients became eligible to use ABCP at the time of PD with the initial EGFR-TKIs. In addition, tumor shrinkage and the accompanying disappearance of tumor invasion to airways or vessels were the major reasons for being classified as “ABCP fit”. Of note, some “BEV unfit” patients at baseline might benefit from the ABCP regimen after EGFR-TKI therapy. To the best of our knowledge, this is the first study to focus on the sequential changes in the qualification for BEV-containing combination therapy in real-world settings, and our findings might be useful in determining the target population for future clinical trials.
This study has several limitations. First, this was a retrospective, nonrandomized study that was conducted at a single institution; thus, the possibility of unintentional selection bias in the selection of patients could not be completely excluded. Second, the therapeutic effects of second or later lines of treatment, including osimertinib, and the status of the acquired T790M mutation at the time of PD with initial EGFR-TKIs were not assessed. Successive osimertinib use would affect patient survival, which could be another potential bias. At present, osimertinib is available in first-line settings for patients with NSCLC harboring EGFR mutations (Soria et al. 2018). In this study, we excluded patients treated with osimertinib as the initial EGFR-TKI, which could impair the generalizability of our results. Third, the assessment of the qualifications for BEV use via a bronchoscopy was ineligible in many cases at the time of PD, although almost all patients were examined at baseline with both CT scans and a bronchoscopy; this could impair the reliability of the assessment at the time of PD. However, these might be inevitable limitations of any retrospective study. Hence, future prospective studies with larger cohorts are warranted to validate the findings of this study.
Conclusions
The qualification for BEV use itself represents a prognostic factor for patients with advanced non-SQ NSCLC harboring EGFR mutations. This study suggests that some patients harboring EGFR mutations, including those who are “BEV unfit” at baseline, are potentially eligible for the ABCP regimen after the initial EGFR-TKI failure.
Abbreviations
- NSCLC:
-
Non-small cell lung cancer
- EGFR:
-
Epidermal growth factor receptor
- TKI:
-
Tyrosine kinase inhibitor
- BEV:
-
Bevacizumab
- VEGF:
-
Vascular endothelial growth factor
- ASCO:
-
American Society of Clinical Oncology
- EB:
-
Erlotinib and bevacizumab
- PFS:
-
Progression-free survival
- HR:
-
Hazard ratio
- CI:
-
Confidence interval
- ICI:
-
Immune checkpoint inhibitor
- ABCP:
-
Atezolizumab plus carboplatin, paclitaxel, and bevacizumab
- ALK:
-
Anaplastic lymphoma kinase
- non-SQ:
-
Non-squamous
- CT:
-
Computed tomography
- ECOG-PS:
-
Eastern Cooperative Oncology Group-Performance Status
- OS:
-
Overall survival
- NOS:
-
Not other specified
- PD:
-
Progressive disease
- CEA:
-
Carcinoembryonic antigen
- IQR:
-
Interquartile range
- RAM:
-
Ramucirumab
References
Akamatsu H et al (2018) Osimertinib with ramucirumab in EGFR-mutated, T790M-positive patients with progression during EGFR-TKI therapy: phase Ib study. Clin Lung Cancer 19(6):e871–e874
Akamatsu H et al (2019) Phase I/II study of osimertinib with bevacizumab in EGFR-mutated, T790M-positive patients with progressed EGFR-TKIs: West Japan Oncology Group 8715L (WJOG8715L). Clin Lung Cancer 20:e492–e494
Amin DN et al (2006) Tumor endothelial cells express epidermal growth factor receptor (EGFR) but not ErbB3 and are responsive to EGF and to EGFR kinase inhibitors. Cancer Res 66(4):2173–2180
Bruns CJ et al (2000) Blockade of the epidermal growth factor receptor signaling by a novel tyrosine kinase inhibitor leads to apoptosis of endothelial cells and therapy of human pancreatic carcinoma. Cancer Res 60(11):2926–2935
Byers LA, Heymach JV (2007) Dual targeting of the vascular endothelial growth factor and epidermal growth factor receptor pathways: rationale and clinical applications for non-small-cell lung cancer. Clin Lung Cancer 8(Suppl 2):S79–S85
Ciardiello F et al (2000) Antiangiogenic and antitumor activity of anti-epidermal growth factor receptor C225 monoclonal antibody in combination with vascular endothelial growth factor antisense oligonucleotide in human GEO colon cancer cells. Clin Cancer Res 6(9):3739–3747
Ciardiello F et al (2001) Inhibition of growth factor production and angiogenesis in human cancer cells by ZD1839 (Iressa), a selective epidermal growth factor receptor tyrosine kinase inhibitor. Clin Cancer Res 7(5):1459–1465
Dingemans AM et al (2011) First-line erlotinib and bevacizumab in patients with locally advanced and/or metastatic non-small-cell lung cancer: a phase II study including molecular imaging. Ann Oncol 22(3):559–566
Goldstraw P et al (2016) The IASLC lung cancer staging project: proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J Thorac Oncol 11(1):39–51
Gridelli C et al (2016) BEVERLY: rationale and design of a randomized open-label phase III trial comparing bevacizumab plus erlotinib versus erlotinib alone as first-line treatment of patients with EGFR-mutated advanced nonsquamous non-small-cell lung cancer. Clin Lung Cancer 17(5):461–465
Hattori Y et al (2015) A phase 2 study of bevacizumab in combination with carboplatin and paclitaxel in patients with non-squamous non-small-cell lung cancer harboring mutations of epidermal growth factor receptor (EGFR) after failing first-line EGFR-tyrosine kinase inhibitors (HANSHIN Oncology Group 0109). Lung Cancer 87(2):136–140
Herbst RS et al (2011) Efficacy of bevacizumab plus erlotinib versus erlotinib alone in advanced non-small-cell lung cancer after failure of standard first-line chemotherapy (BeTa): a double-blind, placebo-controlled, phase 3 trial. Lancet 377(9780):1846–1854
Hiranuma O et al (2019) Rationale and design of a phase II trial of osimertinib combined with bevacizumab in patients with untreated epidermal growth factor receptor-mutated non-small-cell lung cancer and malignant pleural and/or pericardial effusion (spiral II study). Clin Lung Cancer 20(3):e402–e406
Ichihara E et al (2015) Phase II trial of gefitinib in combination with bevacizumab as first-line therapy for advanced non-small cell lung cancer with activating EGFR gene mutations: the Okayama Lung Cancer Study Group Trial 1001. J Thorac Oncol 10(3):486–491
Johnson DH et al (2004) Randomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol 22(11):2184–2191
Kurata T et al (2017) Phase I/II study of erlotinib, carboplatin, pemetrexed, and bevacizumab in chemotherapy-naive patients with advanced non-squamous non-small cell lung cancer harboring epidermal growth factor receptor mutation. Genes Cancer 8(5–6):559–565
Lee CK et al (2017) Checkpoint inhibitors in metastatic EGFR-mutated non-small cell lung cancer—a meta-analysis. J Thorac Oncol 12(2):403–407
Luwor RB et al (2005) The antiepidermal growth factor receptor monoclonal antibody cetuximab/C225 reduces hypoxia-inducible factor-1 alpha, leading to transcriptional inhibition of vascular endothelial growth factor expression. Oncogene 24(27):4433–4441
Mok TS et al (2017) Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med 376(7):629–640
Nakagawa K et al (2019) RELAY: A multinational, double-blind, randomized Phase 3 study of erlotinib (ERL) in combination with ramucirumab (RAM) or placebo (PL) in previously untreated patients with epidermal growth factor receptor mutation-positive (EGFRm) metastatic non-small cell lung cancer (NSCLC). J Clin Oncol 37(15_suppl):9000
Ninomiya T et al (2018) A phase I trial of afatinib and bevacizumab in chemo-naive patients with advanced non-small-cell lung cancer harboring EGFR mutations: okayama Lung Cancer Study Group Trial 1404. Lung Cancer 115:103–108
Paez JG et al (2004) EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 304(5676):1497–1500
Pore N et al (2006) EGFR tyrosine kinase inhibitors decrease VEGF expression by both hypoxia-inducible factor (HIF)-1-independent and HIF-1-dependent mechanisms. Cancer Res 66(6):3197–3204
Reck M et al (2009) Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAiL. J Clin Oncol 27(8):1227–1234
Rosell R et al (2017) Erlotinib and bevacizumab in patients with advanced non-small-cell lung cancer and activating EGFR mutations (BELIEF): an international, multicentre, single-arm, phase 2 trial. Lancet Respir Med 5(5):435–444
Saito H et al (2019) Erlotinib plus bevacizumab versus erlotinib alone in patients with EGFR-positive advanced non-squamous non-small-cell lung cancer (NEJ026): interim analysis of an open-label, randomised, multicentre, phase 3 trial. Lancet Oncol. 20(5):625–635
Sandler A et al (2006) Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 355(24):2542–2550
Sannomiya M et al (2004) Application of preparative high-speed counter-current chromatography for the separation of flavonoids from the leaves of Byrsonima crassa Niedenzu (IK). J Chromatogr A 1035(1):47–51
Seto T et al (2014) Erlotinib alone or with bevacizumab as first-line therapy in patients with advanced non-squamous non-small-cell lung cancer harboring EGFR mutations (JO25567): an open-label, randomised, multicentre, phase 2 study. Lancet Oncol 15(11):1236–1244
Socinski MA et al (2018) Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med 378(24):2288–2301
Solorzano CC et al (2001) Optimization for the blockade of epidermal growth factor receptor signaling for therapy of human pancreatic carcinoma. Clin Cancer Res 7(8):2563–2572
Soria JC et al (2018) Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med 378(2):113–125
Swinson DE, O’Byrne KJ (2006) Interactions between hypoxia and epidermal growth factor receptor in non-small-cell lung cancer. Clin Lung Cancer 7(4):250–256
Swinson DE et al (2004) Hypoxia-inducible factor-1 alpha in non small cell lung cancer: relation to growth factor, protease and apoptosis pathways. Int J Cancer 111(1):43–50
Takagi Y et al (2013) Eligibility for bevacizumab as an independent prognostic factor for patients with advanced non-squamous non-small cell lung cancer: a retrospective cohort study. PLoS One 8(3):e59700
Takano T et al (2005) Epidermal growth factor receptor gene mutations and increased copy numbers predict gefitinib sensitivity in patients with recurrent non-small-cell lung cancer. J Clin Oncol 23(28):6829–6837
Tamiya M et al (2018) Phase1 study of cisplatin plus pemetrexed with erlotinib and bevacizumab for chemotherapy-naive advanced non-squamous non-small cell lung cancer with EGFR mutations. Invest New Drugs 36(4):608–614
Viloria-Petit A et al (2001) Acquired resistance to the antitumor effect of epidermal growth factor receptor-blocking antibodies in vivo: a role for altered tumor angiogenesis. Cancer Res 61(13):5090–5101
Yamamoto N et al (2018) Erlotinib plus bevacizumab (EB) versus erlotinib alone (E) as first-line treatment for advanced EGFR mutation-positive non-squamous non-small-cell lung cancer (NSCLC): survival follow-up results of JO25567. J Clin Oncol 36(15_suppl):9007–9007
Zhao B et al (2018) Erlotinib in combination with bevacizumab has potential benefit in non-small cell lung cancer: a systematic review and meta-analysis of randomized clinical trials. Lung Cancer 122:10–21
Zhou C et al (2015) BEYOND: a randomized, double-blind, placebo-controlled, multicenter, phase III study of first-line carboplatin/paclitaxel plus bevacizumab or placebo in Chinese patients with advanced or recurrent nonsquamous non-small-cell lung cancer. J Clin Oncol 33(19):2197–2204
Zinner RG et al (2015) PRONOUNCE: randomized, open-label, phase III study of first-line pemetrexed + carboplatin followed by maintenance pemetrexed versus paclitaxel + carboplatin + bevacizumab followed by maintenance bevacizumab in patients ith advanced nonsquamous non-small-cell lung cancer. J Thorac Oncol 10(1):134–142
Acknowledgements
We thank Enago (https://www.enago.jp/) for the English language review.
Funding
This study did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
TH, YO, and KH acquired the clinical data. TH and YO drafted the manuscript. TH, YO, KH, and YH interpreted the data.
Corresponding author
Ethics declarations
Conflict of interest
None.
Ethical approval
This study protocol was approved by the Ethics Committee of the Tokyo Metropolitan Cancer and Infectious Diseases Center Komagome Hospital and was conducted in accordance with the tenets of the Declaration of Helsinki.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hakozaki, T., Okuma, Y., Hashimoto, K. et al. Correlation between the qualification for bevacizumab use and the survival of patients with non-small cell lung cancer harboring the epidermal growth factor receptor mutation: a retrospective analysis. J Cancer Res Clin Oncol 145, 2555–2564 (2019). https://doi.org/10.1007/s00432-019-02985-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-019-02985-1